Summary
Twenty five patients were treated with proton beam therapy to 45 Gy(RBE), 52.5 Gy(RBE), or 60 Gy(RBE) in 15 fractions using a 3+3 study design. Dose constraints were based on biologically equivalent doses to standard fractionated treatment. Two patients experienced high-grade toxicity, both possibly related to radiation therapy. We consider this approach to be a good option for patients who are not candidates for concurrent chemoradiation.
Background
Many patients with locally advanced non-small cell lung cancer (NSCLC) cannot undergo concurrent chemotherapy because of comorbidities or poor performance status. Hypofractionated radiation regimens, if tolerable, may provide an option to these patients for effective local control.
Patients and Methods
Twenty five patients were enrolled in a phase I dose-escalation trial of proton beam therapy (PBT) from September 2010 through July 2012. Eligible patients had histologically documented lung cancer, thymic tumors, carcinoid tumors, or metastatic thyroid tumors. Concurrent chemotherapy was not allowed, but concurrent treatment with biologic agents was. The dose-escalation schema comprised 15 fractions of 3 Gy(RBE)/fraction, 3.5 Gy(RBE)/fraction, or 4 Gy(RBE)/fraction. Dose constraints were derived from biologically equivalent doses of standard fractionated treatment.
Results
The median follow-up time for patients alive at the time of analysis was 13 months (range 8-28 months). Fifteen patients received treatment to hilar or mediastinal lymph nodes. Two patients experienced dose-limiting toxicity possibly related to treatment; one received 3.5-Gy(RBE) fractions and developed an in-field tracheoesophageal fistula 9 months after PBT and 1 month after bevacizumab. The other patient received 4-Gy(RBE) fractions and was hospitalized for bacterial pneumonia/radiation pneumonitis 4 months after PBT.
Conclusion
Hypofractionated PBT to the thorax delivered over 3 weeks was well tolerated even with significant doses to the lungs and mediastinal structures. Phase II/III trials are needed to compare the efficacy of this technique with standard treatment for locally advanced NSCLC.
Keywords: proton therapy, lung cancer, biologically equivalent dose
Introduction
Non-small cell lung cancer (NSCLC) is frequently diagnosed in patients of an advanced age that are likely to have additional comorbid conditions and poor performance status, deeming them unable to tolerate standard chemoradiation therapy. [1] [2] [3]. Effective regimens that omit concurrent systemic therapy are needed for such patients. In addition, if shorter radiation courses could be delivered for locally advanced disease, patients who could tolerate it could receive systemic doses of sequential chemotherapy sooner than would otherwise be possible, which could reduce the risk of distant metastases and increase the cost-effectiveness of radiation by reducing the number of fractions to be given.
Numerous studies have been published regarding hypofractionated stereotactic radiotherapy for early-stage lung cancer [4], but analyses of hypofractionated regimens for locally advanced disease are limited. One institution reported two studies in which 45 Gy in 3-Gy fractions was given to patients with poor performance status and noted that rates of response and locoregional control were comparable despite poor prognostic factors [5, 6]. The advent of increasingly conformal techniques may allow further dose escalation to improve the likelihood of local control while sparing surrounding normal structures.
To address this possibility, we undertook this prospective phase I study to assess the safety of dose-escalating hypofractionated proton therapy, given without chemotherapy, for NSCLC. Our hypothesis was that the favorable dose-distribution characteristics of proton beam therapy (PBT) would render doses of up to 60 Gy(RBE) [biologically equivalent dose [BED] 84 Gy) feasible even for patients with poor performance status.
Patients and Methods
This study was approved by the appropriate institutional review board. Eligibility criteria included histologically or cytologically documented NSCLC. Multiple histologies were initially allowed due to the phase I nature of the study, but in order to maintain uniformity, only the results with NSCLC are included in this report. Concurrent chemotherapy was not allowed, but concurrent treatment with biologic agents such as epidermal growth factor receptor (EGFR) or vascular endothelial growth factor (VEGF) inhibitors was allowed. Other exclusion criteria were prior radiotherapy to the chest or a life expectancy of <6 months.
