Significance
Events at conception shape the future growth and health of offspring, to impact life course potential and disease susceptibility. The environment and experiences of both parents contribute to programming offspring phenotype through epigenetic modifications imparted before embryo implantation. How the father transmits this information remains elusive. Possible pathways include the sperm genome and epigenome, postejaculatory effects of seminal fluid on sperm, and indirect actions of seminal fluid on various female factors regulating embryo development. In this study, we provide evidence that seminal fluid acts to influence both sperm integrity and the balance of embryotrophic and embryotoxic signals in the female reproductive tract, in turn affecting embryo development and programming of future adiposity and metabolic phenotype in male offspring.
Keywords: embryo development, programming, metabolic disorder, fertility, growth factors
Abstract
Paternal characteristics and exposures influence physiology and disease risks in progeny, but the mechanisms are mostly unknown. Seminal fluid, which affects female reproductive tract gene expression as well as sperm survival and integrity, provides one potential pathway. We evaluated in mice the consequences for offspring of ablating the plasma fraction of seminal fluid by surgical excision of the seminal vesicle gland. Conception was substantially impaired and, when pregnancy did occur, placental hypertrophy was evident in late gestation. After birth, the growth trajectory and metabolic parameters of progeny were altered, most profoundly in males, which exhibited obesity, distorted metabolic hormones, reduced glucose tolerance, and hypertension. Altered offspring phenotype was partly attributable to sperm damage and partly to an effect of seminal fluid deficiency on the female tract, because increased adiposity was also evident in adult male progeny when normal two-cell embryos were transferred to females mated with seminal vesicle-excised males. Moreover, embryos developed in female tracts not exposed to seminal plasma were abnormal from the early cleavage stages, but culture in vitro partly alleviated this. Absence of seminal plasma was accompanied by down-regulation of the embryotrophic factors Lif, Csf2, Il6, and Egf and up-regulation of the apoptosis-inducing factor Trail in the oviduct. These findings show that paternal seminal fluid composition affects the growth and health of male offspring, and reveal that its impact on the periconception environment involves not only sperm protection but also indirect effects on preimplantation embryos via oviduct expression of embryotrophic cytokines.
The plasma fraction of seminal fluid contains a complex mix of bioactive proteins and other agents produced by male accessory sex organs, which act after intromission to maximize the chances of successful conception by promoting sperm survival and functional competence (1). Seminal plasma can also affect reproductive events independent of sperm. Seminal fluid regulation of female reproductive physiology is well-known in insects, where effects on female reproductive organs, immune system, and behavioral responses promote fertilization and transmission of the male germ line (2). Rodent, porcine, and human studies show that seminal fluid also exerts a substantial influence on female reproductive tract physiology in vertebrates (3, 4). TGF-β and E-series prostaglandins, produced in the seminal vesicle and other male accessory glands, are major male–female signaling agents in mammalian seminal fluid (5). These factors induce the female reproductive tract to synthesize cytokines and chemokines that in turn influence the immune response to facilitate tolerance of male gametes and the conceptus (3).
The periconception phase is a time of developmental plasticity, when the embryo is highly responsive to cues affecting later fetal and postnatal development (6). Greater susceptibility of adult offspring to metabolic disease can result from perturbation at this early time (7). Maternal tract effects at conception are mediated by nutrient availability and other signals to the embryo (6). Embryo sensing of local cytokine and growth factor balance is one underlying mechanism, with oviduct-secreted factors such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and leukemia inhibitory factor (LIF) acting to shape embryo programming of later growth and phenotype characteristics in offspring (8–11).
Less is known about the father’s contribution to the conception environment. A pathway of potential influence is seminal fluid regulation of growth factors and cytokines with embryotrophic effects (3). Although it is known that pregnancy can occur without female exposure to seminal plasma, the full physiological role of seminal fluid at conception and its impact on progeny have not been adequately investigated. Here we test the hypothesis that paternal seminal plasma acts at conception to influence the progression of pregnancy and postnatal outcomes in offspring through effects on female reproductive tissues as well as sperm.
Results
Seminal Fluid Deficiency Reduces Fecundity and Alters Placental Development.
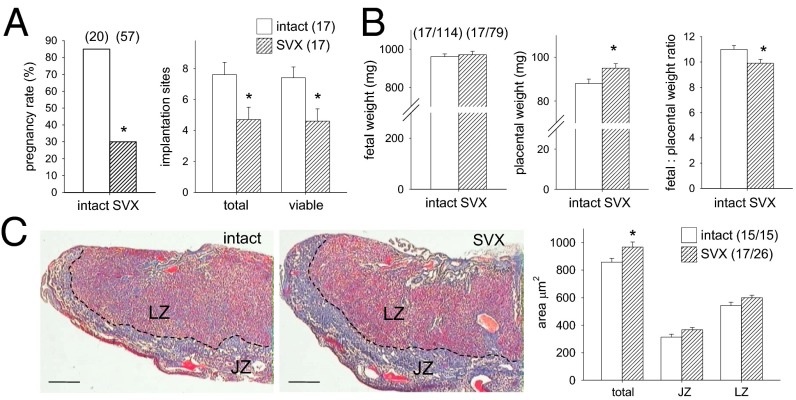
To investigate the contribution of seminal plasma to reproductive outcome, we excised seminal vesicles from male BALB/c mice (SVX males) (5). Male sexual behavior with CBAF1 females was not changed in SVX males. However, the rate of progression to pregnancy was only 35% of that with intact control males (Fig. 1A; P < 0.001). In late-gestation pregnancies sired by SVX males, the number of viable implantation sites was 37% fewer (Fig. 1A; P = 0.016). There was no increase in dead fetuses or resorbing implantation sites, indicating the diminished fecundity of SVX males was due to fewer implanting embryos. This is consistent with earlier studies, where excision of seminal vesicles impaired fertilization and reduced embryo implantation (12, 13). Fetal weight was similar regardless of whether pregnancies were sired by intact or SVX males (Fig. 1B), and fetuses were overtly normal. However, placentas were 8% heavier than in control pregnancies, even after taking litter size into account (Fig. 1B), conferring a 10% reduction in the fetal-to-placental weight ratio (Fig. 1B; P = 0.021), a measure of placental efficiency (14). Placentas from SVX matings had a 13% greater total cross-sectional area than controls, with expansion in both the junctional and labyrinthine regions (Fig. 1C; P = 0.021).
Fig. 1.
