Significance
Homologous chromosome pairing ensures that each product of meiosis contains a full haploid genome complement and is particularly important in allopolyploid plants which contain multiple, genetically related sets of chromosomes. In domesticated allohexaploid wheat, the Ph1 locus drives homologous bivalent formation and is required to maintain genome stability and disomic inheritance. In this study, we show that the most closely related gene in Arabidopsis, the cyclin-dependent protein kinase CDKG1, also functions in chromosome pairing and is required for the completion of synapsis in male meiocytes at high ambient temperature. This class of CDK has been previously implicated in RNA splicing and may be responsible for the intrinsic temperature sensitivity of meiosis in males.
Abstract
The Arabidopsis cyclin-dependent kinase G (CDKG) gene defines a clade of cyclin-dependent protein kinases related to CDK10 and CDK11, as well as to the enigmatic Ph1-related kinases that are implicated in controlling homeologous chromosome pairing in wheat. Here we demonstrate that the CDKG1/CYCLINL complex is essential for synapsis and recombination during male meiosis. A transfer-DNA insertional mutation in the cdkg1 gene leads to a temperature-sensitive failure of meiosis in late Zygotene/Pachytene that is associated with defective formation of the synaptonemal complex, reduced bivalent formation and crossing over, and aneuploid gametes. An aphenotypic insertion in the cyclin L gene, a cognate cyclin for CDKG, strongly enhances the phenotype of cdkg1–1 mutants, indicating that this cdk–cyclin complex is essential for male meiosis. Since CYCLINL, CDKG, and their mammalian homologs have been previously shown to affect mRNA processing, particularly alternative splicing, our observations also suggest a mechanism to explain the widespread phenomenon of thermal sensitivity in male meiosis.
Replication and segregation of DNA underpins the reproduction of all cells. In organisms that undergo sexual reproduction, the nucleus carries two copies of each chromosome (or homologs) that replicate and recognize each other before the meiotic reduction division. This process allows the homologs to pair and exchange genetic material before segregation. A proteinaceous structure, known as the synaptonemal complex (SC), forms along the length of each set of paired chromosomes and subsequently forms physical connections between the homologs. Recombination between the homologs involves first the formation of DNA double-strand breaks (DSBs) that are either repaired as noncrossover or crossover (CO) products. Chiasmata, the cytological manifestation of COs, provide a physical connection between the homologs that persists after the SC is disassembled.
Many of the proteins involved in meiotic recombination and homologous chromosome synapsis have been identified. At the leptotene stage of prophase I, the chromosome axes are elaborated along the conjoined bases of the sister chromatids. Concomitantly, Spo11-dependent DSBs form and are processed at the axes where the recombination machinery is assembled. This enables the alignment of homologous chromosomes and is dependent on the strand-exchange proteins Rad51 and DMC1. During zygotene, the SC starts to form between the homologs through the polymerization of the central element protein ZYP1 which brings the homologous chromosomes into close apposition. SC formation is complete by pachytene, during which the final stages of recombination are complete. The SC breaks down at the end of pachytene, and the chromosomes condense further during diplotene/diakinesis. At metaphase the bivalents held together by chiasmata are arranged at the metaphase plate, and the first meiotic division occurs.
Although many of the structural proteins involved in the progression of meiotic prophase have been identified (reviewed by ref. 1), regulators are still poorly understood. Cyclin-dependent protein kinases (CDKs) regulate diverse cellular functions in both mitotic and meiotic cell cycles. Cdc28 in budding yeast has a role in Spo11-dependent DSB formation and is necessary for SC assembly (2). CDK2 is required to prevent nonhomologous chromosome pairing in mice (3), and the Arabidopsis homolog CDKA;1 is necessary for the meiotic process (4), perhaps through regulation of retinoblastoma-related protein (Rbr) activity (5). Ph1, a complex locus containing multiple copies of CDK-like genes (6), enhances the fidelity of chromosome pairing in hexaploid wheat (7), such that homologous chromosomes cross over but homeologs do not (8). The pachytene checkpoint that couples pairing and recombination in animals can be overcome by protein phosphatase inhibitors (9). Ph1 also affects histone phosphorylation and chromatin structure (7), but its precise mechanism remains unclear. Although the Arabidopsis genome does not contain a homolog of Ph1 kinases, we previously identified CDKG as the most closely related kinase (10), one which is also conserved across plants and animals. We therefore undertook a detailed study of CDKG function in Arabidopsis using a combined genetic and cytological approach and demonstrate that the CDKG/CYCLINL complex is essential for the final steps of chromosome synapsis during male gamete formation.
