Significance
We developed a novel proteomic platform that can easily be translated into a protein biomarker diagnostic. This platform integrates microfluidic technology with electrical impedance sensing and embodies a unique two-chamber architecture in which the capture/reaction and detection steps are physically separated from one another. This two-chamber design is a deviation from traditional proteomic biosensors and offers the potential for greater sensitivity as the capture/reaction and detection steps can be independently optimized. We demonstrate the implementation and feasibility of this platform in assaying both protein abundance, using the human cytokine interleukin 6, and activity, using Abelson tyrosine kinase.
Keywords: biosensor, decoupled architecture, bead-based assay
Abstract
Global studies of the human proteome have revealed a plethora of putative protein biomarkers. However, their application for early disease detection remains at a standstill without suitable methods to realize their utility in the clinical setting. There thus continues to be tremendous interest in developing new technology for sensitive protein detection that is both low in cost and carries a small footprint to be able to be used at the point of care. The current gold standard method for protein biomarker detection is the ELISA, which measures protein abundance using bulky fluorescent scanners that lack portability. Here, we present a digital microfluidic platform for protein biomarker detection that is low in cost compared with standard optical detection methods, without any compromise in sensitivity. This platform furthermore makes use of simple electronics, enabling its translation into a portable handheld device, and has been developed in a manner that can easily be adapted to assay different types of proteomic biomarkers. We demonstrate its utility in quantifying not only protein abundance, but also activity. Interleukin-6 abundance could be assayed from concentrations as low as 50 pM (an order of magnitude lower than that detectable by a comparable laboratory designed ELISA) using less than 5 μL of sample, and Abelson tyrosine kinase activity was detectable in samples containing 100 pM of kinase.
Recent decades of research have shown that the molecular mechanisms underlying human disease are much more complex than originally appreciated, with one or more rare genetic events often playing key roles in the transformation of a normal cell into a diseased cell. Not surprisingly, the diagnosis and treatment of many human diseases continue to struggle from the use of simplified models that are based on a handful of highly expressed biomarkers. Thanks to recent advances in microarray and next-generation sequencing technologies, a steadily increasing number of complete genomes have been sequenced, and considerable progress has been made using genomic biomarkers to better personalize healthcare. However, the ability to provide complete personalized healthcare demands the ability to monitor genetic biomarkers as well as protein biomarkers, and the development of proteomic technologies suitable for analyzing human samples has lagged considerably behind.
To be well suited as a clinical diagnostic, a proteomic technology must be sensitive enough to detect endogenous levels of low abundance proteins, require low sample volumes, have a short assay time, and ideally be portable as to allow informed treatment decisions to be made at the point of care. Microfluidics, with its inherent advantages of low sample and reagent volumes, multiplex and automated capabilities, and precise control over the microenvironment, has emerged as a promising operation platform with which to develop sensitive proteomic technologies. Several groups have used microfluidic sandwich immunoassays and enzymatic assays to demonstrate improvements in assay cost (1), amount of reagents and the starting sample needed (2, 3), assay times (4), and, most importantly, sensitivity (5–10). However, to move away from “chips in labs” and to develop true “lab on a chip” technologies that can potentially be portable and even handheld, it is necessary to both miniaturize the fluidics and minimize the footprint of the instrumentation as a whole. Such miniaturization can be accomplished by scaling down the optical readout instrumentation, but also by using electronic detection, which offers the added benefit of being low in cost (11). To date, several attempts at label-free electronic detection, such as detection based on impedance spectroscopy at the surface of microelectrodes (12), capacitive sensing-based approaches (13–15), and field affect transistor-based approaches (16), have been made with varying success. The main challenge with these approaches is the requirement for operation in low salt concentrations. High salt concentrations screen any field emitted from charged macromolecules, such as proteins, making the device unsuitable for analyzing complex biological mixtures like serum, which have salt concentrations in the range of several hundred millimolar. In addition, alternative methods to optical and electrical detection, such as magnetic detection, have also been explored. For example, much promise has been shown in achieving matrix-independent protein detection using nanomagnetic tags together with magnetic-sensing techniques such as giant magnetoresistive (GMR) sensing (17) and Hall sensing (18).
Traditional affinity-based protein biosensors, such as those described above, typically work by immobilizing a specific probe molecule onto an active transducing region and are therefore designed such that the capture/reaction and detection steps are coupled to one other. This coupled architecture is limiting in sensitivity as the constraints for designing an optimal capture/reaction chamber inherently contradict with that for an optimal detection chamber. For example, increasing the area of the capture/reaction chamber will increase the sensitivity of the transducer but decrease the rate at which target molecules are captured. Conversely, decreasing the area of the capture/reaction chamber will increase the rate at which target molecules are captured but decrease the sensitivity of the transducer.
We set out to develop a digital proteomic technology that has the potential to be more sensitive than existing microfluidic platforms. Our technology builds on a bead-based immunoassay involving bulk electrical impedance measurement of beads (19) but uniquely embodies a decoupled architecture in which the capture/reaction and detection steps are physically separated and thus able to be independently optimized. This decoupled architecture allows us to interrogate a surface several thousand squared micrometers in area and still maintain single bead detection. Here, we demonstrate the feasibility and implementation of this technology as a method to assay both protein abundance using the human cytokine interleukin 6 (IL-6) and enzyme activity using the human tyrosine Abelson (Abl) kinase.