Treatment evaluations
Chest imaging with computed tomography (CT) or positron emission tomography (PET)/CT was required before study entry. Baseline pulmonary function tests were also obtained, including forced expiratory volume in one second (FEV1) and diffusion capacity (DLCO). During treatment, patients were monitored weekly for adverse events, which were scored according to the Common Terminology Criteria for Adverse Events, version 4.0. Toxicity assessment after PBT was performed at 6 weeks after treatment ended and then every 3 months for the first two years, or earlier at the physician's discretion.
Radiation therapy simulation and volume delineation
All patients underwent treatment simulation on a CT scanner with 4D imaging for internal motion assessment. The gross tumor volume (GTV), clinical target volume (CTV), and internal target volume (ITV) were delineated on each planning scan. The suggested GTV-to-CTV margin was 8 mm, with adjustment by the treating physician to account for potential microscopic spread. In PBT planning, each beam has a unique expansion of the planning target volume (PTV) from the ITV. In the plane perpendicular to the proton beam axis, the PTV was expanded by a fixed distance of 5 mm from the ITV to account for setup uncertainties. In the parallel axis, both the distal and the proximal expansion was based on the range uncertainty of the beam, and the margins calculated based on published formulae [7-9].
Radiation dose constraints
The dose constraints for the organs at risk were derived by using a BED calculation with an α/β ratio of 3 and determining the total dose in 15 fractions that would be equivalent to our institution's standard fractionated regimen constraints. Use of this ratio provided conservative estimates of dose constraints (Table 1). PBT doses were defined in Gy(RBE). If dose constraints could not be met, patients were treated at the next lowest dose, if applicable.
Table 1. Dose Constraints to Organs at Risk.
| Target | Current Dose-Volume Constraints for Standard Fractionated Regimens at our Institution (2-Gy Fractions to 60-74 Gy) | Dose-Volume Constraints in Current Study [BED dose assuming α/β=3 with 15-fraction regimen) |
|---|---|---|
| Total Lung | V20 <40% Mean Lung Dose <20 Gy |
V17<40% Mean Lung Dose <17.5 Gy(RBE) [17.1 Gy(RBE)] |
| Liver | 40% <50 Gy | 40% <40 Gy(RBE) [38.9 Gy(RBE)] |
| Kidneys (both) | 1/3 <20 Gy | 1/3 <18 Gy(RBE) [17.1 Gy(RBE)] |
| Esophagus | 20% <70 Gy 50% <50 Gy |
20% <55 Gy(RBE) [52.65 Gy(RBE)] 50% <40 Gy(RBE) [38.9 Gy(RBE)] |
| Heart | 50% <30 Gy 40% <40 Gy |
50% <25 Gy(RBE) [24.6 Gy(RBE)] 40%<32 Gy(RBE) [31.9 Gy(RBE)] |
| Spinal Cord | Maximum dose 45 Gy | Maximum dose 36 Gy(RBE) [35.4 Gy(RBE)] |
| Brachial Plexus | Maximum dose <60 Gy | Dose to <1 cm3 must not exceed 50 Gy(RBE) [45.6 Gy(RBE)] |
Study design and statistical considerations
The phase I study design developed by Ji, Li, and Bekele [10] was used to find the maximum tolerated dose as follows. Three dose levels [45 Gy(RBE) in 3-Gy(RBE) fractions, 52.5 Gy(RBE) in 3.5-Gy(RBE) fractions, and 60 Gy(RBE) in 4-Gy(RBE) fractions] were to be tested. Dose-limiting toxicity (DLT) was defined as any grade 4 toxicity involving the esophagus or skin, or any grade ≥3 complications of the lung, liver, kidney, or gastrointestinal tract. The rate of DLTs could not exceed 25% in any dosage group. Thirty patients were to be enrolled and tested in groups of 3, starting at the lowest dose of 45 Gy(RBE). Each group was monitored for 3 months from the start of radiation therapy before the next-higher dose was given (Figure 1).
Figure 1.