Results
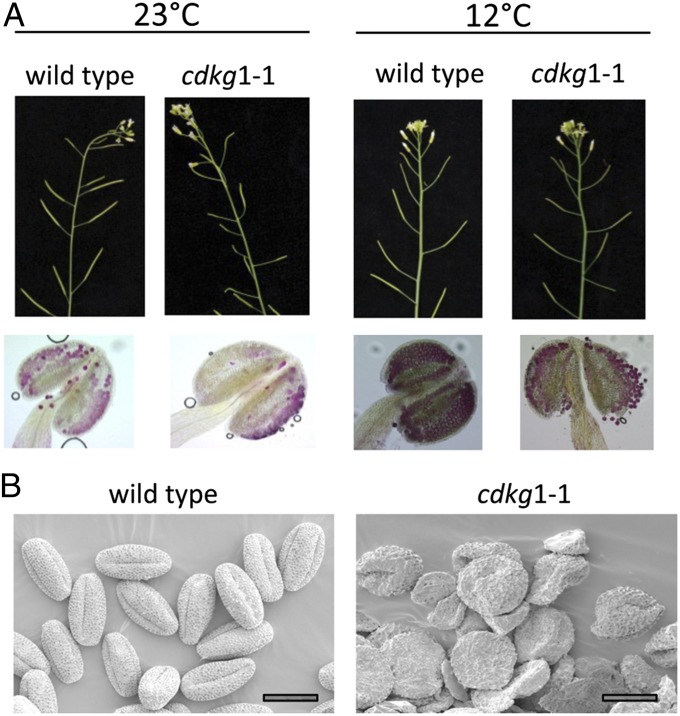
A transfer-DNA (T-DNA) insertion in CDKG1 (At5g63370) was verified in the line, SALK_075762 (11), using PCR and sequencing of the resultant PCR product. In homozygous mutant plants, CDKG1 transcript was not detectable by RT-PCR (Fig. S1), indicating that cdkg1–1 is effectively a null allele. When grown under standard greenhouse conditions in summer [25 °C average (effective range 14–30 °C), 16 h day], homozygous mutant siliques varied in length, indicating that fertility was compromised. Seed number was reduced and variable in homozygous mutants: only 25–40% mutant siliques contained any seed, whereas more than 95% siliques contained seed in all WT plants (n = 10 for both lines). The reduction in the number of seed per silique was also dramatic, with only 12.1 (±4.9) per silique (n = 20) in mutant and 39.3 (±2.7) per silique (n = 20) in WT. The variation in seed set between mutant pods suggested that there might be an environmental variable affecting phenotype. We therefore explored different variables (water regime, lighting, and temperature) and found that altering the temperature had a reproducible effect on pod length (Fig. 1) and seed set. Homozygous cdkg1–1 plants grown in controlled environment rooms (CERs) at a constant temperature of 16 °C were essentially fully fertile (94% filled siliques, n = 100). Grown at 23 °C, cdkg1–1 mutants were almost completely sterile with only 2% (n = 100) siliques containing any seed (1–2 seeds per silique), with most being completely empty. This indicated that CDKG is required only at the higher temperature.
Fig. 1.
Mutation of CDKG1 produces a temperature-sensitive fertility defect. (A) Floral shoots of WT and mutant (cdkg1–1) grown at 12 and 23 °C. A single anther from each plant, stained with Alexander’s Stain to illustrate pollen viability (living pollen stains red), is shown below. Viability scores are given in Table S1. (B) SEM of WT and mutant pollen. (Scale bar, 20 μm.)
To confirm that these effects were due to the insertion in CDKG1, we introduced a 5.0-kbp genomic fragment, gCDKG1 (−1657 to +3379), containing the complete WT CDKG1 gene and neighboring regulatory regions into a homozygous cdkg1 mutant. Of 22 independent transgenic lines, 16 lines were restored to normal fertility under all tested conditions, with more than 95% filled siliques, confirming that the fertility defect was due to the insertion event in CDKG1.
To determine if the defect is male-specific, female-specific, or affects both sexes, we did a reciprocal cross between homozygous mutant and WT plants. WT pollen on mutant stigmas produced siliques with similar numbers of seed to WT pollen on WT stigmas, but pollen from homozygous mutant plants produced no normal siliques on both mutant and WT (Table 1). Pollen from WT plants was highly viable, but that from homozygous mutant plants grown at high temperature were largely nonviable (Fig. 1A), as judged by Alexander staining. Examination of mature pollen by scanning electron microscopy (SEM) showed that most pollen from mutant plants were collapsed and appeared lysed (Fig. 1B). When pollen from a heterozygous mutant (CDKG1/cdkg1–1) was used to pollinate a WT plant, seed set was normal, and the transmission frequency of the mutant allele (as judged by genotyping the resultant progeny) was 95%, which is not statistically different from the WT allele. Taken together, these data indicate that the defect is specific to the male line before or during meiosis.