Results
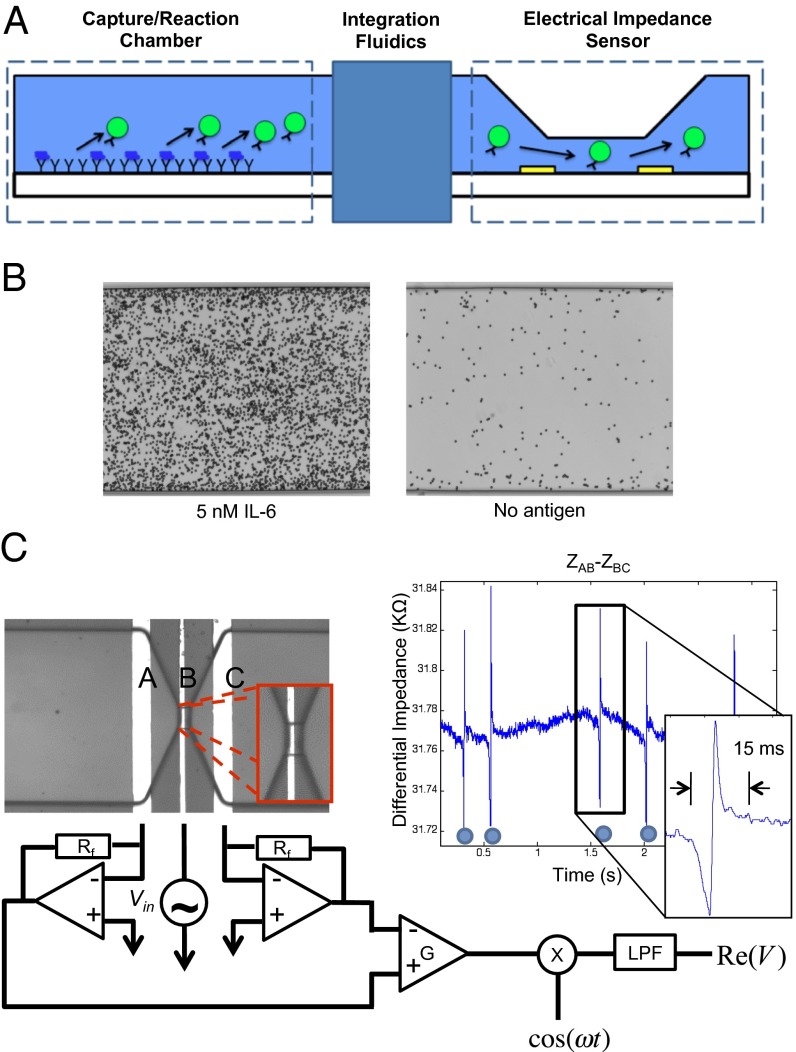
Our platform consists of two components: a capture/reaction chamber that has probe molecules specific to the target of interest functionalized on its surface and an electrical impedance sensor consisting of a micropore sandwiched between two gold electrodes (Fig. 1A). Although this platform has been designed to be adaptable to different types of proteomic assays, initial efforts were focused on the development of a chip to assay protein abundance using a standard sandwich immunoassay strategy. In this adaptation, antibodies specific for the target of interest are both functionalized onto the surface of the capture/reaction chamber and conjugated to micrometer-sized beads. The sample to be analyzed is first injected into the capture/reaction chamber and allowed to incubate for 1 h. The capture/reaction chamber is then emptied of the sample, and detection is performed by packing the chamber with the antibody-conjugated micrometer-sized beads. The presence of the target of interest in the sample thus results in the formation of the immuno-sandwich structure, capturing the beads onto the surface within seconds. Any unbound and nonspecifically bound beads are washed out of the channel, and the specifically bound beads are eluted and directed toward the electrical impedance sensor where they are counted electrically. Each bead when passed singly through the active electrodes will induce a change in resistance. Thus, each change in resistance will correlate to a specifically bound bead, and in turn, counting the changes in resistance will correlate with the abundance of target protein present in the sample.
Fig. 1.
Strategy overview. Our digital microfluidic biosensor has a decoupled architecture consisting of a capture/reaction chamber that is physically separated from an electrical impedance sensor (A). To assay protein abundance, a standard sandwich immunoassay strategy was used where antibodies against the target of interest were both immobilized onto the surface of the capture/reaction chamber and conjugated to micrometer-sized beads. Shown are representative micrographs of specifically bound beads in the capture/reaction chamber (B) and the electrical impedance sensor, as well as a representative current trace of beads being digitally counted (C).
Capture/Reaction Chamber Design.
In such a bead-based microfluidic assay, there are several parameters that contribute to the sensitivity of the capture/reaction step. These parameters include the area of the probe surface, the flow rate with which the sample is introduced into the capture/reaction chamber, the flow rate during the various wash steps, the flow rate used to elute the beads to the detection chamber, and the size and type of the beads used. With these parameters in mind, we fabricated a relatively wide (300 µm) and long (4 mm) capture/reaction chamber that was 30 µm tall to maximize the number of interactions between the target protein and the functionalized surface. The bead type, bead size, and flow rates mentioned herein were used because they complemented our large capture/reaction chamber in maximizing sensitivity. Magnetic beads (2.8 µm) were optimal in terms of being able to be easily manipulated, having no aggregation behavior and rapid settling onto the probe surface in the capture/reaction chamber, and being small in size to maximize the area of the probe surface that is interrogated. Shown in Fig. 1B is a representative micrograph of immunobound beads in the capture/reaction chamber.
Fluidic Integration.
Although the capture/reaction chamber is physically separated from the detection chamber, the two chambers are in fluid contact with one another, and thus much attention was paid to the integration of the two chambers. Fluidic integration posed two main challenges, the first being that the large capture/reaction chamber needed to be tapered down to a small micropore with single bead sensitivity. As a result, simply fabricating a chip with the two chambers connected in a continuous fashion resulted in beads clogging the micropore. The second challenge was that during the detection step when the beads are injected into the capture/reaction chamber, the beads had a tendency to sediment and nonspecifically bind to the surface of the inlet well. Consequently, during the elution step, in addition to the beads that are specifically bound in the capture/reaction chamber, these nonspecifically bound beads were also eluted and counted, therefore resulting in a high level of background and compromising our platform’s sensitivity.