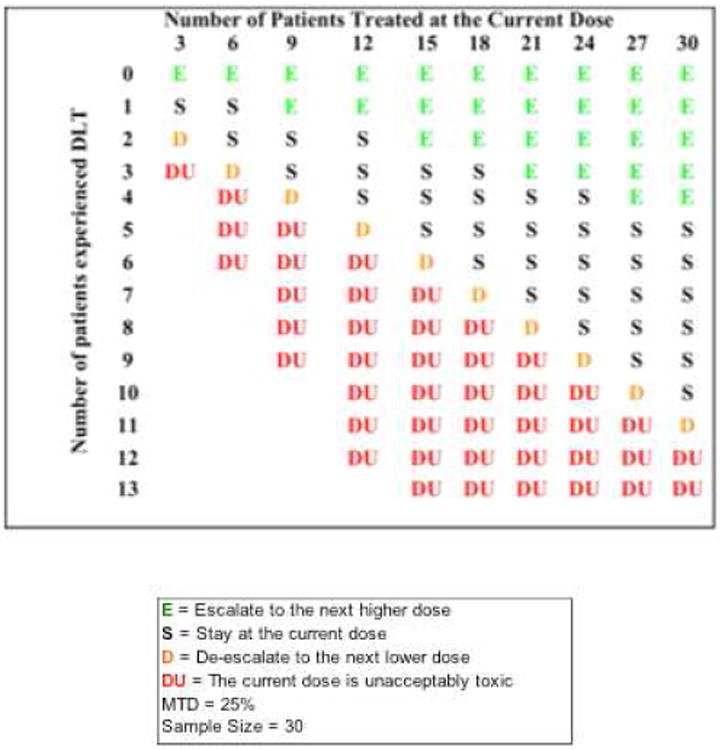
Phase I dose-finding trial monitoring chart.
Results
Twenty five patients with NSCLC enrolled on this trial. Patient characteristics are shown in Table 2. Sixty four percent of patients had mediastinal involvement, including those who did not have NSCLC. Patients without mediastinal involvement were included in this study if their tumors were thought to be too large (typically >4 cm) or too centralized (i.e., adjacent to the mediastinum or bronchial tree) to be treated with stereotactic ablative body radiation. No DLT was experienced during the evaluation period (90 days from the start of PBT), and thus the dose was escalated after every 3 patients according to the statistical design; thus most patients received 60 Gy(RBE) (Table 2). In two patients, dose constraints could not be met at the intended dose level [one patient was to receive 52.5 Gy(RBE) and the other 60 Gy(RBE)], and the dose was thus reduced to the next dosing level [45 Gy(RBE) and 52.5 Gy(RBE), respectively]. Only one patient received concurrent therapy with a biologic agent (cetuximab), which they had also taken several months before radiation. Note that the decision to withhold concurrent chemotherapy was made by the consulting medical oncologist, and typically pertained to a patient's performance status (36% of patients with PS=2), age (median age in the study 74 years), and/or progression of disease through prior systemic regimens.
Table 2. Patient Characteristics (n=25).
| Characteristic | Value or No. of Patients (%) |
|---|---|
| Age at diagnosis, years | |
| Median (range) | 74 (40–85) |
| Sex | |
| Male | 14 (56%) |
| Female | 11 (44%) |
| ECOG PS | |
| 0 | 2 (8%) |
| 1 | 14 (56%) |
| 2 | 9 (36%) |
| T Status | |
| T1 | 8 (32%) |
| T2 | 12 (48%) |
| T3 | 4 (20%) |
| T4 | 1 (4%) |
| N Status | |
| N0 | 10 (40%) |
| N1 | 4 (16%) |
| N2 | 7 (28%) |
| N3 | 4 (20%) |
| Pre-radiation FEV1, L | |
| Median (range) | 2.4 (1.6–4.4) |
| Pre-radiation DLCO, % of expected value | |
| Median (range) | 71 (17–105) |
| Prescribed Dose† | |
| 45 Gy(RBE) | 3 (12%) |
| 52.5 Gy(RBE) | 4 (16%) |
| 60 Gy(RBE) | 18 (72%) |
| Gross Tumor Volume, cm3 | |
| Median (range) | 53 (3.1–419.6) |
| Clinical Target Volume, cm3 | |
| Median (range) | 212 (52.2–674.7) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status score; NSCLC, non-small cell lung cancer, FEV1, forced expiratory volume in 1 second, DLCO, diffusing capacity of the lung
All doses were to be delivered in 15 fractions of 3 Gy(RBE), 3.5 Gy(RBE), or 4 Gy(RBE).