Table 1.
Reciprocal cross between cdkg1–1 mutant and WT plants
| Parental genotype (Female × Male) | Seed number | Total siliques | Average number per silique |
| Col0 × Col0 | 221 | 10 | 22.1 |
| cdkg1–1 × Col0 | 243 | 12 | 20.2 |
| Col0 × cdkg1–1 | 5 | 10 | 0.5 |
| cdkg1–1 × cdkg1–1 | 6 | 10 | 0.6 |
CDKG1 Is Required for Successful Meiosis in the Pollen Mother Cell.
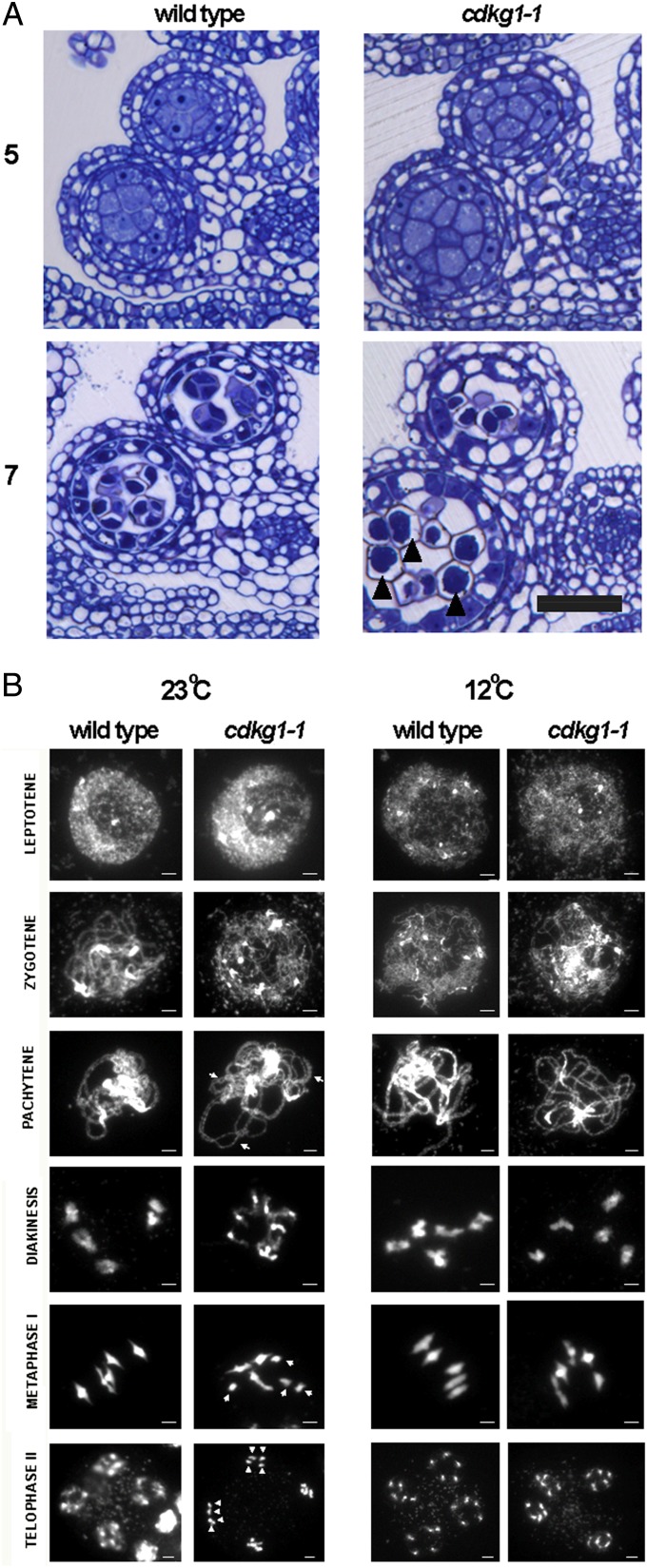
Because cdkg1–1 segregation data suggested a sporophytic or early meiotic defect, we examined resin sections taken from anthers at defined stages (12) to determine the earliest stage at which WT and mutant showed differences in development. No visible difference between mutant and WT meiocytes was detected before stage 6, but differences first appear at and are dramatic from stage 7 (defined by the completion of meiosis and formation of a tetrad of four haploid microspores). Almost all tetrads in mutant anthers were abnormal compared with those in WT (Fig. 2A).
Fig. 2.
cdkg1–1 mutants are conditionally defective in meiosis. (A) Resin sections of staged (5, 7) anthers from WT and cdkg1–1 mutant plants illustrating normal tetrads in WT stage 7 anthers and aborted tetrads in the mutant (arrowheads). (Scale bar, 25 μm.) (B) Chromatin configuration at different stages (as indicated to the Left) of meiosis in WT and mutant grown at 23 and 12 °C. Arrows indicate unsynapsed regions in a pachytene nucleus and univalents in metaphase I, and arrowheads indicate missegregated meiotic products. (Scale bar, 2 μm.)
Loss of cdkg1 Affects Synapsis and Chromosome Segregation.
Using the DNA-specific dye, DAPI, clear differences between mutant and WT meiocytes were first detected during prophase I (Fig. 2B). In WT meiocytes at both temperatures and mutant meiocytes at 12 °C, the chromosomes underwent synapsis and were fully synapsed at pachytene, and the five bivalents were arranged on the metaphase plate at metaphase I. Bivalents then segregated into two sets of five half-bivalents and eventually gave rise to four products in telophase II. The first visible abnormality in the cdkg1-1 homozygous mutant grown at 23 °C was incomplete synapsis during a stage akin to pachytene in most nuclei. Consistent with this observation, mixtures of bivalents and univalents were found at metaphase I (arrowed). Univalents often segregated unevenly into the daughter cells at telophase II (arrowheads). We next compared the bivalent/univalent ratio in mutant and WT meiocytes at metaphase I. In WT, 100% of meiocytes have five bivalents aligned on the metaphase plate, whereas only 8.6% meiocytes from the mutant have five bivalents (Fig. S2).
ZYP1 Loading and Chromosome Pairing Is Temperature-Dependent in the Absence of CDKG1.
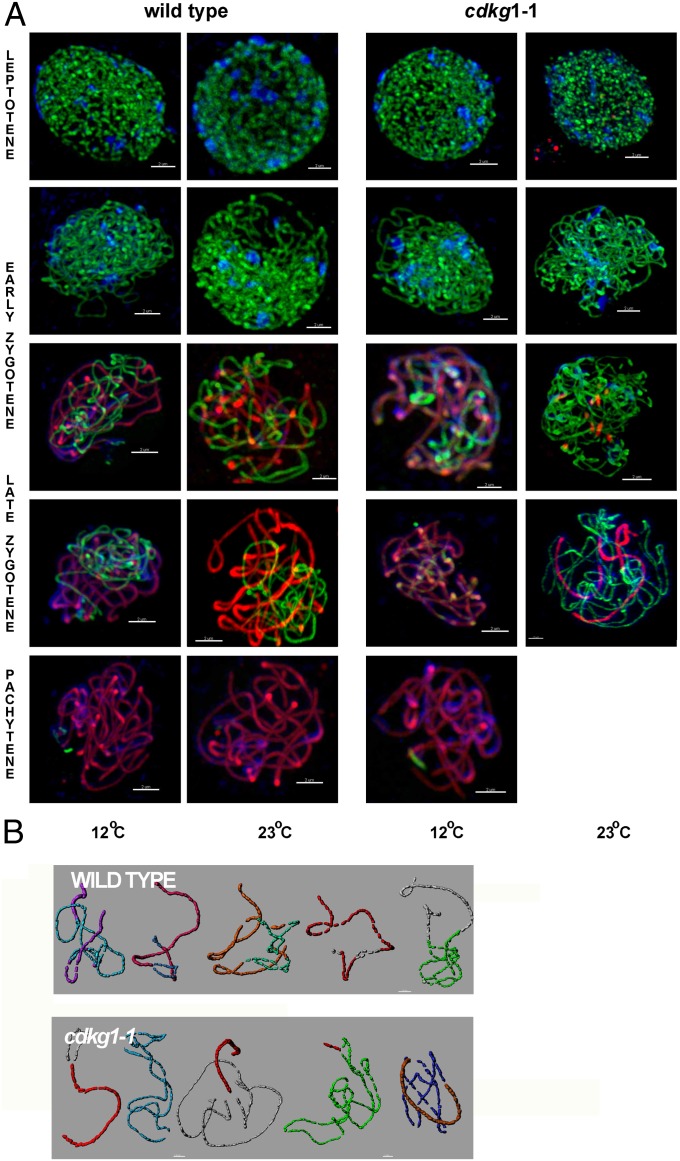
To ascertain the basis of the prophase defect, we immunolocalized ASY1, an axis-associated protein, and a SC protein, ZYP1, in polyacrylamide-embedded meiocytes. In WT meiocytes, the ASY1 protein loads onto chromatin during G2/leptotene and marks unpaired regions, whereas the transverse filament protein ZYP1 marks synapsed regions that initially appear as foci during leptotene, short linear stretches at zygotene, and at pachytene all of the chromosomes are fully synapsed with continuous ZYP1 signal (13).