To prevent the micropore from becoming clogged by beads during the detection step, we introduced a cascade of filters between the capture/reaction chamber and the micropore to trap any contaminating particles larger than 7 µm in diameter from reaching the electrical impedance sensor (SI Materials and Methods). These filters also helped to break up any bead aggregates, ensuring that the beads passed singly through the micropore. We in addition made use of extremely low noise electronic readout circuitry to maximize the size of the micropore such that bead clogging could be avoided, but single 2.8-µm bead sensitivity was still maintained. We experimentally tested a range of micropore sizes and determined that that the micropore must be at least 40 µm wide and 10 µm tall.
To prevent nonspecifically bound beads in the inlet well from getting counted along with the specifically bound beads, we fabricated a bead trap structure between the inlet well and the capture/reaction chamber. This bead trap was designed with an overhang, which reduced the channel height leading into the capture/reaction chamber to 15 µm (SI Materials and Methods). After washing the unbound or weakly bound antibody-conjugated beads from the capture/reaction chamber, 20-µm followed by 8-µm polystyrene beads were injected into the channel. These larger beads, which get trapped by the overhang, essentially formed a barrier to any 2.8-µm beads present in the inlet well from entering the capture/reaction chamber during the elution step.
Electrical Impedance Sensor Design.
To detect single 2.8-μm beads, we implemented a differential measurement configuration. Three electrodes, A, B, and C, were each spaced 50 µm apart, with electrode B positioned in the middle of the micropore at the base (Fig. 1C). The impedance was measured between electrodes A and B (ZAB) and also B and C (ZBC), and the difference between ZAB between ZBC was calculated. Each bead passing through the micropore resulted in a negative peak followed by a positive peak in the impedance. This differential measurement configuration, compared with a single-ended configuration, was found to minimize the common-mode signal and increase the signal-to-noise ratio. This differential measurement configuration furthermore offered a processing gain by having a double peak signature result from a single event that was even further amplified by applying a matched filter to the measured signal.
A lock-in-amplification measurement was also used; one electrode was excited with a 300-mV AC signal high enough in frequency (700 kHz) to bypass the interfacial parasitic capacitance at the surface of the electrodes, and an output electrode was tied to a transimpedance amplifier. The transimpedance amplifier fed into a mixer to rectify the signal and then a low-pass filter to measure the envelope of the output signal. We used Zurich Instruments Hf2A lock-in-amplifier that in our frequency range of interest had an input current noise as low as 1 pA/Hz1/2.
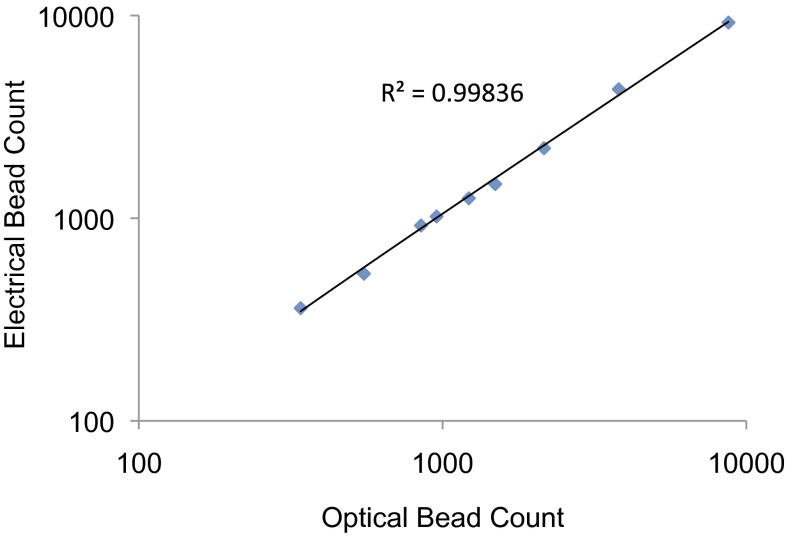
To demonstrate the accuracy of the design of our electrical impedance sensor, we optically quantified immunobound beads on the surface of the capture/reaction chamber and then chemically eluted them to the electrical impedance sensor where they were recounted electrically. Various concentrations of immunobound beads were quantified. As shown in Fig. 2, we observed a linear trend with an R2 value of 0.9984 for electrical bead counts compared with optical bead counts.
Fig. 2.
Evaluation of electrical impedance sensor accuracy. Varying concentrations of immunobound beads in the capture/reaction chamber were optically quantified before being eluted. The eluted beads were directed toward the detection chamber where they were recounted electrically. Shown is a graph of optical bead counts as a function of electrical bead counts. A linear trend with R2 = 0.9984 was observed.
IL-6 Immunoassay.