The distribution of doses delivered to critical structures in the patients in this trial is illustrated in Figure 2. The substantial portions of patients who received high doses to the esophagus [8 patients (32%) with a maximum esophagus dose >60 Gy(RBE)] and lung [10 patients (40%) with a mean lung dose >12 Gy(RBE)] reflect the prevalence of mediastinal involvement among this group. In contrast, the cardiac and spinal cord doses in most patients were modest, with only a few patients receiving doses that approached tolerance levels.
Figure 2.
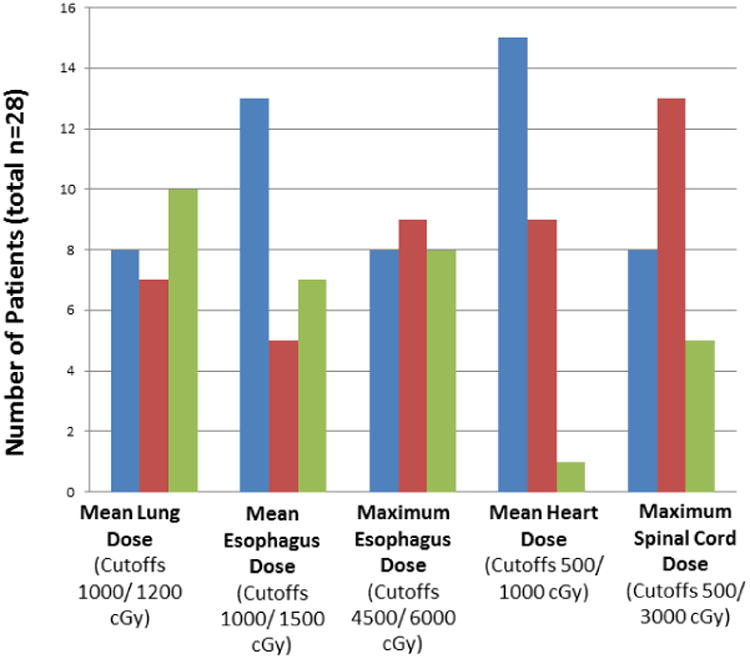
Distribution of radiation doses to normal structures. Blue bars represent the numbers of patients with doses less than the first cutoff value; red bars, patients with doses between the first and second cutoff values; and the green bars, patients with doses above the second cutoff value. The cutoff values were chosen based on the distribution of doses actually received rather than the dose constraints (see Table 1).
Toxicity
All patients completed the evaluation follow-up period (90 days from the start of PBT). The median follow-up time for all patients was 13 months (range 8–28 months). The most common toxic effects were fatigue and Grade 2 esophagitis (e.g. requiring narcotic intervention) (Table 3). Two patients experienced grade ≥3 toxicity defined as DLTs. One of those patients had locally advanced NSCLC and received 6 cycles of induction chemotherapy followed by PBT to a total dose of 52.5 Gy(RBE) in 15 fractions. The mean esophageal dose was 21.0 Gy(RBE), and the maximum esophageal dose was 55.6 Gy(RBE), with an esophageal V40 of 39% (Figure 3). Approximately 8 months after PBT, bevacizumab was begun when recurrent disease was discovered, and 1 month after that, the patient reported persistent coughing/choking. A tracheoesophageal fistula, without tumor recurrence, was found on endoscopy and in an overlapping region of the treatment field. Over the next several weeks, both a “button seal” and a stent were placed in attempts to relieve the patient's symptoms, but the patient experienced an episode of fatal hemoptysis and died 7 weeks after discovery of the fistula.