ASY1 loading to meiotic chromosomes is not affected by the absence of CDKG1 function. However, and as previously reported for wheat–rye hybrids lacking the Ph1 locus (14), in mutant meiocytes grown at 23 °C the staining appeared less sharp, suggesting an altered chromatin structure (compare mutant and WT panels in Fig. 3A). ZYP1 loading and synapsis, however, was temperature-sensitive in cdkg1–1 mutants (Fig. 3A). At 12 °C, ZYP1 loading was indistinguishable in mutant and WT meiocytes. Small linear segments of ZYP1 were detected at zygotene, and a continuous ZYP1 signal was observed at pachytene, coincident with synapsis. In zygotene/pachytene nuclei from mutant meiocytes (23 °C), segments of ZYP1 loading of various sizes were observed, but full ZYP1 decoration of synapsed bivalents was not observed, and consequently, pachytene stage was not observed (Fig. S3), although homologous chromosomes were prealigned (Fig. 3A). Three-dimensional reconstruction of nuclei, used to examine bivalent synapsis in more detail [Fig. 3B and Fig. S4 (15)], revealed considerable variation in ZYP1 loading between mutant meiocytes (Fig. S3), with individual chromosome pairs being variably synapsed and partially ZYP-loaded. In WT, all bivalents were normally synapsed, whereas this was never observed in the mutant nuclei, with bivalents synapsing an average of 29.92% (range 0–73.76%, SD = 0.185, n = 10). These observations indicate that the initial loading of ZYP1 (and bivalent recognition) is independent of CDKG1, but that the extended loading of ZYP1 from these initiation sites is CDKG-dependent at high ambient temperature.
Fig. 3.
Synaptonemal complex formation in WT and cdkg1–1 mutant meiocyte nuclei, isolated from plants grown at 12 or 23 °C. (A) Nuclei at different meiotic stages immunostained with ASY1 (green) and ZYP1 (red) with DAPI counterstain (blue). (Scale bar, 2 μm.) (B) Three-dimensional reconstruction of individual bivalents from a pachytene WT nucleus (Upper) and pachytene-like mutant nucleus (Lower) at 23 °C. The nuclei were processed using Imaris, and each bivalent pair was isolated and false-colored. Quantification of ZYP1 loading is given in Fig. S3.
To evaluate whether recombination-related processes were also defective in the mutant, we used immunostaining with antibodies against the recombination proteins, DMC1 and RAD51 (16). Both these proteins were recruited normally in WT and mutant and at both temperatures (Fig. 4A), suggesting that the processes associated with DSB formation and early repair are not affected. Mature recombination nodules were detected with α−MLH1 antibody (17, 18) (Fig. 4B). As expected, MLH1 foci were found to always be associated with ZYP1 segments in both mutant and WT nuclei. In WT nuclei grown at 23 °C the average number of MLH1 foci at zygotene/pachytene stages was 9.5 ± 2.1(n = 15), and this number was reduced to 2.5 ± 2.4 (n = 24) in the mutant at the same temperature (P value 4.9 × 10−11, P < 0.001). However, at 12 °C there was no significant difference (P value 0.015, P < 0.001) between the number of MLH1 foci in the WT (9.9 ± 1.4, n = 9) and the cdkg1–1 mutant nuclei (7.9 ± 1.8, n = 10), indicating that CDKG1 is required to maintain chiasma frequency at higher temperatures. These data also compare very well with estimates of chiasma frequency at metaphase I in summer-grown greenhouse material using the method described by ref. 19: 3.8 per cell in cdkg1–1, compared with 9.7 per cell in WT.
Fig. 4.
Dual immunolocalization of SC proteins with recombination pathway proteins in WT and cdkg1–1 nuclei at 23 °C. DAPI-stained chromatin is shown in blue. (Scale bars, 2 μm.) (A) Leptotene nuclei immunostained with ASY1 (red) and DMC1 (green). (B) Leptotene nuclei immunostained with ASY1 (red) and RAD51 (green). (C) Late zygotene (Top) and pachytene or pachytene-like (Middle) nuclei of WT and cdkg1–1 mutants at 23 °C. ASY1 (red), ZYP1 (gray), and MLH1 (green). (Bottom) Detail of MLH1 foci on ZYP1-loaded regions in both WT and mutant.
CDKG1–CyclinL Complex Is Required for Male Fertility.