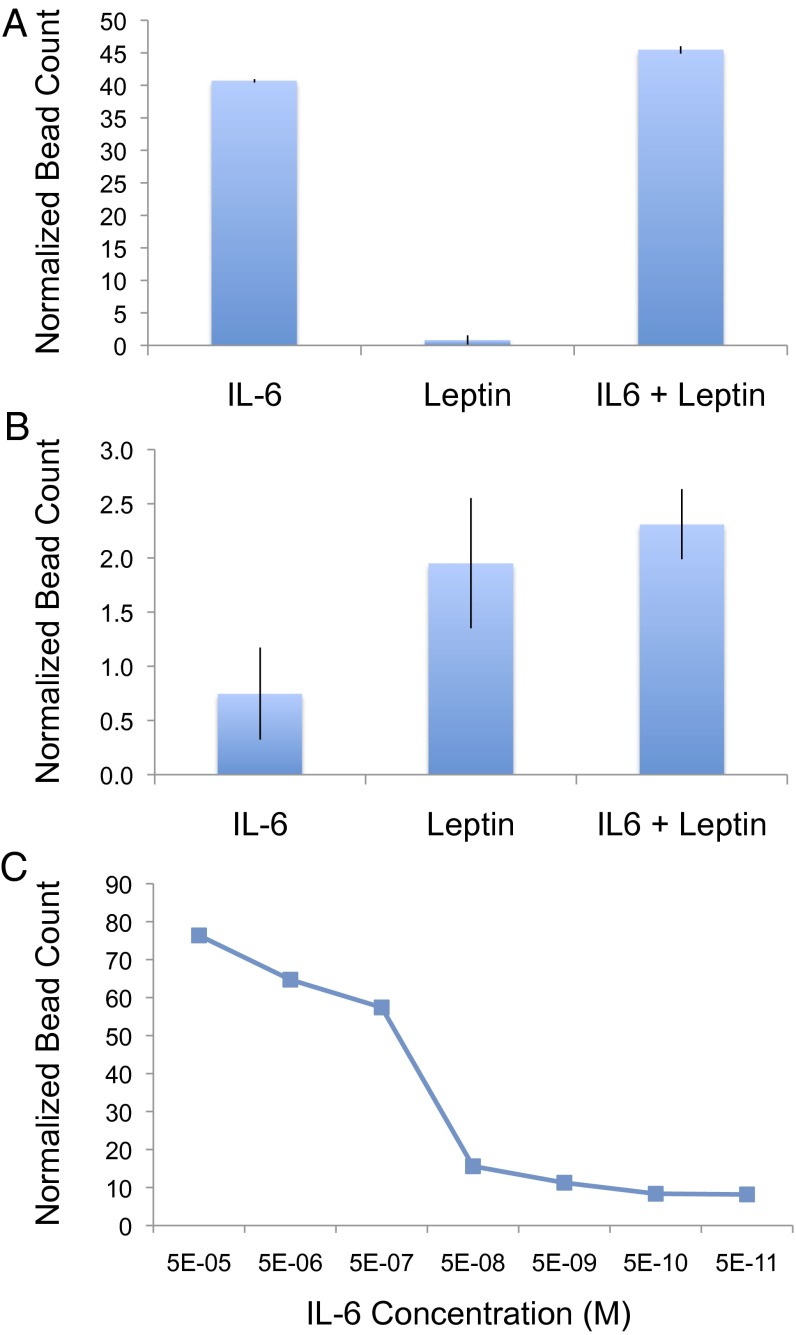
With continued interest in developing methods to quantify cancer-related cytokines for early detection, we chose to use IL-6 for a proof-of-concept demonstration of an immunoassay application. IL-6 is a multifunctional cytokine known to play key roles in a wide range of biological activities in different cell types and has been implicated to be involved in the host immune defense mechanism, as well as the regulation of growth and differentiation in various cancers. To show the specificity of the microfluidic immunoassay, antibodies specific for IL-6 were both immobilized on the surface of the reaction chamber and conjugated to 2.8-μm beads. Using our IL-6 chips, we assayed three samples: 5 nM IL-6, 600 nM leptin, and 5 nM IL-6 and 600 nM leptin mixed together. Each sample was prepared using 50% human serum, and IL-6 abundance was measured by optically quantifying micrographs of the reaction chamber to determine the resulting number of specifically bound beads. Incubation of each sample in the reaction chamber was performed under standard conditions of a 1-h incubation at 30 °C and with continuous flow at a rate of 0.08 μL/min. As shown in Fig. 3A, the normalized bead counts were over fourfold above background in both the IL-6 alone and IL-6 with leptin samples but were at background levels for the leptin alone sample. As a control, these same samples were also assayed using leptin chips, where anti-leptin antibodies were instead immobilized on the surface of the reaction chamber and conjugated to the beads; complementary results were observed where normalized bead counts were elevated for the leptin alone and IL-6 with leptin samples but were at background for the IL-6 alone sample (Fig. 3B).
Fig. 3.
IL-6 and leptin microfluidic immunoassays; 50% human sera was spiked with recombinant IL-6 (5 nM), leptin (600 nM), or IL-6 and leptin mixed together. Each sample was analyzed using both anti–IL-6 (A) and anti-leptin (B) chips to demonstrate specificity of our microfluidic sandwich immunoassay approach. Titrating concentrations of IL-6 were also analyzed using anti–IL-6 chips under the same conditions to evaluate dynamic range and sensitivity (C). Detection of the bound cytokine of interest was performed using anti–IL-6– or anti-leptin–conjugated beads. Bound beads were optically quantified, and normalized bead counts were calculated by dividing by the number of beads observed to nonspecifically be bound in a control channel (no antigen, 50% human serum) performed in parallel. A dynamic range of at least six orders or magnitude and a limit of detection of 50 pM was observed.
In addition to specificity, we also investigated the performance of our microfluidic immunoassay with a titration in which varying concentrations of IL-6 were assayed (Fig. 3C). With a flow rate of 0.08 μL/min, 4.8 μL of sample for each concentration was analyzed over the course of the 1-h incubation. We observed a dynamic range greater than six orders of magnitude and a limit of detection of at least 50 pM. Notably, our titration curve revealed an apparent sublinearity in the dynamic range both at its high and low end. This sublinearity of the dynamic range is a commonly observed effect in bead-based immunoassays, which can be attributed to what is often referred to as “analog to digital conversion noise” resulting from the translation of the actual number of antigens captured (analog) to the quantity of beads bound (digital). Given the large size of the bead relative to the antibodies and antigens, not every molecule on the probe surface is interrogated; each bead interrogates multiple neighboring probe molecules. Thus, the number of antigen/antibody interactions that hold down a specifically bound bead likely varies from a handful to several hundred. This fact is particularly relevant for extremely low concentrations, where it is possible that at the flow rate we used during the wash step, a single immunocomplex is insufficient to hold down a bead.