Table 3. Toxicity.
| Grade | Dermatitis | Pneumonitis | Esophagitis | Fatigue |
|---|---|---|---|---|
| Grade 0 | 18 | 7 | 15 | 10 |
| Grade 1 | 6 | 13 | 0 | 6 |
| Grade 2 | 1 | 4 | 9 | 9 |
| Grade ≥3 | 0 | 1 | 1 | 0 |
Figure 3.
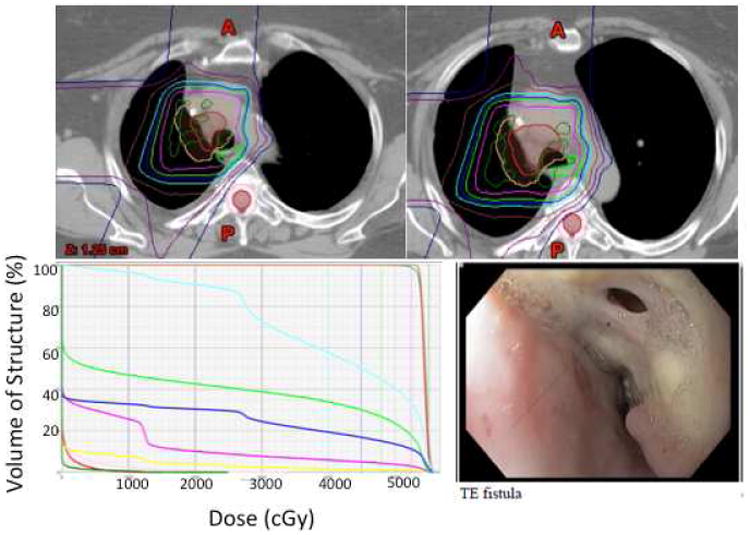
Top, isodose distribution on axial CT slices in the patient who developed a transesophageal fistula. Pink, 52.5 Gy(RBE), dark blue, 45 Gy(RBE), aqua, 40 Gy(RBE), brown, 30 Gy(RBE). Bottom left, dose-volume histogram of normal tissue structures for the same patient. Green, esophagus, dark blue, total lung, pink, heart, aqua, right lung, red, spinal cord. Bottom right, endoscopic image of transesophageal fistula, which had been stented before the patient experienced massive hemoptysis.
The second patient was treated for NSCLC to a total dose of 60 Gy(RBE) in 4 Gy(RBE) fractions after two cycles of induction chemotherapy; the mean lung dose was 16.9 Gy(RBE) and the total lung V17 was 32%. Approximately 4 months later, the presence of increased dyspnea without fever prompted a thoracic CT scan, which showed opacities throughout the right lung, both within and outside of the radiation field. Gram-negative rods were also found in the sputum. Supportive treatment included bronchodilators and wide-spectrum antibiotics, after which the patient's condition improved and the opacities ultimately resolved. Because this patient may have had both radiation pneumonitis (as suggested by the interval after radiation, the high lung dose, the presence of opacities in the treatment field, and increased dyspnea) and pneumonia, this case was coded as “possible” grade 3 radiation pneumonitis.
Finally, we examined whether target volumes and tumor locations were associated with esophagitis and pneumonitis. All patients with grade ≥2 esophagitis had either nodal metastases or tumors directly involving the mediastinum. The median value of the maximum esophagus dose for these patients was 57.6 Gy(RBE) and the median value of the mean esophagus dose was 20.2 Gy(RBE), both substantially higher than the median values for the entire group [50.0 Gy(RBE) and 9.9 Gy(RBE), respectively]. In addition, the median GTV of the patients with grade ≥2 esophagitis was 147 cm3, versus 53 cm3 for the entire group. The patient who experienced a tracheoesophageal fistula had a GTV of 147 cm3. Similarly, the mean lung dose of patients experiencing grade ≥2 RP was 13.0 Gy as compared with 11.3 Gy for the entire group. The GTV in the patients who experienced grade 2 or higher RP was 125 cm3 versus 53.0 cm3 in the entire group.