A systematic protein–protein interaction study (20) indicated that that CYCLINL is the cognate cyclin for CDKG. We therefore examined the phenotypes and genetic interactions of insertion mutants in these three genes. Knockout mutations in the CYCLINL gene (mos12–2) are lethal (21), so we sought alleles with partial loss of function. An allele with a T-DNA insertion in the 3′ end of the gene leading to the production of partial cyclinL mRNA was identified and confirmed by RT-PCR (Fig. S1) and called cycL1–1. cycL1–1 homozygous lines were fully fertile at both 12 and 23 °C. Pairwise crosses were made between cdkg1–1 and cyclinL lines to produce heterozygous F1 individuals. Double-homozygous mutant cycL1–1/cdkg1–1 plants, produced by allowing an F2 plant with the genotype cycL1–1/cycL1–1; cdkg1–1/+ to self-pollinate and genotyping the progeny, were essentially sterile (fewer than 15 seeds per plant) under all conditions tested (Fig. S5A). Loss of CYCLINL in a cdkg1–1 homozygous background, therefore, strongly enhanced the meiotic phenotype, such that the double-homozygous mutant grown at either temperature resembled the cdkg1–1 homozygous mutant grown at 23 °C with regard to the cytology of meiosis (Fig. S5B) and pollen formation (Fig. S5C). Loading of ZYP1 at 12 and 23 °C was essentially identical to the single homozygous cdkg1–1 mutant at 23 °C with small segments of ZYP1 loading, coinciding with limited regions of synapsis and supporting the notion that the CDKG1–CYCLINL complex is required for complete polymerization of ZYP1.
Discussion
The mutual recognition and physical association of homologous chromosomes as structurally connected bivalents during the first reduction division of meiosis is critically important for their accurate segregation. Missegregation defects underlie many genetic diseases in animals and humans. Conversely, the ability to manipulate the stringency of pairing could release novel variation and be an important tool in plant breeding where there is an urgent need to introduce new traits by means of recombination using crosses between phenotypically distinct varieties (22). As potential regulators that can themselves be manipulated, kinases could be attractive tools in breeding (23). Loss of CDKG1–CYCLINL function leads to a failure to consummate the physical association of cognate homologs and has dramatic consequences on the fidelity of chromosome segregation.
CDK–Cyclin Regulation of Meiosis.
As with mitosis, reversible phosphorylation of key proteins plays diverse regulatory roles in meiosis. Several CDKs and cyclins have been identified where mutations lead to a variety of specific defects. CDKA (or CDK1 in animals) is central to mitotic progression and is also required for the meiotic program (4). A phosphomimic substitution, T161D, in T-loop of CDKA partially restores the primary vegetative defect of cdka-1 mutants but is unable to support the meiotic program, indicating that CDKA function is perhaps even more critical during meiosis. At least two different cyclins that interact with CDKA also lead to meiotic defects. TARDY ASYNCHRONOUS MEIOSIS encodes CYCLIN A1;2 (CYCA1;2), and tam mutants form dyads instead of tetrads (24). Meiosis progresses asynchronously with delays in pachytene of both meiosis I and meiosis II. A null mutant leads to progressive polyploidization and loss of fertility (25). SOLO DANCERS (SDS), a plant-specific cyclin, is required for chromosome pairing and/or synapsis of homologous chromosomes during prophase I, the absence of which leads to greatly reduced levels of meiotic recombination (26). In mammals, the structurally related kinase CDK2 is essential for fertility, blocking meiosis at pachytene with incomplete synapsis (3). This differs radically from the cdkg1–1 phenotype, however, in that the chromosomes are extensively tangled by nonhomologous synapsis.
Other kinases have been implicated specifically in chromosome pairing in plants. The Ph1 locus of chromosome 5B of allohexaploid wheat contains (among other elements) a cluster of defective CDK-related genes (23, 27) that suppress the expression of unlinked, but structurally related, CDK genes and CDK activity as measured by Histone H1 phosphorylation (7). The transsilencing of these CDKs by Ph1 appears to drive homologous bivalent formation by preventing pairing between homeologs from its three constituent chromosome sets. The increased stringency of meiotic chromosome pairing may be mediated via changes in replication and reduced histone H1 phosphorylation, but specific mechanisms were not elaborated. Deletion of Ph1 increases CDK activity and permits homeologous chromosome pairing, whereas increasing the dosage of Ph1 (as in lines triisomic for the long arm of Chromosome 5B) suppresses synapsis and chiasma formation even between homologs (28). The activity of CDKG1 in Arabidopsis therefore parallels that of Ph1 in bread wheat, insofar as the mutant has defective pairing that phenocopies the effect of additional copies of the Ph1 locus in wheat. Given the effect of okadaic acid on the mammalian pachytene checkpoint (9), these data indicate that phosphorylation also modulates the degree of pairing in plants.
The origin and function of the Ph-1–related CDKs is intriguing as they appear to be an orphan group, restricted to the monocotyledonous lineage. Based on BLAST against nonplant protein sequences and 3D modeling onto known protein structures, Ph1 kinases have been reported to resemble mammalian CDK2 in terms of predicted 3D structure (6). CDKG1 is the most similar plant kinase to Ph1 kinases in terms of primary sequence (10) and, consistent with this relationship, the data presented here indicate that CDKG1 acts on the same biological process, namely chromosome pairing. It will be interesting to undertake high-resolution characterization of the prophase defect in ph1 mutant wheat when specific probes become available, but one might predict a similar defect in late zygotene/pachytene.
Bivalent formation relies on homologous recombination and synaptonemal complex polymerization, which physically binds homologs together via the establishment of COs (29–31). The normal localization of DMC1 and RAD51 proteins suggests that DSB formation and the early stages of repair are not affected by the loss of CDKG1. Elevated levels of DNA fragmentation were not observed in the mutant, implying that all DSBs are effectively repaired either via a non-CO recombination or via repair using the sister chromatid as a template. In addition, the lack of chromatin bridges at anaphase I indicates that DSBs were not repaired by nonhomologous end joining. In the absence of CDKG1, the level of class I crossovers, as detected by MutL homolog 1 (MLH1) staining, is dramatically reduced. The reduction of MLH1 foci in the mutant is likely a result of the reduced level of synapsis, as DSBs in unsynapsed regions lack the opportunity to engage in interhomolog recombination and are likely repaired via intersister recombination similar to what is observed during meiosis in haploid Arabidopsis (32).