Seminal vesicle excision reduces fertility and fecundity and affects placental size. (A) Female mice mated with SVX males achieved pregnancy infrequently, compared with controls mated with intact males. In pregnancies sired by SVX males, total and viable implantation sites visible in the uterus were fewer than in controls on gd 17.5. Numbers of mated and pregnant females are in parentheses. (B) In pregnancies sired by SVX males, fetal weight was not changed but placental weight was larger, and fetal-to-placental weight ratio was smaller, than in controls. (C) Total cross-sectional area of midsagittal sections of placentas in pregnancies sired by SVX males, stained with Masson’s trichrome, was increased compared with controls, with an increase in both the labyrinthine zone (LZ) and junctional zone (JZ). See Fig. S4 for an enlargement. (Scale bars, 450 μm.) The dotted line is the JZ/LZ boundary. Pregnancy rate is analyzed by χ2 test; other data are the estimated marginal mean ± SEM and were compared by mixed-model ANOVA, with mother as subject and litter size as covariate (*P < 0.05). Numbers of mated dams and implantation sites are in parentheses.
Seminal Fluid Deficiency Alters Postnatal Growth in Progeny.
Placental hypertrophy is an adaptive response to compromised placental transport function and/or disturbances to fetal growth, and is often associated with altered fetal programming (15). In offspring of SVX males, whereas litter size at birth was reduced by two pups on average (Fig. 2A), paternal seminal fluid deficiency did not affect birth weight (Fig. 2B) or sex ratio (46.1% and 53.3% males in litters of intact and SVX sires, n = 180 and n = 120, respectively; P > 0.05). Postnatally, pups of SVX sires were moderately growth-impaired (Fig. 2B). Then, after puberty, growth accelerated progressively and progeny were heavier than controls from 11 wk, independent of sex (Fig. 2 B and C and Fig. S1; P < 0.05).
Fig. 2.
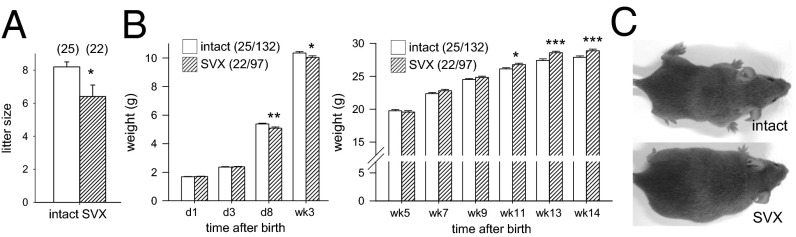
Seminal vesicle excision alters postnatal growth and weight of adult offspring. (A) Litters sired by SVX males contained fewer pups at birth, compared with control litters sired by intact males. (B) Postnatal weight of pups sired by SVX males was unchanged on days 1 and 3 after birth but reduced at days 8 and 21, compared with control pups. Postweaning growth trajectory of progeny sired by SVX males was accelerated compared with control progeny. Data are the estimated marginal mean ± SEM, and the effect of seminal fluid was determined by mixed-model ANOVA, with litter size and sex as covariates (*P < 0.05; **P < 0.01; ***P < 0.001). See Fig. S1 for offspring growth data according to sex. Numbers of mated dams and progeny are in parentheses. (C) Representative male progeny at 14 wk.
Seminal Fluid Deficiency Increases Adiposity in Male Progeny.
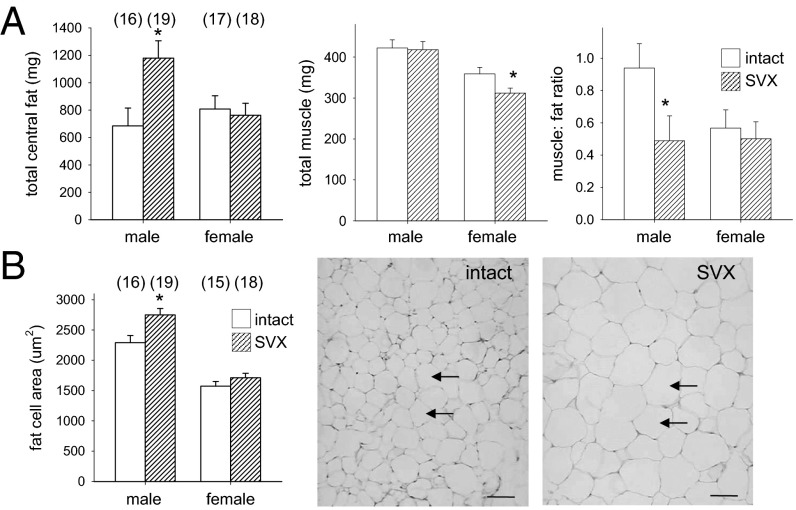
Distorted growth patterns in utero and after birth are linked with obesity and metabolic disorder in adults (16). Analysis of body morphometry at 14 wk showed male progeny of SVX sires had substantially more fat than controls, with a 72% increase in absolute mass of central adipose tissue (Fig. 3A; P = 0.012), due to increases in epididymal, retroperitoneal, and perirenal fat (Table S1). Altered fat mass conferred a 48% reduction in the muscle-to-fat ratio (Fig. 3A; P = 0.047), accompanied by a 20% increase in the mean transsectional area of adipocyte cells (Fig. 3B; P = 0.017). Total central fat mass and adipocyte size were not affected in female progeny (Fig. 3 A and B). Tissue mass was unchanged in female progeny relative to controls (Table S1), except that absolute combined muscle mass was reduced by 13% (Fig. 3A; P = 0.025).
Fig. 3.
Seminal vesicle excision increases central fat in adult offspring. (A) In adult progeny of SVX males at 14 wk, the total central fat mass was increased in males, the total muscle mass was reduced in females, and the muscle-to-fat ratio was reduced in males, compared with control adult progeny sired by intact males. (B) Average adipocyte area in retroperitoneal fat was increased in male progeny of SVX males, compared with controls. (Scale bars, 50 µm.) Arrows indicate adipocytes. See Fig. S4 for an enlargement. Data are the estimated marginal mean ± SEM, and the effect of seminal fluid was evaluated for each sex by mixed-model ANOVA, with litter size as covariate (*P < 0.05). Numbers of mated dams and progeny are in parentheses.
Seminal Fluid Deficiency Alters Metabolic Status and Blood Pressure in Male Progeny.