Discussion
Pertinent findings from this phase I dose-escalation study of an intermediate hypofractionated regimen for NSCLC were as follows. First, the modified dose constraints used were effective, both in allowing treatment of patients with extensive mediastinal disease and in limiting the rate of high-grade acute toxicity. Second, hypofractionated regimens up to 60 Gy(RBE) in 15 fractions were tolerated well in most cases, even when patients were not candidates for systemic therapy because of poor performance status or advanced age. Finally, such regimens should be considered with caution for patients receiving VEGF inhibitors such as bevacizumab before, during, or after treatment because of an apparent increased risk of tracheoesophageal fistulas, particularly for tumors that directly invade the esophagus or trachea.
Limited reports of hypofractionated radiation therapy without chemotherapy for locally advanced or inoperable NSCLC have been published over the past decade, all of which demonstrated acceptable toxicity results. Investigators from the University of Wisconsin performed a dose-escalation trial of hypofractionated tomotherapy in which 46 patients received 25 fractions in escalating doses from 2.28 Gy to 3.22 Gy. No patients in that study experienced grade ≥3 toxicity, and the rate of grade ≥2 pneumonitis was approximately 15%, leading the investigators to conclude that this dose fractionation regimen was safe and could be considered for patients with locally advanced or inoperable NSCLC [11]. More recently, investigators at Fudan University in China treated 34 patients with stage III NSCLC, all of whom received hypofractionated radiation therapy to 50 Gy in 20 fractions followed by escalated doses, in 3-Gy fractions, to total doses of 65–68 Gy. All patients had received induction chemotherapy. Two of the patients in that study (6%) experienced grade ≥3 esophagitis and 1 (3%) had grade ≥3 pneumonitis; the locoregional progression-free survival rate at 3 years was 61% [12]. Another study in Italy assessed the safety of hypofractionated three-dimensional conformal radiation therapy for patients with inoperable advanced NSCLC; these patients were given 60 Gy in 20 fractions, and only 3 developed grade ≥3 toxicity, 1 with esophagitis and 2 with pneumonitis [13].
The fractionation regimen in this study utilized a high dose per fraction relative to similar analyses examining this question, and with comparable low rates of similar toxicity. As alluded to above, it is our assessment that these doses were achievable due to the unique combination of adjusted dose constraints and the use of a very conformal technique. Novel dose constraints were used, as there are limited parameters for intermediate fractionated regimens, as compared to the more established constraints for fractionation schemes up to 10 total fractions. While we acknowledge the proposed limitations and potential inaccuracies of using the BED paradigm for particle therapy given in large fractions [14], we believe that the approximation is accurate enough to estimate the effects of the fraction sizes that we used (up to 4 Gy). Furthermore, this method provides a relatively simple way to apply these same concepts to institution-specific dose constraints.
With regard to the modality of choice for this study, PBT was utilized to reduce the risk to surrounding structures, particularly the low dose regions in critical structures such as the heart, lung, and esophagus. However, it is also notable that several studies, including those cited above, have also demonstrated promising results in the setting of definitive treatment with NSCLC using either IMRT or 3D-conformal therapy [12, 13, 15, 16]. Our recommendation based on the results of this study is that all patients in whom the below dose constraints can be met would be eligible for fractionation regimens up to 60 Gy in 15 fractions. However, in all patients we do advocate using image guidance and a four dimensional CT scan, with frequent image-guided setup. Given the short course of the treatment regimen, we do not believe that adaptive planning is compulsory (and was not performed on any of the patients in this study), though can be considered during week 2 for patients with very large tumors or those near critical structures.