Although the loading of the axis-associated protein ASY1 is not affected in the mutant, polymerization of the SC transverse filament protein ZYP1 is compromised, resulting in partial asynapsis. Links between chromosome structure and recombination have been proposed (29), and CDKG1 might act in this pathway. Another attractive possibility is that CDKG1 is part of a checkpoint mechanism that detects recombination defects and leads to meiotic arrest, as proposed for the RBR protein in Arabidopsis (5).
Temperature Stress, Splicing, and Male Meiosis.
A possible alternative mechanism for CDKG1 action is less direct, via the regulation of gene expression. The large isoform of CDK11 (the mammalian homolog) promotes pre-mRNA splicing through its interaction with CyclinL (33), and a similar function has been proposed for CyclinL in plants (21), where it is required for the correct splicing of disease resistance transcripts. Indeed, CDKG1 was found to be associated with the spliceosome and regulates the splicing of a gene involved in pollen cell wall formation (34), indicating a general role in regulating several different aspects of pollen development.
The effect of temperature on meiosis and recombination frequency has long been recognized (35), and this study suggests a mechanism at least for male meiosis. In many species, pollen formation is particularly sensitive to stress, and plants are often sterile under conditions where vegetative growth can continue, leading to reductions in yield (reviewed by ref. 36). Moreover, male and female meiosis in Arabidopsis differ in terms of recombination rates (37). Reduction in CDKG activity reveals a pathway affecting the frequency of COs that is inherently temperature-sensitive and male-specific. Other work suggests that pairing and recombination is exquisitely sensitive to environmental conditions—a difference of 8 °C is sufficient to alter the balance of COs along the length of the chromosome in barley (38), and meiosis is more sensitive to temperature in polyploid wheat compared with its diploid relatives (39), but no mechanism was proposed in either case. The insertion mutation in CDKG1 leads to a loss of detectable transcript and could reveal the presence of a temperature-sensitive pathway. An alternative, and not mutually exclusive, mechanism would be that CDKG1 participates in a process that is itself thermodynamically unstable, and loss of CDKG1 enhances the inherent temperature sensitivity of the pathway. CDKG1 is evolutionarily conserved across both plants and animals and is implicated in mRNA processing (10), perhaps via regulation of alternative splicing (34). Alternative splicing often depends on the active selection of nonconsensus splice sites that are thermodynamically unfavorable and have been suggested as a mechanism for allowing biological processes to adjust to ambient temperature variation (40, 41). Defining other targets of CDKG1–CYCLINL should provide further insight into the regulation of homologous chromosome pairing and holds promise for manipulating recombination in breeding, particularly of cereals, where the production of favorable recombinants can be rate-limiting.
Materials and Methods
The WT Columbia (Col-0) and mutant stocks [SALK_075762 (cdkg1–1); and SAIL_285_G10 (cycL1–1)] used in this study were obtained from the Nottingham Arabidopsis Stock Centre. Moist sterilized seeds were pretreated for 3 d at 4 °C in dark, then germinated on Petri-plates and transferred to soil after 1 wk or sown direct to soil. Plants were grown in a glasshouse (temperature range 14 to 25 °C) or in a growth chamber (16 h light; 60–70% relative humidity) at 23, 16, or 12 °C.
Genotyping and Cloning.
Homozygous lines for the T-DNA insertion were identified by PCR using the LBa1 primer and a gene-specific primer (Table S2) and confirmed by sequencing. For the construction of the CDKG1 complementation plasmid, the CDKG1 promoter attB1 and Flg CDKG1 attB2 primers (Table S2) were used to amplify the entire CDKG1 coding sequence with flanking 5′ and 3′ UTR regions from genomic DNA. The PCR product was cloned into the binary vector pMDC123 by using the GateWay recombination system. The constructs were confirmed by DNA sequencing (carried out by Genome Centre of John Innes Centre).
RNA Extraction and RT-PCR.
Tissue samples were collected and immediately frozen in liquid nitrogen. Tissues were ground in liquid nitrogen, and total RNA was isolated using the RNeasy mini kit (Qiagen). DNase treatment was carried out on 2 mg of total RNA using the Dnase-free kit (Promega). One microgram of total RNA was reverse-transcribed using SuperScript III (Invitrogen) following the manufacturer’s instructions using the primers indicated in Table S2.
Meiotic Spread Preparation.
Whole inflorescences from Arabidopsis were fixed in 3:1 ethanol:acetic acid and stored at 4 °C. Flower buds in the size range 0.3–0.9 mm were used to prepare the squashes as described (42). Slides were then stained with DAPI for observation or used for FISH. FISH was used to identify individual bivalents, and chiasmata counts were recorded by light microscopy according to ref. 43.
Cytological Procedures.
Mutant and WT pollen mother cells were examined by light microscopy in using FISH and DAPI stained spreads described by ref. 19. Pollen viability was assessed as described in ref. 44. Tissue samples were fixed in 2.5% (vol/vol) glutaraldehyde in 0.05 M sodium cacodylate, pH 7.3, vacuum-infiltrated, washed and dehydrated in an ethanol series (10, 20, 30, 50, 70, 95, and 100% ethanol) before infiltrating with and embedding in LR White resin (London Resin Company). For light microscopy, 0.5-μm–thick sections were dried onto glass slides and stained with 0.5% (wt/vol) Toluidine blue “O” in 0.5% (wt/vol) borax.
Immunolabeling Arabidopsis Pollen Mother Cells.
Meiocytes from WT and mutant plants growing at the different temperatures where embedded in acrylamide to preserve their 3D structure and used for the immunolocalization studies as described in ref. 22 with the following modifications. Buds of Arabidopsis plants in the prophase I meiotic stage (size range 0.3–0.9 mm) were isolated into Buffer A and fixed in 2% paraformaldehyde for 30 min. After 2 × 10-min washes in Buffer A the buds were macerated with a brass rod, and the suspension was then embedded in acrylamide. Immunolocalization using α−ASY1 (1:250), α−ZYP1 (1:200), α−DMC1 (1:200), α−RAD51 (1:200), and α−MLH1 (1:250) antibodies was performed, and images were acquired using a Leica TCS SP5II confocal microscope and Z-stacks deconvolved using AutoQuant ×2 (media Cybernetics). Bivalent tracking and analysis was performed using Imaris 7.3 (Bitplane).
