Abstract
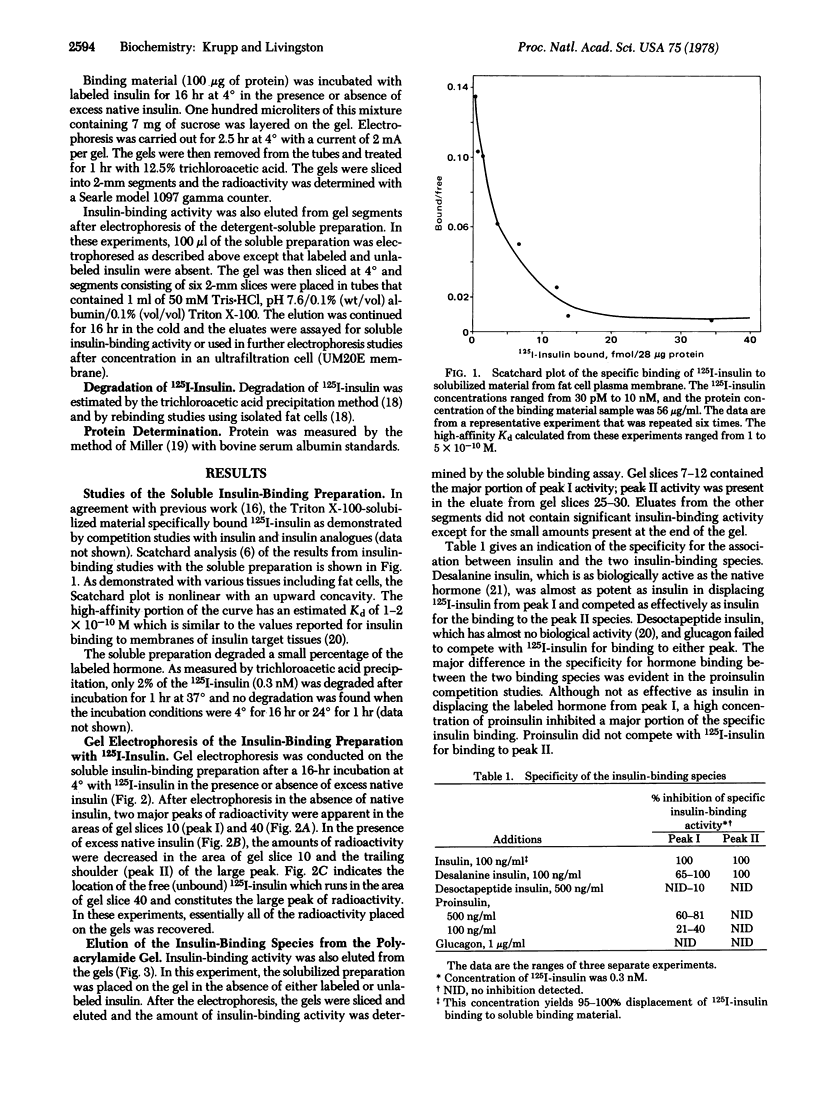
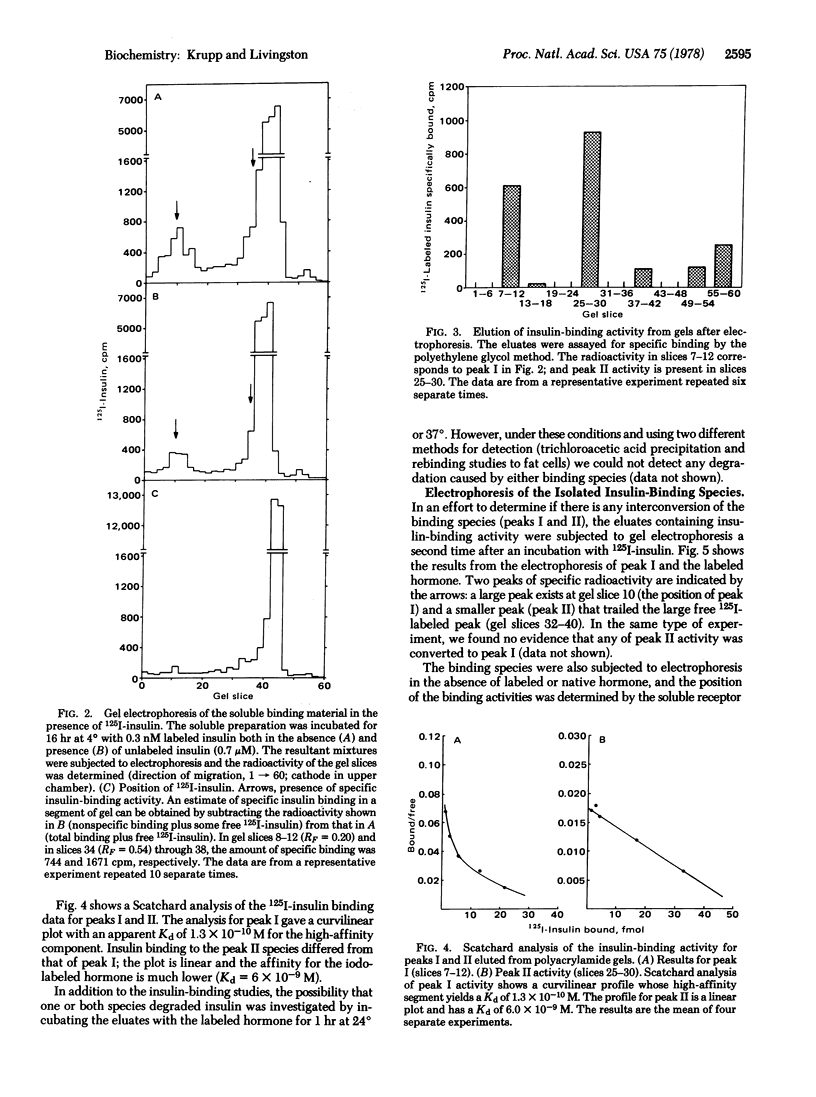
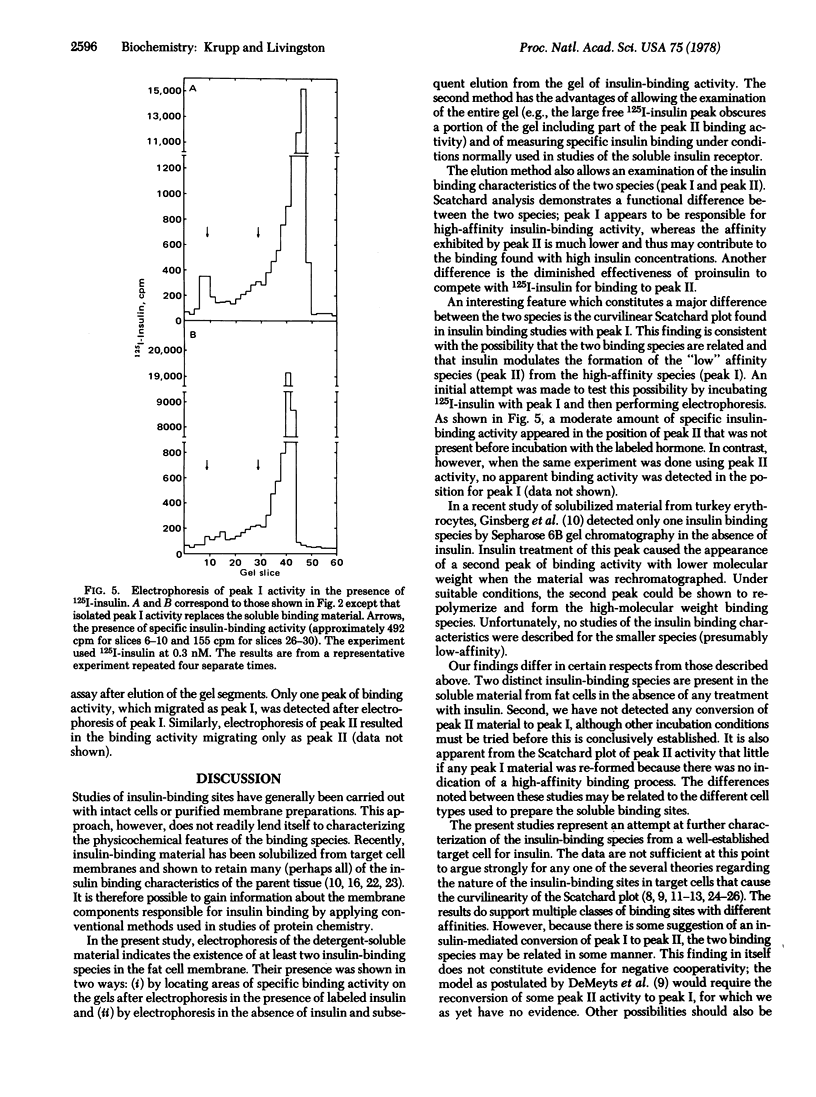
The components of fat cell membranes responsible for the binding of insulin were solubilized by treatment with the nonionic detergent Triton X-100. By using a polyethylene glycol precipitation method to assay specific insulin binding, the soluble preparation was shown to have insulin-binding characteristics similar to those of intact fat cells. Further studies of this preparation by polyacrylamide gel electrophoresis in the presence of 125I-labeled insulin demonstrated two distinct insulin binding activities, designated species I and II. The two species were separated by electrophoresis in the absence of iodo-labeled hormone and eluted from the gel. Scatchard analysis of the insulin binding data for species I showed a curvilinear plot with the initial portion having a Kd of 1.3 × 10-10 M. The Scatchard plot for species II was linear with a Kd of 6.0 × 10-9 M. Desoctapeptide insulin and glucagon failed to compete for the insulin-binding sites in both species whereas desalanine insulin was an effective competitor. High concentrations of proinsulin competed with the iodo-labeled hormone for binding to species I but not to species II. In the presence of a low concentration of 125I-labeled insulin (0.3 nM) some species I activity appeared to be converted to species II activity; there was no evidence of interconversion between the two species in the absence of insulin. Neither species degraded insulin as measured by trichloroacetic acid precipitation or rebinding to intact fat cells. These findings indicate the existence in the adipocyte plasma membrane of two insulin-binding species that have distinct physicochemical properties.