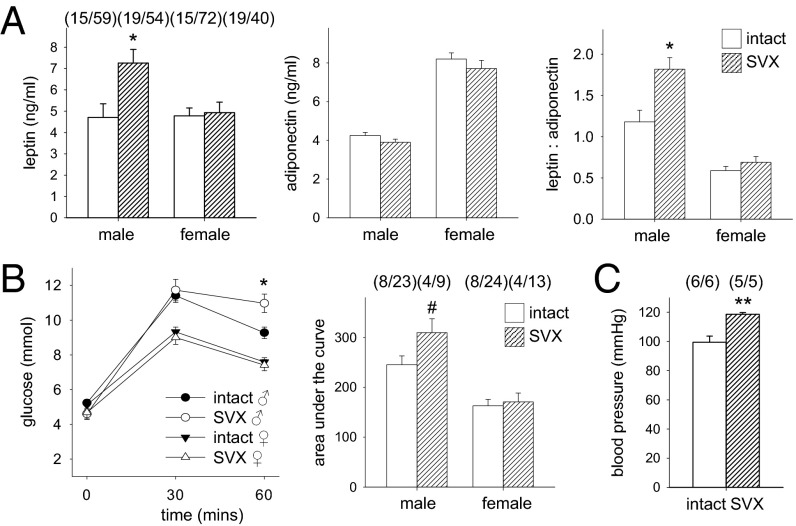
Altered metabolism and regulation of energy balance are consequences of early growth disturbance (16). An altered metabolic profile was seen in male progeny of SVX sires, with a 54% increase in both plasma leptin and the leptin-to-adiponectin ratio (Fig. 4A; P = 0.006 and P = 0.002, respectively), whereas the metabolic profile in females was unchanged other than reduced plasma free fatty acid content (Fig. S1). Delayed glucose clearance, a measure of insulin resistance, was evident in male but not female progeny of SVX sires (Fig. 4B). We elected to evaluate blood pressure only in male mice, as metabolic changes were not observed in females. Male progeny of SVX sires showed a 15% increase in systolic blood pressure compared with controls (Fig. 4C; P = 0.003). These changes in male offspring are hallmark features of metabolic syndrome (17).
Fig. 4.
Seminal vesicle excision alters metabolic parameters in adult offspring. (A) In adult male progeny of SVX males at 14 wk, plasma leptin and leptin-to-adiponectin ratio were elevated, compared with control male progeny sired by intact males. (B) Glucose clearance assessed at 60 min after challenge and as the area under the curve was slowed compared with control males at 14 wk. (C) Resting systolic blood pressure of male progeny was elevated, compared with control males at 14 wk. Data are the estimated marginal mean ± SEM, and the effect of seminal fluid was evaluated by mixed-model ANOVA (*P < 0.05; **P < 0.01; #P = 0.062). Numbers of mated dams and progeny are in parentheses.
Seminal Fluid Deficiency Disrupts Blastocyst Development.
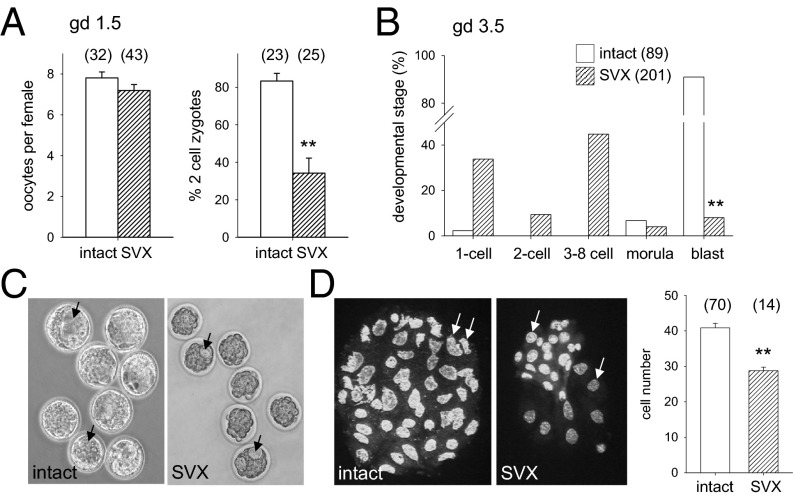
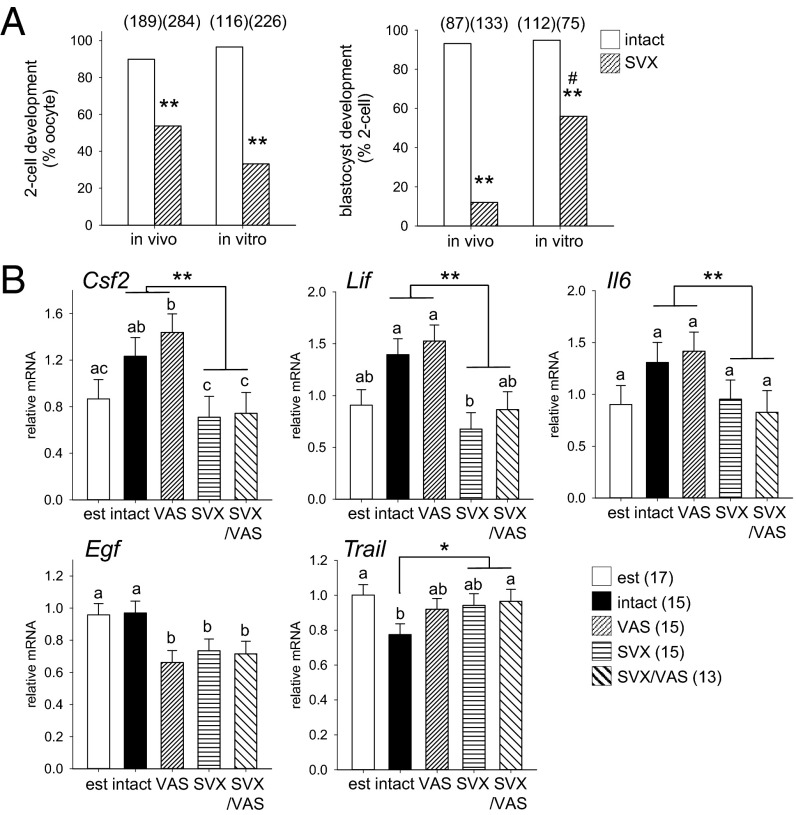
Compromised development in utero can originate in disturbances during early embryo development (18, 19). When two-cell embryos were flushed from oviducts of females 10–12 h after mating with SVX males, abnormalities in development were already evident. Oocytes were normal in number, but the rate of fertilization and cleavage to two-cell stage was reduced by 60% with SVX sires compared with controls (Fig. 5A; P < 0.001). When embryos were flushed just before implantation, the number of blastocysts was reduced by 90% (Fig. 5B; P < 0.001). Most embryos of SVX sires were developmentally delayed or arrested, at the eight-cell stage or earlier. Blastocysts were smaller and showed abnormal blastocoel cavities, with fewer total blastomeres than controls (Fig. 5 C and D), although inner cell mass and trophectoderm cells could not be reliably quantified due to the high incidence of apoptotic cells. Thus, the low fecundity of SVX sires is due to disrupted oocyte fertilization and impaired embryo development. This implies that later changes to placental development may originate in the preimplantation embryo.