Supplementary Material
Acknowledgments
We thank Susan Bunnywell for assistance with cytology and sectioning; Kim Findlay for scanning electron microscopy; and Chris Franklin for critical reading of the manuscript. We thank Chris Franklin for DMC1 and RAD51 antibodies and Liudmila Chelisheva for the MLH1 antibodies. Funding: EU FP7 program AGRON-OMICS network Grant 037704 and National Science Foundation of China Grant 31271438 (to T.Z.); C.N. was partly supported by Leverhulme Grant F00424R; BBSRC Grant BB/F018754/1 (to S.J.A., G.J., C.N., and D.W.P.); Euporean Union Framework 7 Knowledge-Based Bio-Economy collaborative network 222883 'Meiosys (to S.J.A.); and Biotechnology and Biological Sciences Research Council Grant BB/J004405/1 (to J.H.D. and C.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318460111/-/DCSupplemental.
References
- 1.Osman K, Higgins JD, Sanchez-Moran E, Armstrong SJ, Franklin FC. Pathways to meiotic recombination in Arabidopsis thaliana. New Phytol. 2011;190(3):523–544. doi: 10.1111/j.1469-8137.2011.03665.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Z, et al. Cyclin-dependent kinase promotes formation of the synaptonemal complex in yeast meiosis. Genes Cells. 2010;15(10):1036–1050. doi: 10.1111/j.1365-2443.2010.01440.x. [DOI] [PubMed] [Google Scholar]
- 3.Viera A, et al. CDK2 is required for proper homologous pairing, recombination and sex-body formation during male mouse meiosis. J Cell Sci. 2009;122(Pt 12):2149–2159. doi: 10.1242/jcs.046706. [DOI] [PubMed] [Google Scholar]
- 4.Dissmeyer N, et al. T-loop phosphorylation of Arabidopsis CDKA;1 is required for its function and can be partially substituted by an aspartate residue. Plant Cell. 2007;19(3):972–985. doi: 10.1105/tpc.107.050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z, et al. Retinoblastoma protein is essential for early meiotic events in Arabidopsis. EMBO J. 2011;30(4):744–755. doi: 10.1038/emboj.2010.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yousafzai FK, Al-Kaff N, Moore G. Structural and functional relationship between the Ph1 locus protein 5B2 in wheat and CDK2 in mammals. Funct Integr Genomics. 2010;10(2):157–166. doi: 10.1007/s10142-010-0170-7. [DOI] [PubMed] [Google Scholar]
- 7.Greer E, et al. The Ph1 locus suppresses Cdk2-type activity during premeiosis and meiosis in wheat. Plant Cell. 2012;24(1):152–162. doi: 10.1105/tpc.111.094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins G. Chromosome pairing in Triticum aestivum cv. Chinese Spring. Carlsberg Res Commun. 1983;48:255–283. [Google Scholar]
- 9.Li XC, Schimenti JC. Mouse pachytene checkpoint 2 (trip13) is required for completing meiotic recombination but not synapsis. PLoS Genet. 2007;3(8):e130. doi: 10.1371/journal.pgen.0030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doonan JH, Kitsios G. Functional evolution of cyclin-dependent kinases. Mol Biotechnol. 2009;42(1):14–29. doi: 10.1007/s12033-008-9126-8. [DOI] [PubMed] [Google Scholar]
- 11.Alonso JM, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301(5633):653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- 12.Sanders PM, et al. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. (Translated from English) Sex Plant Reprod. 1999;11(6):297–322. [Google Scholar]
- 13.Higgins JD, Sanchez-Moran E, Armstrong SJ, Jones GH, Franklin FC. The Arabidopsis synaptonemal complex protein ZYP1 is required for chromosome synapsis and normal fidelity of crossing over. Genes Dev. 2005;19(20):2488–2500. doi: 10.1101/gad.354705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knight E, et al. Inducing chromosome pairing through premature condensation: analysis of wheat interspecific hybrids. Funct Integr Genomics. 2010;10(4):603–608. doi: 10.1007/s10142-010-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips D, Nibau C, Wnetrzak J, Jenkins G. High resolution analysis of meiotic chromosome structure and behaviour in barley (Hordeum vulgare L.) PLoS ONE. 2012;7(6):e39539. doi: 10.1371/journal.pone.0039539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neale MJ, Keeney S. Clarifying the mechanics of DNA strand exchange in meiotic recombination. Nature. 2006;442(7099):153–158. doi: 10.1038/nature04885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moens PB, et al. The time course and chromosomal localization of recombination-related proteins at meiosis in the mouse are compatible with models that can resolve the early DNA-DNA interactions without reciprocal recombination. J Cell Sci. 2002;115(Pt 8):1611–1622. doi: 10.1242/jcs.115.8.1611. [DOI] [PubMed] [Google Scholar]
- 18.Jackson N, et al. Reduced meiotic crossovers and delayed prophase I progression in AtMLH3-deficient Arabidopsis. EMBO J. 2006;25(6):1315–1323. doi: 10.1038/sj.emboj.7600992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JD, Armstrong SJ, Franklin FC, Jones GH. The Arabidopsis MutS homolog AtMSH4 functions at an early step in recombination: Evidence for two classes of recombination in Arabidopsis. Genes Dev. 2004;18(20):2557–2570. doi: 10.1101/gad.317504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Leene J, et al. Targeted interactomics reveals a complex core cell cycle machinery in Arabidopsis thaliana. Mol Syst Biol. 2010;6:397. doi: 10.1038/msb.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu F, Xu S, Wiermer M, Zhang Y, Li X. The cyclin L homolog MOS12 and the MOS4-associated complex are required for the proper splicing of plant resistance genes. Plant J. 2012;70(6):916–928. doi: 10.1111/j.1365-313X.2012.04906.x. [DOI] [PubMed] [Google Scholar]