To evaluate how the sensitivity of our platform compared with current gold standard methods, we performed a similar titration using ELISA in a standard 96-well plate (Table 1). The same reagents as our microfluidic immunoassay were used; however, the anti–IL-6 capture antibodies were instead adsorbed onto the surface of the ELISA plate with an overnight incubation at 4 °C, and detection was achieved using a fluorescent secondary antibody in place of the bead (SI Materials and Methods). Because the 96-well format requires larger volumes to ensure uniform wetting of the surface of the well, 100 μL of sample was used for each reaction performed in the 96-well plate. A titration of IL-6 performed using a 3-h binding step at room temperature showed that the limit of detection of the IL-6 ELISA in the 96-well format to be 500 pM. Although 500 pM is more than three orders of magnitude above the limit of detection reported for commercially available IL-6 ELISA kits, the antibody pairing used in these commercial ELISA kits are often proprietary, and because we wanted to perform a direct platform comparison, we used our own antibody pairing to develop a laboratory IL-6 ELISA. We expect that with further optimization of the antibody pairs, both the detection limit of the IL-6 ELISA and our digital microfluidic IL-6 assay would be significantly improved. Our platform comparison thus shows our biosensor proves to be an order of magnitude more sensitive than ELISA without any further optimizations of the reaction chamber size, flow rate during the reaction step, or flow rate during the wash steps. This increased sensitivity of our platform is even more evident when taking in account the smaller sample volume needed; our platform is capable of detecting roughly two orders of magnitude fewer molecules than ELISA, with 4.8 μL of 50 pM being roughly equivalent to 1 × 108 molecules and 100 μL of 500 pM being equivalent to 3 × 1010 molecules. This capability of detecting fewer molecules could prove to have value in applications targeting protein biomarkers that are present in only a small subgroup of cells within a large population, such as early-stage solid-state tumors.
Table 1.
Platform comparison of IL-6 immunoassay
| Assay specifications | Our platform | ELISA |
| Volume needed | 4.8 μL | 100 μL |
| Limit of detection | 50 pM | 500 pM |
| No. of molecules | 1 × 108 molecules | 3 × 1010 molecules |
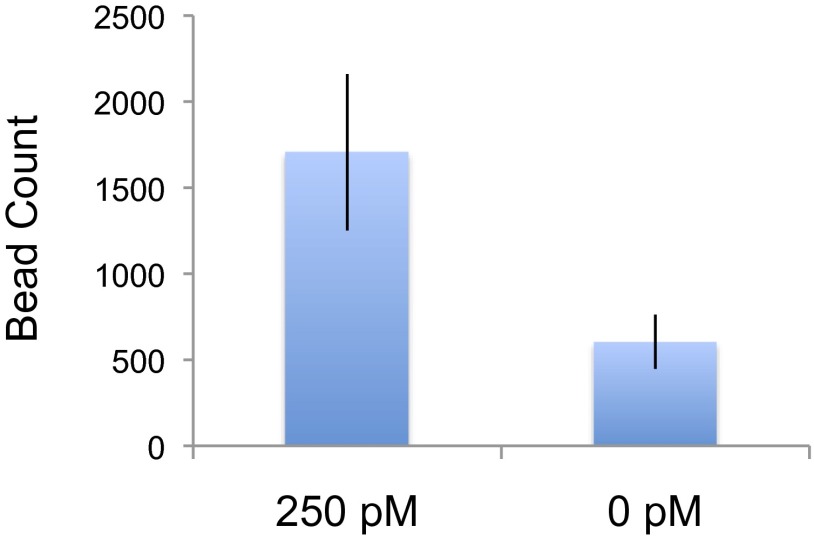
We next set out to demonstrate the ability of our technology to assay protein abundance using electrical detection: 250 pM of IL-6 was assayed using the same standard conditions of a 1-h incubation at 30 °C and with continuous flow at a rate of 0.08 μL/min. After washing away nonspecifically bound beads, the immunobound beads in the capture/reaction were eluted and directed toward the electrical impedance sensor by washing with 1 M NaOH at a flow rate of 0.05 μL/min. As shown in Fig. 4, nearly a threefold increase in signal over background was observed (P < 0.04).
Fig. 4.
IL-6 electrical detection: 250 pM IL-6 was assayed using our digital chip and resulted in nearly a threefold increase in signal over background, with P = 0.04.
Abl Kinase Assay.
We also focused our efforts on demonstrating the ability of our technology to measure protein activity. Given the lack of methods to easily and quantitatively detect posttranslational modifications, we adapted our biosensor to measure kinase activity and chose to use the human Abl kinase, which is known to have oncogenic activity when constitutively active, for a proof of concept. Instead of antibodies against the protein of interest, for the Abl kinase assay, the surface of the capture/reaction chamber was functionalized with peptides containing an optimized sequence of amino acids known to be targeted by Abl, and the micrometer-sized beads were conjugated with antibodies specific for the phosphorylated form of the peptide. The kinase reaction was performed on chip, and here, counting the changes in resistance will correlate with the extent of phosphorylation of the peptides and in turn represent a measure of kinase activity present in the sample.