Fig. 5.
Seminal vesicle excision impairs preimplantation embryo development. (A) Embryos flushed on gestational day (gd) 1.5 from the oviducts of females mated with SVX males were comparable in number but the rate of zygote cleavage to two-cell stage was reduced, compared with control embryos sired by intact males. (B) Embryos flushed from the uterus on gd 3.5 showed impaired development to blastocyst stage when sired by SVX males, compared with controls. (C and D) Blastocysts sired by SVX males showed frequent irregularities in blastocoel cavity formation (arrows) (C) and contained fewer blastomeres compared with control blastocysts (D). Embryo developmental stage was analyzed by χ2 test; other data are mean ± SEM and were compared by one-way ANOVA (**P < 0.01). Numbers of mated dams and embryos are in parentheses.
Intrinsic and Extrinsic Factors both Impact Embryo Quality.
Embryo survival and developmental competence are subject to both intrinsic oocyte- and sperm-derived factors and extrinsic maternal tract signals (18). There is good evidence that seminal plasma deficiency exerts adverse effects on sperm integrity, in turn affecting embryo development (12, 13), but we sought to evaluate whether indirect effects may also contribute. When two-cell zygotes were flushed from females mated with SVX males and developed in vitro, partial rescue of the developmental impairment was seen, with a fourfold increase in the rate of two-cell progression to blastocyst (Fig. 6A; P < 0.01). This shows that in vivo environment contributes to impaired development of embryos sired by SVX males.
Fig. 6.
Seminal vesicle excision imparts maternal tract-mediated inhibitory effects and alters oviduct expression of embryotrophic cytokines. (A) The rate of cleavage to two-cell stage in zygotes from females mated with SVX males was less compared with control zygotes sired by intact males when zygotes were developed in vivo or flushed from the oviducts on gd 0.5 and developed in vitro (**P < 0.01). In contrast, development to blastocyst in two-cell embryos sired by SVX males was increased when embryos were developed in vitro, compared with in vivo (#P < 0.01), but less than in controls (**P < 0.01). (B) Oviduct expression of the cytokines Csf2, Lif, Il6, Egf, and Trail on gd 0.5 after mating with intact, VAS, SVX, SVX/VAS, or estrous females (est). Data are the estimated marginal mean ± SEM, and the effect of seminal fluid was evaluated by mixed-model ANOVA. Different superscripts represent statistical difference between groups. *P < 0.05, **P < 0.01 compared with females exposed to seminal plasma. Numbers of embryos and mated females are in parentheses.
Adiposity in Male Progeny Is Imparted by the Oviduct.
To evaluate whether seminal fluid priming of the female tract influences obesity in male offspring, we used an embryo transfer strategy. Embryos recovered at two-cell stage from females mated with intact males were transferred to the oviducts of recipients made pseudopregnant by mating to vasectomized males with intact or excised seminal vesicles (VAS and SVX/VAS males, respectively). In additional recipients, embryos were transferred at blastocyst stage to the uterus of recipients mated to VAS or SVX/VAS males. In recipients killed in late gestation, there was no effect of recipient exposure to seminal plasma on pregnancy rate, proportion of embryos implanted, or fetal or placental weights (Fig. S2).
In the progeny of recipients progressed to term, body morphometry analysis at 14 wk revealed differences in fat deposition associated with female tract exposure to seminal fluid. Male progeny originating from two-cell transfers into recipients mated with SVX/VAS sires had 28% more total central fat than controls (Table 1; P = 0.05), due to increased epididymal and retroperitoneal fat deposits. Significant effects of recipient dam seminal fluid exposure were not evident in other parameters of adult metabolic function or body morphometry (Table S2); however, it seems likely that changes were masked by embryo transfer itself (Table S3), as reported in previous studies (8). However, male offspring of blastocyst transfers showed similar fat deposits regardless of recipient female exposure to seminal plasma (Table 1 and S4). This indicates that the environment from two-cell to blastocyst stage is crucial for programming adult metabolic phenotype, and shows the adverse effect of paternal seminal fluid deficiency is partly imparted by the maternal tract environment.
Table 1.
Effect of recipient exposure to seminal fluid on adiposity in male progeny after embryo transfer at two-cell or blastocyst stage
| Fat parameter | VAS (relative), % | VAS/SVX (relative), % | P value | VAS (absolute) | VAS/SVX (absolute) | P value |
| Progeny of two-cell transfers | ||||||
| Total central fat (%), mg‡ | 2.33 ± 0.22 | 2.98 ± 0.24* | 0.050 | 749 ± 78 | 981 ± 87† | 0.054 |
| Epididymal fat (%), mg | 1.71 ± 0.17 | 2.23 ± 0.19* | 0.046 | 550 ± 60 | 733 ± 66* | 0.048 |
| Retroperitoneal fat (%), mg | 0.40 ± 0.04 | 0.49 ± 0.04 | 0.096 | 128 ± 13 | 161 ± 13 | 0.098 |
| Renal fat (%), mg | 0.22 ± 0.02 | 0.26 ± 0.02 | NS | 71 ± 7 | 87 ± 7 | NS |
| Progeny of blastocyst transfers | ||||||
| Total central fat (%), mg‡ | 4.10 ± 0.61 | 3.67 ± 0.61 | NS | 1,411 ± 220 | 1,271 ± 220 | NS |
| Epididymal fat (%), mg | 3.14 ± 0.48 | 2.74 ± 0.48 | NS | 1,079 ± 172 | 952 ± 172 | NS |
| Retroperitoneal fat (%), mg | 0.64 ± 0.09 | 0.59 ± 0.09 | NS | 221 ± 30 | 202 ± 30 | NS |
| Renal fat (%), mg | 0.32 ± 0.06 | 0.34 ± 0.06 | NS | 111 ± 23 | 118 ± 23 | NS |
Data for two-cell transfer are n = 21 male progeny from 12 litters in recipients mated with VAS males, and n = 17 male progeny from 10 litters in recipients mated with VAS/SVX males. Data for blastocyst transfers are n = 9 male progeny from 9 litters in recipients mated with VAS males, and n = 9 male progeny from 9 litters in recipients mated with VAS/SVX males. See Tables S2 and S4 for complete data on body morphometry analysis of progeny from two-cell and blastocyst transfer. Data are estimated marginal mean ± SEM. Relative or absolute weight and effect of seminal fluid composition were compared by mixed-model linear repeated-measures ANOVA. Litter size was not a significant covariate. P values are given when P < 0.1. †P < 0.06; *P < 0.05; compared with VAS group. NS, not significant.