- 22.Phillips D, Nibau C, Ramsay L, Waugh R, Jenkins G. Development of a molecular cytogenetic recombination assay for barley. Cytogenet Genome Res. 2010;129(1–3):154–161. doi: 10.1159/000314335. [DOI] [PubMed] [Google Scholar]
- 23.Al-Kaff N, et al. Detailed dissection of the chromosomal region containing the Ph1 locus in wheat Triticum aestivum: with deletion mutants and expression profiling. Ann Bot (Lond) 2008;101(6):863–872. doi: 10.1093/aob/mcm252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Magnard JL, McCormick S, Yang M. Progression through meiosis I and meiosis II in Arabidopsis anthers is regulated by an A-type cyclin predominately expressed in prophase I. Plant Physiol. 2004;136(4):4127–4135. doi: 10.1104/pp.104.051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Jha AK, Chen R, Doonan JH, Yang M. Polyploidy-associated genomic instability in Arabidopsis thaliana. Genesis. 2010;48(4):254–263. doi: 10.1002/dvg.20610. [DOI] [PubMed] [Google Scholar]
- 26.Azumi Y, et al. Homolog interaction during meiotic prophase I in Arabidopsis requires the SOLO DANCERS gene encoding a novel cyclin-like protein. EMBO J. 2002;21(12):3081–3095. doi: 10.1093/emboj/cdf285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffiths S, et al. Molecular characterization of Ph1 as a major chromosome pairing locus in polyploid wheat. Nature. 2006;439(7077):749–752. doi: 10.1038/nature04434. [DOI] [PubMed] [Google Scholar]
- 28.Holm PB, Wang X. The effect of chromosome 5B on synapsis and chiasma formation in wheat, Triticum aestivum cv Chinese Spring. Carlsberg Res Commun. 1988;53:191–208. [Google Scholar]
- 29.Kleckner N. Meiosis: How could it work? Proc Natl Acad Sci USA. 1996;93(16):8167–8174. doi: 10.1073/pnas.93.16.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roeder GS. Meiotic chromosomes: It takes two to tango. Genes Dev. 1997;11(20):2600–2621. doi: 10.1101/gad.11.20.2600. [DOI] [PubMed] [Google Scholar]
- 31.Zickler D, Kleckner N. The leptotene-zygotene transition of meiosis. Annu Rev Genet. 1998;32:619–697. doi: 10.1146/annurev.genet.32.1.619. [DOI] [PubMed] [Google Scholar]
- 32.Cifuentes M, Rivard M, Pereira L, Chelysheva L, Mercier R. Haploid meiosis in Arabidopsis: double-strand breaks are formed and repaired but without synapsis and crossovers. PLoS ONE. 2013;8(8):e72431. doi: 10.1371/journal.pone.0072431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loyer P, et al. Characterization of cyclin L1 and L2 interactions with CDK11 and splicing factors: Influence of cyclin L isoforms on splice site selection. J Biol Chem. 2008;283(12):7721–7732. doi: 10.1074/jbc.M708188200. [DOI] [PubMed] [Google Scholar]
- 34.Huang XY, et al. CYCLIN-DEPENDENT KINASE G1 is associated with the spliceosome to regulate CALLOSE SYNTHASE5 splicing and pollen wall formation in Arabidopsis. Plant Cell. 2013;25(2):637–648. doi: 10.1105/tpc.112.107896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dowrick GJ. The influence of temperature on meiosis. Heredity. 1957;11:37–49. [Google Scholar]
- 36.Zinn KE, Tunc-Ozdemir M, Harper JF. Temperature stress and plant sexual reproduction: uncovering the weakest links. J Exp Bot. 2010;61(7):1959–1968. doi: 10.1093/jxb/erq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giraut L, et al. Genome-wide crossover distribution in Arabidopsis thaliana meiosis reveals sex-specific patterns along chromosomes. PLoS Genet. 2011;7(11):e1002354. doi: 10.1371/journal.pgen.1002354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins JD, et al. Spatiotemporal asymmetry of the meiotic program underlies the predominantly distal distribution of meiotic crossovers in barley. Plant Cell. 2012;24(10):4096–4109. doi: 10.1105/tpc.112.102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rezaei M, Arzani A, Sayed-Tabatabaei BE. Meiotic behaviour of tetraploid wheats (Triticum turgidum L.) and their synthetic hexaploid wheat derivates influenced by meiotic restitution and heat stress. J Genet. 2010;89(4):401–407. doi: 10.1007/s12041-010-0058-2. [DOI] [PubMed] [Google Scholar]
- 40.James AB, et al. Alternative splicing mediates responses of the Arabidopsis circadian clock to temperature changes. Plant Cell. 2012;24(3):961–981. doi: 10.1105/tpc.111.093948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diernfellner A, et al. Long and short isoforms of Neurospora clock protein FRQ support temperature-compensated circadian rhythms. FEBS Lett. 2007;581(30):5759–5764. doi: 10.1016/j.febslet.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armstrong SJ, Franklin FC, Jones GH. Nucleolus-associated telomere clustering and pairing precede meiotic chromosome synapsis in Arabidopsis thaliana. J Cell Sci. 2001;114(Pt 23):4207–4217. doi: 10.1242/jcs.114.23.4207. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez Moran E, Armstrong SJ, Santos JL, Franklin FC, Jones GH. Chiasma formation in Arabidopsis thaliana accession Wassileskija and in two meiotic mutants. Chromosome Res. 2001;9(2):121–128. doi: 10.1023/a:1009278902994. [DOI] [PubMed] [Google Scholar]
- 44.Alexander MP. Differential staining of aborted and nonaborted pollen. Stain Technol. 1969;44(3):117–122. doi: 10.3109/10520296909063335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.