We attempted to elucidate predictors of toxicity in this setting, to assess if certain factors (i.e. tumor location and size) are of greater importance when predicting serious adverse events. It appears that both characteristics are of clinical significance in treating with this fractionation regimen. That is, patients with target volumes that are either central in location, due to nodal disease or direct involvement of the mediastinum, leading to maximum esophagus doses of approximately >55 Gy (RBE), or patients with larger tumors (>125-150 cc) appear to be at a greater risk of Grade ≥2 esophagus or lung toxicity, though these do not represent precise patient thresholds due to limited patient numbers. However, it should be noted that due to the small proportion of high grade (dose-limiting) toxicity in the entire patient cohort, we conclude that there is no patient or target volume subgroup elucidated in this analysis in which the dose of 60 Gy (RBE) in 15 fractions is contraindicated.
Bevacizumab has been linked with the formation of tracheoesophageal fistulas in several case reports, sometimes occurring up to 2 years after the completion of RT [17, 18]. In 2010, investigators from the Sarah Cannon Research Institute reported tracheoesophageal fistulas occurring in two prospective trials involving bevacizumab with chemoradiation, one for SCLC and the other for NSCLC [19]. Both trials were closed early as a result of these events, and information on risk has since been added to the drug information for this agent. Because the window of risk for developing this complication after radiation therapy has not been established, the contribution of PBT to the fistula in the current study is unclear, particularly because it appeared while the patient was receiving only bevacizumab. Nevertheless, we recommend that bevacizumab be avoided immediately before (e.g., within 3 months) and during radiation therapy, particularly therapy given in this fractionation scheme. Patients should also be informed of the risk of this severe toxicity if they receive bevacizumab any time after the radiation.
This study had several limitations. First, although the incidence of toxicity was low, because the median follow-up time was 13 months, much of what was reported could be considered acute effects. Further follow-up will be needed to assess late toxicity, which may be of greater consequence in patients given hypofractionated therapy. Second, because this was a phase I study, with limited numbers of patients and limited numbers of events, we could not assess the statistical significance of potential predictors of toxicity. Third, while PBT demonstrates favorable dose distribution characteristics, particularly with regard to the region proximal to the tumor, there are also many challenges with this technique that need to be overcome, such as range uncertainties, the inability to determine a simple and reproducible planning target volume, and the capacity to account for tumor motion in a robust fashion, particularly in non-passive scattering techniques. These obstacles are well described in a recent review by Englesman et al.[20], which presents difficulties in applying this technique across institutions that are likely to be addressed over the next several years.
Finally, because this analysis focused on toxicity, the patient group included those with disease of a wide range of stages, and so obtaining even preliminary data on local control and disease-free survival was not feasible. Any estimates of overall survival would be confounded by the advanced age and poor performance status of many of the patients. However, this regimen is likely to be used for such patients, as well as those who cannot tolerate concurrent chemoradiation for other reasons, and thus survival outcomes in this context would be valuable nonetheless. Indeed, a randomized phase III trial was recently activated that compares radiation given in 15 fractions of 4 Gy to 33 fractions of 2 Gy for patients who are not candidates for concurrent chemotherapy; the primary endpoint in this trial is overall survival. Our institution is actively participating in this trial and will be frequently using PBT to achieve this dose.
Acknowledgments
Supported in part by NCI Cancer Center Support (core) Grant CA16672 and P01 CA021239. We thank Christine Wogan, MS, ELS, for her valuable input in reviewing and editing this manuscript.