Total central fat is the sum of all fat depots measured.
Seminal Fluid Deficiency Alters Oviduct Cytokines.
Because seminal plasma can regulate expression of colony-stimulating factor 2 (Csf2), interleukin-6 (Il6), and other cytokines in the uterus (5), we investigated whether oviduct synthesis of growth factors and cytokines was similarly regulated (11, 20). Quantitative RT-PCR analysis in oviducts at gestation day (gd) 0.5 after mating with intact, SVX, VAS, or SVX/VAS males showed substantial effects of seminal fluid composition on cytokine profile. Oviduct expression of Csf2, Lif, and Il6 was reduced in mated females not exposed to seminal plasma (SVX and VAS/SVX groups), compared with females exposed to seminal plasma (intact and VAS groups; Fig. 6B; all P < 0.05). epidermal growth factor (Egf) expression was suppressed after mating unless females were exposed to intact seminal fluid (P < 0.05). Additionally, oviduct expression of the apoptosis-inducing factor TNF-related apoptosis-inducing ligand (Trail) (21) was suppressed after mating with intact males, but not sperm- or seminal plasma-deficient males (Fig. 6B; P < 0.05), and the ratio of trophic cytokines to Trail showed a strong effect of seminal fluid (Fig. S3). Correlation analysis showed that seminal plasma, as opposed to sperm, zygotes, or mating itself, was the major determinant of Csf2, Lif, and Il6 (Table S5; all P < 0.01), whereas Egf and Trail were positively and negatively correlated with the presence of zygotes (both P < 0.05). Given that embryos deprived of GM-CSF exhibit metabolic disturbance in offspring (8), this implies that lack of oviduct trophic support is a mechanism underlying the indirect effects of paternal seminal fluid deficiency on embryo development and adverse offspring outcomes.
Discussion
The local milieu at conception has a crucial influence on programming an embryo’s future growth, phenotype, and susceptibility to disease in later life (18). Here we report that a key determinant of conception environment is the plasma fraction of paternal seminal fluid, which as well as protecting sperm integrity also regulates female tract expression of embryotrophic cytokines to exert a profound effect on the development and metabolic phenotype of the resulting progeny. Our experimental strategy enabled distinction of a subtle role for seminal plasma–oviduct interactions in contributing to preimplantation embryo programming of metabolic phenotype in male offspring, against a background of major effects of seminal fluid deficiency on fertilization and embryo quality attributable to defective sperm. Specifically, seminal plasma induces oviduct synthesis of GM-CSF, LIF, and IL6, which promote blastomere survival, program optimal developmental competence, and suppress TRAIL, which activates blastomere apoptosis. These results expand understanding of the male contribution to events at conception and the significance of paternal factors for offspring health (18).
Paternal seminal plasma deficiency profoundly impacted progeny, particularly in males, which showed hallmark characteristics of metabolic syndrome (17). Changes in phenotype were evident from before birth as placental hypertrophy. This is a common response to insults in utero, whereby expanded placental size compensates for compromised placental transport efficiency (15). The finding of stronger effects in male offspring is consistent with previously described sex-specific effects of cytokine perturbation in early life. Susceptibility to metabolic disturbance after GM-CSF deprivation is greater in male than in female progeny (8), with disproportionate loss of male fetuses in Csf2-null mutant mice (22). Sexual dimorphism is also seen in dietary, culture-induced, and physiochemical models of metabolic programming, where males are consistently more vulnerable (6, 23, 24). These differential responses to environmental insults originate in sex-dependent transcriptional differences in several molecular pathways controlling glucose metabolism, protein metabolism, DNA methylation, and epigenetic regulation (25, 26).
In humans, variable incidence of metabolic syndrome across populations has been attributed to genes as well as adult exposures and risk factors. However, failure to find strong genetic determinants (27), and the limited efficacy of adult interventions, has shifted the focus to developmental origins. Animal studies show that perturbations to the nutritional, physiochemical, growth factor, or other aspects of the maternal environment, as well as ex vivo conception or culture of embryos, cause altered fetal and placental growth followed by metabolic disorder in offspring (18). Altered conditions at conception generally induce adaptations to protect the fetus from immediate growth constraints, but the consequence is elevated risk of later metabolic disease (28, 29). However, maternal factors do not provide a full explanation and attention has turned to the father, where there is now compelling evidence that stress, chemical exposures, and dietary conditions experienced by a male can influence the metabolic and fertility phenotype of his progeny (30, 31).
Possible pathways by which paternal effects are transmitted include the sperm genome and epigenome, postejaculatory effects of seminal fluid on sperm, and seminal fluid regulation of various female events that impact embryo development (32). In the current study, we sought to test this third alternative. Consistent with earlier studies in rodents (12, 13), the major impact of seminal vesicle excision from sires was impaired fertilization, disrupted blastocyst development, and lower embryo implantation rate. These overt effects on early cleavage stages are most reasonably attributable to sperm DNA damage or epigenetic changes in the sperm, as opposed to maternal tract effects. This is in line with a role for seminal plasma in protecting sperm from DNA fragmentation due to oxidative injury in the female tract (33), which may be aggravated by the absence of copulatory plug formation with seminal vesicle-excised males. This interpretation fits with the relatively normal implantation rate seen when embryos were transferred to recipients not exposed to seminal plasma.
However, the effect on progeny phenotype, and particularly the differential effect on males, is less readily attributable to sperm DNA damage and is more likely to result from nongenomic epigenetic mechanisms influencing sperm and/or the embryo. Paternal interventions that alter methylation and acetylation patterns in sperm and consequently the preimplantation embryo are an important determinant of offspring phenotype (34, 35). A previous indication that seminal plasma may influence progeny is reported in hamsters, where excision of paternal accessory sex glands caused reduced postnatal growth and elevated anxiety in adults (36). In this model, reduced acetylation in male pronuclei and retarded kinetics of demethylation and remethylation in cleavage-stage embryos were associated with dysregulated expression of paternally expressed Igf2 and Dlk1 (37). An epigenetic pathway is supported by a wide range of studies linking the epigenome in the embryo with metabolic disorder in adult offspring (38). As marked genetic and epigenetic differences exist between the two sexes at the preimplantation stage (25), males and females can respond differently to environmental insults affecting later development (26). Previous studies show that male preimplantation embryos are more vulnerable than females to culture-induced epigenetic programming of later growth disturbance in offspring (8), and intergenerational transmission of glucose intolerance by affected males to F1 and F2 male offspring is reported (24).