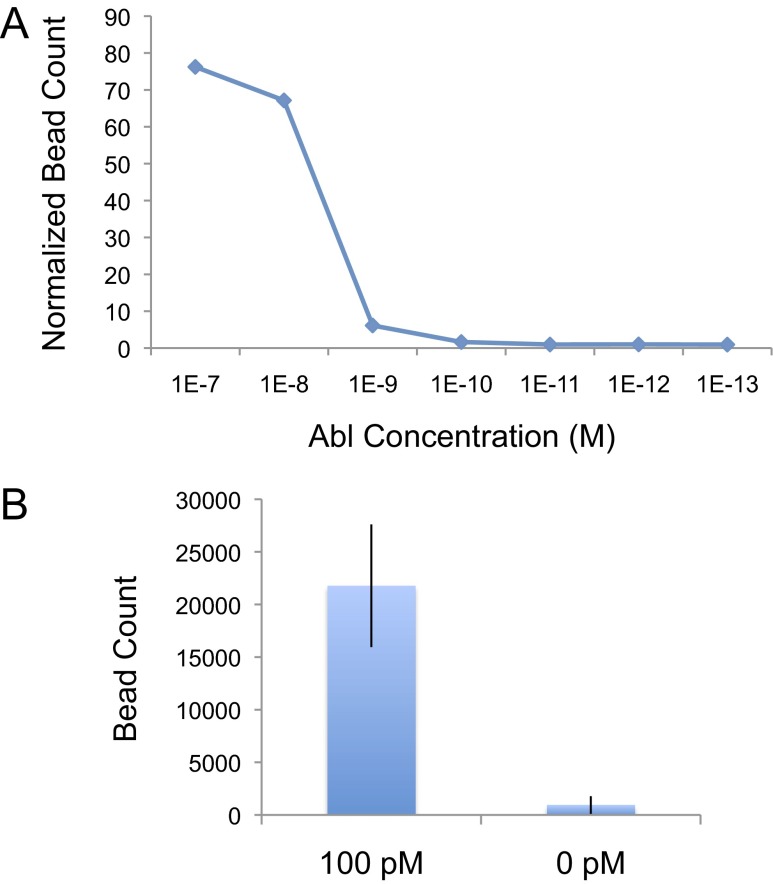
As done with our IL-6 microfluidic immunoassay, we performed a titration of Abl kinase activity and optically quantified the number of beads bound in the capture/reaction chamber before being eluted (Fig. 5A). With a 1-h reaction performed at 30 °C and with continuous flow at a rate of 0.08 μL/min, our biosensor showed a dynamic range of at least four orders of magnitude and a limit of detection of 100 pM in detecting Abl kinase activity. Under these conditions, a total volume of 4.8 μL is assayed, and thus 100 pM is roughly equivalent to 3 × 108 molecules. A similar titration for the Abl kinase assay in a standard 96-well plate format using the same surface chemistry and reagents as our microfluidic kinase assay, but using a fluorescent secondary antibody in place of the bead for detection, showed a limit of detection for the multiwell kinase assay to be 10 pM (SI Materials and Methods). As with the IL-6 ELISA in the 96-well format, 100 μL of sample was used for each kinase reaction performed in the 96-well plate. Thus, 100 μL of 10 pM Abl is equivalent to 6 × 108, or roughly twice as many molecules as was detectable by our microfluidic kinase assay.
Fig. 5.
Abl tyrosine kinase assay. Titrating concentrations of Abl were analyzed using chips functionalized with Abltide (A). Detection of resulting phosphorylated peptides was achieved using anti-phosphotyrosine–conjugated beads. Bound beads were optically quantified, and normalized bead count was calculated by dividing by the number of beads observed to nonspecifically be bound in a control channel (no kinase) performed in parallel. Our Abl kinase assay has a dynamic range of at least four orders of magnitude and a limit of detection 100 pM. 100 pM Abl was also assayed using our digital chip (B) and resulted in a >20-fold increase in signal over background, with P = 0.02.
We also assayed 100 pM of Abl using electrical detection. As with the IL-6 electrical immunoassay, elution of the specifically bound beads was achieved by washing with 1 M NaOH at a flow rate of 0.05 μL/min. As shown in Fig. 5B, a >20-fold increase in signal over background was observed (P < 0.02).
Discussion
In this study, we presented the development of a microfluidic platform capable of digitally detecting proteins. This platform embodies a unique two chamber architecture in which the capture/reaction and detection steps are physically decoupled from one another, allowing for their independent optimization, and therefore has the potential to offer greater sensitivity than traditional affinity-based protein biosensors. Using IL-6 and Abl kinase, we demonstrated the implementation and feasibility of this technology to assay both protein abundance and activity. Furthermore, we showed this technology to reproducibly be comparable in sensitivity to the current gold standard multiwell plate platforms; IL-6 abundance could be assayed from concentrations as low as 50 pM, and Abl kinase activity was detectable in samples containing 100 pM of kinase.
To our knowledge, digital protein detection at this level of sensitivity has not yet been achieved using either label-free or bead-based electrical detection methods because of the inherent matrix dependence of most label-free electronic detection technologies. In addition, electrically detecting single particles over a wide surface area in traditional affinity-based sensors is difficult, if not impossible, due to the need to compromise electronic sensitivity (signal to noise ratio per bound bead) with target protein hit rate (number of target protein interactions with the probe surface per unit of time). By using a decoupled architecture, we eliminated the need to compromise between these two key parameters.
More significantly, however, this work serves as the basis for a clinical diagnostic that can be used by healthcare providers to monitor treatment efficacies. The microfluidic nature and use of electrical impedance-sensing enable this technology to have low sample volumes requirements, a short assay time, and low cost instrumentation. Furthermore, because this platform has been developed using analog electronic amplification circuitry for performing signal readout, this technology has the ability to be easily translated into a portable handheld device for use at the point of care. For the system discussed in this article, we used a commercial bench top lock-in amplifier along with commercial syringe pumps to control the fluid flow, both of which have been widely demonstrated to be capable of being miniaturized to the extent where the whole system can be handheld. Such a device will not only allow informed treatment decisions to be made in a timely fashion, but also facilitate the clinical application of functional protein biomarkers, such as kinase activity, that would otherwise be compromised with freezing and storage.