Keywords: insulin receptor, polyacrylamide gel electrophoresis, adipocyte
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boeynaems J. M., Dumont J. E. The two-step model of ligand-receptor interaction. Mol Cell Endocrinol. 1977 Mar;7(1):33–47. doi: 10.1016/0303-7207(77)90074-0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D. Membrane receptors and hormone action. Adv Protein Chem. 1976;30:251–451. doi: 10.1016/s0065-3233(08)60481-7. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Properties of the insulin receptor of isolated fat cell membranes. J Biol Chem. 1971 Dec 10;246(23):7265–7274. [PubMed] [Google Scholar]
- Czech M. P., Lynn W. S. The plasma membrane of isolated fat cells. I. Identification of trypsin-sensitive membrane peptides by sodium dodecyl sulfate polyacrylamide gel electrophoresis. J Biol Chem. 1973 Jul 25;248(14):5081–5088. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DeMeyts P., Bainco A. R., Roth J. Site-site interactions among insulin receptors. Characterization of the negative cooperativity. J Biol Chem. 1976 Apr 10;251(7):1877–1888. [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Insulin receptors in the liver: specific binding of ( 125 I)insulin to the plasma membrane and its relation to insulin bioactivity. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1833–1837. doi: 10.1073/pnas.68.8.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Buell D. N., Roth J. Preparation of solubilized insulin receptors from human lymphocytes. Biochem Biophys Res Commun. 1972 Nov 1;49(3):870–876. doi: 10.1016/0006-291x(72)90491-3. [DOI] [PubMed] [Google Scholar]
- Ginsberg B. H., Kahn C. R., Roth J., De Meyts P. Insulin-induced dissociation of its receptor into subunits: possible molecular concomitant of negative cooperativity. Biochem Biophys Res Commun. 1976 Dec 20;73(4):1068–1074. doi: 10.1016/0006-291x(76)90232-1. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Gammeltoft S., Vinten J. Insulin receptors in fat cells: relationship between binding and activation. Isr J Med Sci. 1975 Jul;11(7):656–663. [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. The mobile receptor hypothesis and "cooperativity" of hormone binding. Application to insulin. Biochim Biophys Acta. 1976 May 21;433(3):482–495. doi: 10.1016/0005-2736(76)90275-3. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Freychet P., Roth J., Neville D. M., Jr Quantitative aspects of the insulin-receptor interaction in liver plasma membranes. J Biol Chem. 1974 Apr 10;249(7):2249–2257. [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution of D-glucose transport catalyzed by a protein fraction from human erythrocytes in sonicated liposomes. Proc Natl Acad Sci U S A. 1976 Feb;73(2):396–400. doi: 10.1073/pnas.73.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseid S., Beck-Nielsen H., Pedersen O., Sovik O. Decreased binding of insulin to its receptor in patients with congenital generalized lipodystrophy. N Engl J Med. 1977 Feb 3;296(5):245–248. doi: 10.1056/NEJM197702032960503. [DOI] [PubMed] [Google Scholar]
- Pollet R. J., Standaert M. L., Haase B. A. Insulin binding to the human lymphocyte receptor. Evaluation of the negative cooperativity model. J Biol Chem. 1977 Aug 25;252(16):5828–5834. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Roth J. Peptide hormone binding to receptors: a review of direct studies in vitro. Metabolism. 1973 Aug;22(8):1059–1073. doi: 10.1016/0026-0495(73)90225-4. [DOI] [PubMed] [Google Scholar]
- de Haën C. The non-stoichiometric floating receptor model for hormone sensitive adenylyl cyclase. J Theor Biol. 1976 May 21;58(2):383–400. doi: 10.1016/s0022-5193(76)80126-9. [DOI] [PubMed] [Google Scholar]
- de Meyts P., Roth J., Neville D. M., Jr, Gavin J. R., 3rd, Lesniak M. A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973 Nov 1;55(1):154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]