Our findings are consistent with programming mechanisms operating both via sperm and independent of sperm, mediated by the female response to seminal fluid. Evidence against sperm damage as a complete explanation is the improved development of embryos sired by seminal fluid-deficient males after removal from the female tract and culture in vitro, and the altered metabolic phenotype in normally fertilized embryos transferred to tracts of females mated with seminal fluid-deficient males. The female response to seminal fluid involves regulation of oviduct cytokines, and disruption in their balance is strongly implicated in the altered progeny phenotype resulting from seminal plasma deficiency. Oviduct and uterine secretions contain cytokines and growth factors that either promote or constrain normal embryo development from the early cleavage stage (11, 20). Reduced synthesis of LIF, GM-CSF, IL6, and EGF, each of which promotes survival and developmental competence in embryos, together with elevation in TRAIL, which induces apoptosis, provides a mechanism by which the maternal tract contributes to poor embryo development and altered developmental programming after mating with seminal fluid-deficient males.
Pathways linking deprivation in maternal trophic support with epigenetic changes in embryos are postulated (39, 40), but the molecular mechanisms are not yet defined. In mice, LIF maintains inner cell mass cell pluripotency during blastocyst development (41), whereas GM-CSF regulates genes involved in de novo methylation to influence epigenetic reprogramming (10, 42). Absence of GM-CSF compromises blastocyst development by inducing stress response and apoptosis gene pathways (43, 44). IL-6 protects embryos from apoptosis through regulation of Stat3-dependent antiapoptotic microRNAs (45). Because mouse preimplantation embryos are sensitive to deprivation in each factor individually (9, 43–47), it seems reasonable that reduction of all four factors in vivo would impair blastomere survival and impart epigenetic modifications. Effects on progeny growth and metabolism similar to those caused by seminal vesicle excision occur when embryos are deprived of GM-CSF, including greater vulnerability in male offspring (8, 22). LIF neutralization also has adverse effects on offspring phenotype (9, 46). Our data showing increased adiposity in male offspring derived from transfers at the two-cell embryo but not blastocyst stage indicate that seminal plasma-induced maternal tract effects are exerted before the blastocyst stage. Seminal fluid-regulated oviduct signals may interact with and amplify the consequences of sperm defects in embryos, particularly given that suppression of Trail and induction of Egf correspond to the presence of viable zygotes. Male embryos may be more susceptible to cytokine imbalance by virtue of their different metabolic requirements and epigenetic regulation (26), and thus potentially greater sensitivity to signaling factors impacting these pathways. Notably, GM-CSF promotes glucose transport in embryos (43) and, because male embryos are reportedly more glucose-dependent (26), we speculate that impaired glucose uptake secondary to GM-CSF deficiency explains the more profound effect on metabolic programming in males seen herein and previously (8).
In conclusion, this study describes a causal link between paternal seminal plasma and metabolic phenotype in offspring. The findings confirm the importance of seminal plasma in programming phenotype through maintenance of sperm viability and integrity and demonstrate an additional role for seminal plasma involving signaling to the female reproductive tract. These observations raise the prospect that male–female seminal fluid signaling in mammals, as in insects, has an evolutionary benefit in facilitating reproductive success and progeny fitness. It will be of interest to evaluate whether seminal fluid acts similarly in humans, where seminal plasma composition and signaling capacity vary among men (48).
Materials and Methods
Animals.
Pathogen-free CBA × BALB/c (CBAF1) females and BALB/c males from the University of Adelaide Central Animal House were housed in barrier conditions on a 12 h:12 h light–dark cycle and given standard rodent chow (Specialty Foods) and water ad libitum. For natural mating, one to three CBAF1 females (8- to 10-wk-old) were placed with an intact or seminal vesicle-excised BALB/c male (10- to 20-wk-old). The day of detection of a vaginal copulatory plug was designated gd 0.5. For mating with SVX males, which do not produce a copulatory plug, gd 0.5 was defined by sperm detection in vaginal lavage fluid (SVX males) or video of mating behavior using a low-light camera and red light (SVX/VAS males). Animal use was in accordance with the Australian Code of Practice for the Care and Use of Experimental Animals, and experiments were approved by the University of Adelaide Animal Ethics Committee.
Seminal Vesicle Excision and Vasectomy.
Seminal vesicle glands were surgically excised from SVX males at 6–8 wk under anesthesia with 15 μL/g Avertin (2,2,2-tribromomethanol; Sigma-Aldrich) in 2-methyl-2-butanol. Seminal vesicles were ligated with silk suture at the proximal tubule before removal by blunt dissection via a small lateral incision, while retaining the adjacent coagulating glands. In vasectomized males, vas deferens were ligated before bisection using quarterization. Seminal vesicle-excised, vasectomized males were prepared in one surgical procedure.
Pregnancy Parameters.
Females mated with intact or SVX males were euthanized at 1000–1200 hours on gd 17.5. Total, viable, and resorbing implantation sites were counted, and fetuses and placentas were weighed. Placental structure was assessed by histochemical analysis as described (8) and is detailed in SI Materials and Methods.
Postnatal and Adult Outcomes in Progeny.
Pregnant females were housed individually from gd 17.5. Pups were counted and weighed at 12–24 h after birth and at 72 h, 8 d, and 3 wk. Litters were weaned at 3 wk and caged in groups of one to five according to sex. Progeny were fed ad libitum and weighed at 2-wk intervals until 14 wk, when mice were euthanized and postmortem analysis was carried out on one or two randomly selected male and female progeny in each litter. Just before euthanasia at 0900–1000 hours, mice were anesthetized using Avertin and blood was drawn by cardiac puncture for plasma metabolic hormone assay. Postmortem body composition and fat analysis is detailed in SI Materials and Methods.
Blood Pressure, Adipocytokines, and Glucose-Tolerance Test.