Additional effort is needed to fully realize this proof of concept as a portable handheld diagnostic that can be used at the point of care. First, there are several parameters that remain to be investigated that have the potential to further enhance the sensitivity of our platform. For example, much still remains to be examined regarding the architecture of the capture/reaction chamber. A more comprehensive inspection evaluating the practicality of enlarging this chamber’s dimensions even further is needed. In addition, various structures can also be incorporated to facilitate the interaction of the protein of interest and the beads with the probe surface. One possibility is to include herringbone structures that would induce chaotic mixing of the fluids as they move through the capture/reaction chamber to maximize the collisions of target proteins with the probe surface. Another possibility is to include pillar structures on which the probe molecules can be functionalized to increase the area of the probe surface and thus maximize the hit rate of target proteins. However, because the force between the protein of interest or the beads with the probe surface is determined by both the architecture of the capture/reaction chamber and the flow rate applied, each change in chamber architecture will require the flow rates during the capture/reaction, wash, and elution steps to be reoptimized. Second, even though simple electronics has been used, the electrical detection instrumentation will need to be miniaturized. In particular, the lock-in-amplifier will need to be redesigned to have a smaller footprint (20), and a miniaturized fully integrated complementary metal-oxide semiconductor platform will need to be developed. However, we believe that our proof-of-concept demonstration strongly motivates such efforts, and carrying out these future studies will undoubtedly both improve the performance of our digital microfluidic platform for protein detection and strengthen the application of this technology as a low-cost portable clinical diagnostic.
Materials and Methods
Device Fabrication.
Microfluidic channels were fabricated using standard soft lithography techniques (SI Materials and Methods).
On-Chip Microfluidic Immunoassay.
Recombinant human IL-6 (206-IL), anti–IL-6 antibodies (AF-206-NA, MAB206), and anti-leptin antibodies (MAB398, BAM398) were purchased from R&D Systems. Recombinant human leptin (L4146) and human sera (S7023) were purchased from Sigma. Tosyl activated magnetic beads (2.8 μm; 14203) were purchased from Life Technologies. The monoclonal anti–IL-6 antibodies were biotinylated using the EZ-link NHS-PEG4 Biotinylation Kit from Pierce.
For experiments using optical detection, the surface of the reaction chamber was functionalized by incubating 1 μg/mL biotinylated BSA for 15 min at room temperature, followed by 1 mg/mL streptavidin for 15 min and 0.5 mg/mL biotinylated antibody for 20 min. The chamber was then blocked using 1% BSA (in PBS) for 1 h. The sample was incubated in the chamber for 1 h at 30 °C using a flow rate of 0.08 μL/min. Two washes using PBS containing 0.05% Tween-20 (PBST) were performed before introducing the anti–IL-6– or anti-leptin–conjugated beads. Beads were allowed 2 min to settle onto the surface of the chamber before a final wash with PBST to remove the nonspecifically bound beads. All buffer exchanges were performed by manual withdrawal, and flow was applied by controlled withdrawal using a Harvard Apparatus Pump 11 Elite syringe pump. Imaging was done under bright field microscopy using a Zeiss Axiovert 40 CFL microscope. The abundance of the antigen of interest present in each sample was measured by quantifying the number of specifically bound beads of three microscope fields under 20× magnification using ImageJ, calculating the average, and then normalizing by dividing by the average number of beads bound in a parallel reaction performed in the absence of any antigen.
For immunoassay experiments using digital detection, the surface of the reaction chamber was functionalized before channel bonding, but using the same chemistry as with optical detection (SI Materials and Methods). As with the optical immunoassay, the sample was incubated in the chamber for 1 h at 30 °C using a flow rate of 0.08 μL/min, and two washes using PBST were performed before introducing the anti–IL-6–conjugated beads. Beads were allowed 2 min to settle onto the surface of the chamber before a final wash with PBST to remove the nonspecifically bound beads. Specifically bound beads were then eluted and directed toward the electrical impedance sensor by washing with 1 M NaOH at a flow rate of 0.05 μL/min.
On-Chip Microfluidic Kinase Assay.
Recombinant active Abl (14-529), biotin-conjugated Abltide (12-539), and anti-phosphotyrosine antibodies (4G10 Platinum, 05–1050) were purchased from EMD Millipore. Protein G magnetic beads (2.8 μm; 10003D) were purchased from Life Technologies. Antibody-conjugated beads were prepared by resuspending the beads in Tris-buffered saline (TBS) containing 0.05% Tween-20 (TBST), incubating them with antibody for 1 h with agitation, and blocking them four times with 3% (wt/vol) nonfat milk (in TBST) for 15 min each.
For experiments using optical detection, the surface of the reaction chamber was functionalized by incubating 1 μg/mL biotinylated BSA for 15 min followed by 1 mg/mL streptavidin for 15 min and 600 μM biotin-conjugated Abltide for 20 min. Abl kinase was mixed with standard kinase buffer containing PhosSTOP phosphatase inhibitors (Roche) and incubated in the chamber in the presence of 50 mM ATP for 1 h at 30 °C using a flow rate of 0.08 μL/min. To stop the kinase reaction, the chamber was washed with 10 mM Tris⋅HCl, pH 7.5, 140 mM NaCl/0.1% SDS, 2 M NaCl, 2 M NaCl/1% H3PO4, and then with TBST. The chamber was blocked with 3% nonfat milk (in TBS) before introducing the anti-phosphotyrosine–conjugated beads. Beads were allowed 2 min to settle onto the surface of the chamber before a final wash with TBST to remove the nonspecifically bound beads. As with the optical immunoassay, all buffer exchanges were performed by manual withdrawal, flow was applied by controlled withdrawal using a Harvard Apparatus Pump 11 Elite syringe pump, and imaging was done under bright field microscopy using a Zeiss Axiovert 40 CFL microscope. The extent of phosporylation was measured by quantifying the number of specifically bound beads as was done with the optical immunoassay and then normalizing by dividing by the average number of beads bound in a parallel reaction performed in the absence of any kinase.