Footnotes
Conflicts of Interest Notification: The authors declare no conflicts of interest regarding this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langer CJ, Manola J, Bernardo P, et al. Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: implications of Eastern Cooperative Oncology Group 5592, a randomized trial. J Natl Cancer Inst. 2002;94:173–181. doi: 10.1093/jnci/94.3.173. [DOI] [PubMed] [Google Scholar]
- 2.Belani CP, Fossella F. Elderly subgroup analysis of a randomized phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for first-line treatment of advanced nonsmall cell lung carcinoma (TAX 326) Cancer. 2005;104:2766–2774. doi: 10.1002/cncr.21495. [DOI] [PubMed] [Google Scholar]
- 3.Sweeney CJ, Zhu J, Sandler AB, et al. Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: a Phase II trial in patients with metastatic nonsmall cell lung carcinoma. Cancer. 2001;92:2639–2647. doi: 10.1002/1097-0142(20011115)92:10<2639::aid-cncr1617>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen LN, Komaki R, Allen P, et al. Effectiveness of accelerated radiotherapy for patients with inoperable non-small cell lung cancer (NSCLC) and borderline prognostic factors without distant metastasis: a retrospective review. Int J Radiat Oncol Biol Phys. 1999;44:1053–1056. doi: 10.1016/s0360-3016(99)00130-3. [DOI] [PubMed] [Google Scholar]
- 6.Amini A, Lin SH, Wei C, et al. Accelerated hypofractionated radiation therapy compared to conventionally fractionated radiation therapy for the treatment of inoperable non-small cell lung cancer. Radiat Oncol. 2012;7:33. doi: 10.1186/1748-717X-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelsman M, Rietzel E, Kooy HM. Four-dimensional proton treatment planning for lung tumors. Int J Radiat Oncol Biol Phys. 2006;64:1589–1595. doi: 10.1016/j.ijrobp.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Kang Y, Zhang X, Chang JY, et al. 4D Proton treatment planning strategy for mobile lung tumors. Int J Radiat Oncol Biol Phys. 2007;67:906–914. doi: 10.1016/j.ijrobp.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 9.Moyers MF, Miller DW, Bush DA, Slater JD. Methodologies and tools for proton beam design for lung tumors. Int J Radiat Oncol Biol Phys. 2001;49:1429–1438. doi: 10.1016/s0360-3016(00)01555-8. [DOI] [PubMed] [Google Scholar]
- 10.Ji Y, Li Y, Nebiyou Bekele B. Dose-finding in phase I clinical trials based on toxicity probability intervals. Clin Trials. 2007;4:235–244. doi: 10.1177/1740774507079442. [DOI] [PubMed] [Google Scholar]
- 11.Adkison JB, Khuntia D, Bentzen SM, et al. Dose escalated, hypofractionated radiotherapy using helical tomotherapy for inoperable non-small cell lung cancer: preliminary results of a risk-stratified phase I dose escalation study. Technol Cancer Res Treat. 2008;7:441–447. doi: 10.1177/153303460800700605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu ZF, Fan M, Wu KL, et al. A phase II trial of accelerated hypofractionated three-dimensional conformal radiation therapy in locally advanced non-small cell lung cancer. Radiother Oncol. 2011;98:304–308. doi: 10.1016/j.radonc.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Osti MF, Agolli L, Valeriani M, et al. Image Guided Hypofractionated 3-Dimensional Radiation Therapy in Patients With Inoperable Advanced Stage Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Fowler JF. 21 years of biologically effective dose. Br J Radiol. 2010;83:554–568. doi: 10.1259/bjr/31372149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao ZX, Komaki RR, Thames HD, Jr, et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:775–781. doi: 10.1016/j.ijrobp.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Rosenzweig KE, Fox JL, Yorke E, et al. Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer. 2005;103:2118–2127. doi: 10.1002/cncr.21007. [DOI] [PubMed] [Google Scholar]
- 17.Goodgame B, Veeramachaneni N, Patterson A, Govindan R. Tracheo-esophageal fistula with bevacizumab after mediastinal radiation. J Thorac Oncol. 2008;3:1080–1081. doi: 10.1097/JTO.0b013e3181858eba. [DOI] [PubMed] [Google Scholar]
- 18.Gore E, Currey A, Choong N. Tracheoesophageal fistula associated with bevacizumab 21 months after completion of radiation therapy. J Thorac Oncol. 2009;4:1590–1591. doi: 10.1097/JTO.0b013e3181c06a6f. [DOI] [PubMed] [Google Scholar]
- 19.Spigel DR, Hainsworth JD, Yardley DA, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol. 2010;28:43–48. doi: 10.1200/JCO.2009.24.7353. [DOI] [PubMed] [Google Scholar]
- 20.Engelsman M, Schwarz M, Dong L. Physics controversies in proton therapy. Semin Radiat Oncol. 2013;23:88–96. doi: 10.1016/j.semradonc.2012.11.003. [DOI] [PubMed] [Google Scholar]