At 14 wk, resting systolic blood pressure was measured using an ML125 blood pressure controller and tail cuff (ADInstruments) at 1400–1600 hours on the day before euthanasia. Plasma adiponectin, leptin, and insulin were measured by RIA (Linco Research). Glucose and free fatty acids were measured by a Cobas Mira automated centrifugal analyzer (Roche Diagnostic Systems). Glucose tolerance was tested in fasted mice and analyzed by glucometer (Hemocue). Assays are detailed in SI Materials and Methods.
In Vivo and in Vitro Embryo Development.
Embryo development was assessed by flushing embryos at different stages and culture in vitro, as detailed in SI Materials and Methods. Blastocyst-stage embryos were stained with 100 µg/mL propidium iodide and 20 µg/mL Hoechst in Human Tubal Fluid media supplemented with 0.5% BSA (Sigma-Aldrich) and 2.3 mM Hepes (Sigma-Aldrich) for 30 min at 37 °C and examined under UV light to count blastomere nuclei.
Embryo Transfers.
Transfer of two-cell or blastocyst-stage embryos flushed on gd 1.5 or 3.5, respectively, and transfer into oviducts or uteri of recipient CBAF1 females prepared by mating with VAS or SVX/VAS BALB/c males, is detailed in SI Materials and Methods. Pregnancy outcomes were evaluated in two cohorts at gd 17.5 and in offspring at 14 wk.
Reverse Transcription and Quantitative Real-Time PCR.
Quantitative RT-PCR was performed using primer sequences shown in Table S6 and standard techniques described in SI Materials and Methods.
Statistical Analysis.
All data were analyzed using SPSS 12.0 software using mixed-model ANOVA with the mother as subject and sex and litter size as covariates, or other tests as specified, as detailed in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Julie Owens for critical comments; Claire Roberts for advice on placental analysis; David Kennaway, Miles DeBlasio, and David Sharkey for assays; and Camilla Dorian for technical assistance.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305609111/-/DCSupplemental.
References
- 1.Poiani A. Complexity of seminal fluid: A review. Behav Ecol Sociobiol. 2006;60(3):289–310. [Google Scholar]
- 2.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: Identification and function. Annu Rev Entomol. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005;322(1):43–52. doi: 10.1007/s00441-005-1127-3. [DOI] [PubMed] [Google Scholar]
- 4.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188(5):2445–2454. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 5.Tremellen KP, Seamark RF, Robertson SA. Seminal TGFbeta1 stimulates GM-CSF production and inflammatory cell recruitment in the murine uterus. Biol Reprod. 1998;58(5):1217–1225. doi: 10.1095/biolreprod58.5.1217. [DOI] [PubMed] [Google Scholar]
- 6.Fleming TP, Lucas ES, Watkins AJ, Eckert JJ. Adaptive responses of the embryo to maternal diet and consequences for post-implantation development. Reprod Fertil Dev. 2011;24(1):35–44. doi: 10.1071/RD11905. [DOI] [PubMed] [Google Scholar]
- 7.Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: Life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21(4):199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Sjoblom C, Roberts CT, Wikland M, Robertson SA. GM-CSF alleviates adverse consequences of embryo culture on fetal growth trajectory and placental morphogenesis. Endocrinology. 2005;146(5):2142–2153. doi: 10.1210/en.2004-1260. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell MH, Swanson RJ, Oehninger S. In vivo effect of leukemia inhibitory factor (LIF) and an anti-LIF polyclonal antibody on murine embryo and fetal development following exposure at the time of transcervical blastocyst transfer. Biol Reprod. 2002;67(2):460–464. doi: 10.1095/biolreprod67.2.460. [DOI] [PubMed] [Google Scholar]
- 10.Loureiro B, et al. Colony-stimulating factor 2 (CSF-2) improves development and posttransfer survival of bovine embryos produced in vitro. Endocrinology. 2009;150(11):5046–5054. doi: 10.1210/en.2009-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kane MT, Morgan PM, Coonan C. Peptide growth factors and preimplantation development. Hum Reprod Update. 1997;3(2):137–157. doi: 10.1093/humupd/3.2.137. [DOI] [PubMed] [Google Scholar]
- 12.Peitz B, Olds-Clarke P. Effects of seminal vesicle removal on fertility and uterine sperm motility in the house mouse. Biol Reprod. 1986;35(3):608–617. doi: 10.1095/biolreprod35.3.608. [DOI] [PubMed] [Google Scholar]
- 13.O WS, Chen HQ, Chow PH. Effects of male accessory sex gland secretions on early embryonic development in the golden hamster. J Reprod Fertil. 1988;84(1):341–344. doi: 10.1530/jrf.0.0840341. [DOI] [PubMed] [Google Scholar]
- 14.Fowden AL, Forhead AJ, Coan PM, Burton GJ. The placenta and intrauterine programming. J Neuroendocrinol. 2008;20(4):439–450. doi: 10.1111/j.1365-2826.2008.01663.x. [DOI] [PubMed] [Google Scholar]
- 15.Burton GJ, Fowden AL. Review: The placenta and developmental programming: Balancing fetal nutrient demands with maternal resource allocation. Placenta. 2012;33(Suppl):S23–S27. doi: 10.1016/j.placenta.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Cottrell EC, Ozanne SE. Early life programming of obesity and metabolic disease. Physiol Behav. 2008;94(1):17–28. doi: 10.1016/j.physbeh.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Guerre-Millo M. Adipose tissue and adipokines: For better or worse. Diabetes Metab. 2004;30(1):13–19. doi: 10.1016/s1262-3636(07)70084-8. [DOI] [PubMed] [Google Scholar]
- 18.Fleming TP, et al. The embryo and its future. Biol Reprod. 2004;71(4):1046–1054. doi: 10.1095/biolreprod.104.030957. [DOI] [PubMed] [Google Scholar]
- 19.Duranthon V, Watson AJ, Lonergan P. Preimplantation embryo programming: Transcription, epigenetics, and culture environment. Reproduction. 2008;135(2):141–150. doi: 10.1530/REP-07-0324. [DOI] [PubMed] [Google Scholar]
- 20.Hardy K, Spanos S. Growth factor expression and function in the human and mouse preimplantation embryo. J Endocrinol. 2002;172(2):221–236. doi: 10.1677/joe.0.1720221. [DOI] [PubMed] [Google Scholar]
- 21.Riley JK, Heeley JM, Wyman AH, Schlichting EL, Moley KH. TRAIL and KILLER are expressed and induce apoptosis in the murine preimplantation embryo. Biol Reprod. 2004;71(3):871–877. doi: 10.1095/biolreprod.103.026963. [DOI] [PubMed] [Google Scholar]