As with the immunoassay, for kinase assay experiments using digital detection, the surface of the reaction chamber was functionalized before channel bonding (SI Materials and Methods). The kinase reaction was performed, and the channel was blocked as was done in the optical kinase assay. Anti-phosphotyrosine–conjugated beads were introduced into the capture/reaction chamber and allowed 2 min to settle onto the surface before a final wash with TBST to remove the nonspecifically bound beads. Specifically bound beads were then eluted and directed toward the electrical impedance sensor by washing with 1 M NaOH at a flow rate of 0.05 μL/min.
Supplementary Material
Acknowledgments
This work was funded by National Institutes of Health Grant P01 HG000205.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323998111/-/DCSupplemental.
References
- 1.Ge L, Wang SM, Song XR, Ge SG, Yu JH. 3D origami-based multifunction-integrated immunodevice: Low-cost and multiplexed sandwich chemiluminescence immunoassay on microfluidic paper-based analytical device. Lab Chip. 2012;12(17):3150–3158. doi: 10.1039/c2lc40325k. [DOI] [PubMed] [Google Scholar]
- 2.Diercks AH, et al. A microfluidic device for multiplexed protein detection in nano-liter volumes. Anal Biochem. 2009;386(1):30–35. doi: 10.1016/j.ab.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes AJ, Herr AE. Microfluidic Western blotting. Proc Natl Acad Sci USA. 2012;109(52):21450–21455. doi: 10.1073/pnas.1207754110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herr AE, et al. Microfluidic immunoassays as rapid saliva-based clinical diagnostics. Proc Natl Acad Sci USA. 2007;104(13):5268–5273. doi: 10.1073/pnas.0607254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kai J, et al. A novel microfluidic microplate as the next generation assay platform for enzyme linked immunoassays (ELISA) Lab Chip. 2012;12(21):4257–4262. doi: 10.1039/c2lc40585g. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y-G, Moon S, Kuritzkes DR, Demirci U. Quantum dot-based HIV capture and imaging in a microfluidic channel. Biosens Bioelectron. 2009;25(1):253–258. doi: 10.1016/j.bios.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunne J, Reardon H, Trinh V, Li E, Farinas J. Comparison of on-chip and off-chip microfluidic kinase assay formats. Assay Drug Dev Technol. 2004;2(2):121–129. doi: 10.1089/154065804323056468. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Cosgrove BD, Lauffenburger DA, Han J. Microfluidic concentration-enhanced cellular kinase activity assay. J Am Chem Soc. 2009;131(30):10340–10341. doi: 10.1021/ja902594f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulvaney SP, et al. Rapid, femtomolar bioassays in complex matrices combining microfluidics and magnetoelectronics. Biosens Bioelectron. 2007;23(2):191–200. doi: 10.1016/j.bios.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Mulvaney SP, Myers KM, Sheehan PE, Whitman LJ. Attomolar protein detection in complex sample matrices with semi-homogeneous fluidic force discrimination assays. Biosens Bioelectron. 2009;24(5):1109–1115. doi: 10.1016/j.bios.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Tseng D, et al. Lensfree microscopy on a cellphone. Lab Chip. 2010;10(14):1787–1792. doi: 10.1039/c003477k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berggren C, Stålhandske P, Brundell J, Johansson G. A feasibility study of a capacitive biosensor for direct detection of DNA hybridization. Electroanalysis. 1999;11(3):156–160. [Google Scholar]
- 13.Esfandyarpour R, Esfandyarpour H, Harris JS, Davis RW. Simulation and fabrication of a new novel 3D injectable biosensor for high throughput genomics and proteomics in a lab-on-a-chip device. Nanotechnology. 2013;24(46):465301. doi: 10.1088/0957-4484/24/46/465301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esfandyarpour R, Esfandyarpour H, Javanmard M, Harris JS, Davis RW. Microneedle biosensor: A method for direct label-free real time protein detection. Sens Actuators B Chem. 2013;177:848–855. doi: 10.1016/j.snb.2012.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esfandyarpour R, et al. Label-free electronic probing of nucleic acids and proteins at the nanoscale using the nanoneedle biosensor. Biomicrofluidics. 2013;7(4):044114. doi: 10.1063/1.4817771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng G, Patolsky F, Cui Y, Wang WU, Lieber CM. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat Biotechnol. 2005;23(10):1294–1301. doi: 10.1038/nbt1138. [DOI] [PubMed] [Google Scholar]
- 17.Gaster RS, et al. Matrix-insensitive protein assays push the limits of biosensors in medicine. Nat Med. 2009;15(11):1327–1332. doi: 10.1038/nm.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Jensen EC, Megens M, Boser B, Mathies RA. Integrated microfluidic bioprocessor for solid phase capture immunoassays. Lab Chip. 2011;11(18):3106–3112. doi: 10.1039/c1lc20407f. [DOI] [PubMed] [Google Scholar]
- 19.Javanmard M, et al. Electrical detection of protein biomarkers using bioactivated microfluidic channels. Lab Chip. 2009;9(10):1429–1434. doi: 10.1039/b818872f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manickam A, Chevalier A, McDermott M, Ellington AD. A CMOS electrochemical impedance spectroscopy biosensor array for label-free biomolecular detection. IEEE Trans Biomed Circuits Syst. 2010;4(6):379–390. doi: 10.1109/TBCAS.2010.2081669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.