- 22.Robertson SA, Roberts CT, Farr KL, Dunn AR, Seamark RF. Fertility impairment in GM-CSF-deficient mice. Biol Reprod. 1999;60(2):251–261. doi: 10.1095/biolreprod60.2.251. [DOI] [PubMed] [Google Scholar]
- 23.Hochberg Z, et al. Child health, developmental plasticity, and epigenetic programming. Endocr Rev. 2011;32(2):159–224. doi: 10.1210/er.2009-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calle A, et al. Male mice produced by in vitro culture have reduced fertility and transmit organomegaly and glucose intolerance to their male offspring. Biol Reprod. 2012;87(2):34. doi: 10.1095/biolreprod.112.100743. [DOI] [PubMed] [Google Scholar]
- 25.Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A. Sex determines the expression level of one third of the actively expressed genes in bovine blastocysts. Proc Natl Acad Sci USA. 2010;107(8):3394–3399. doi: 10.1073/pnas.0913843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bermejo-Alvarez P, Rizos D, Lonergan P, Gutierrez-Adan A. Transcriptional sexual dimorphism during preimplantation embryo development and its consequences for developmental competence and adult health and disease. Reproduction. 2011;141(5):563–570. doi: 10.1530/REP-10-0482. [DOI] [PubMed] [Google Scholar]
- 27.Manolio TA, et al. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barker DJ, Clark PM. Fetal undernutrition and disease in later life. Rev Reprod. 1997;2(2):105–112. doi: 10.1530/ror.0.0020105. [DOI] [PubMed] [Google Scholar]
- 29.Hanson MA, Gluckman PD. Developmental processes and the induction of cardiovascular function: Conceptual aspects. J Physiol. 2005;565(Pt 1):27–34. doi: 10.1113/jphysiol.2004.082339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng SF, et al. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature. 2010;467(7318):963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 31.Fullston T, et al. Diet-induced paternal obesity in the absence of diabetes diminishes the reproductive health of two subsequent generations of mice. Hum Reprod. 2012;27(5):1391–1400. doi: 10.1093/humrep/des030. [DOI] [PubMed] [Google Scholar]
- 32.Rando OJ. Daddy issues: Paternal effects on phenotype. Cell. 2012;151(4):702–708. doi: 10.1016/j.cell.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O WS, Chen H, Chow PH. Male genital tract antioxidant enzymes—Their ability to preserve sperm DNA integrity. Mol Cell Endocrinol. 2006;250(1-2):80–83. doi: 10.1016/j.mce.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 34.Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE. 2010;5(9):e13100. doi: 10.1371/journal.pone.0013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer NO, Fullston T, Mitchell M, Setchell BP, Lane M. SIRT6 in mouse spermatogenesis is modulated by diet-induced obesity. Reprod Fertil Dev. 2011;23(7):929–939. doi: 10.1071/RD10326. [DOI] [PubMed] [Google Scholar]
- 36.Wong CL, et al. Ablation of paternal accessory sex glands imparts physical and behavioural abnormalities to the progeny: An in vivo study in the golden hamster. Theriogenology. 2007;68(4):654–662. doi: 10.1016/j.theriogenology.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 37.Poon HK, Lee KH, Wong CL, O WS, Chow PH. Absence of paternal accessory sex gland secretions disturbs epigenetic reprogramming and expression of Igf2 and Dlk1 in golden hamster embryos. Theriogenology. 2009;71(9):1367–1380. doi: 10.1016/j.theriogenology.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 38.Seki Y, Williams L, Vuguin PM, Charron MJ. Minireview: Epigenetic programming of diabetes and obesity: Animal models. Endocrinology. 2012;153(3):1031–1038. doi: 10.1210/en.2011-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinclair KD, Young LE, Wilmut I, McEvoy TG. In-utero overgrowth in ruminants following embryo culture: Lessons from mice and a warning to men. Hum Reprod. 2000;15(Suppl 5):68–86. doi: 10.1093/humrep/15.suppl_5.68. [DOI] [PubMed] [Google Scholar]
- 40.Chason RJ, Csokmay J, Segars JH, DeCherney AH, Armant DR. Environmental and epigenetic effects upon preimplantation embryo metabolism and development. Trends Endocrinol Metab. 2011;22(10):412–420. doi: 10.1016/j.tem.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyanari Y, Torres-Padilla ME. Control of ground-state pluripotency by allelic regulation of Nanog. Nature. 2012;483(7390):470–473. doi: 10.1038/nature10807. [DOI] [PubMed] [Google Scholar]
- 42.Zhao XY, et al. Granulocyte-macrophage colony-stimulating factor induces de novo methylation of the p15 CpG island in hematopoietic cells. Cytokine. 2005;31(3):203–212. doi: 10.1016/j.cyto.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Robertson SA, Sjöblom C, Jasper MJ, Norman RJ, Seamark RF. Granulocyte-macrophage colony-stimulating factor promotes glucose transport and blastomere viability in murine preimplantation embryos. Biol Reprod. 2001;64(4):1206–1215. doi: 10.1095/biolreprod64.4.1206. [DOI] [PubMed] [Google Scholar]
- 44.Chin PY, Macpherson AM, Thompson JG, Lane M, Robertson SA. Stress response genes are suppressed in mouse preimplantation embryos by granulocyte-macrophage colony-stimulating factor (GM-CSF) Hum Reprod. 2009;24(12):2997–3009. doi: 10.1093/humrep/dep307. [DOI] [PubMed] [Google Scholar]
- 45.Shen XH, Han YJ, Zhang DX, Cui XS, Kim NH. A link between the interleukin-6/Stat3 anti-apoptotic pathway and microRNA-21 in preimplantation mouse embryos. Mol Reprod Dev. 2009;76(9):854–862. doi: 10.1002/mrd.21048. [DOI] [PubMed] [Google Scholar]
- 46.Cheung LP, Leung HY, Bongso A. Effect of supplementation of leukemia inhibitory factor and epidermal growth factor on murine embryonic development in vitro, implantation, and outcome of offspring. Fertil Steril. 2003;80(Suppl 2):727–735. doi: 10.1016/s0015-0282(03)00772-6. [DOI] [PubMed] [Google Scholar]
- 47.Cheng TC, et al. Leukemia inhibitory factor antisense oligonucleotide inhibits the development of murine embryos at preimplantation stages. Biol Reprod. 2004;70(5):1270–1276. doi: 10.1095/biolreprod.103.023283. [DOI] [PubMed] [Google Scholar]
- 48.Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod. 2007;13(7):491–501. doi: 10.1093/molehr/gam028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.