Abstract
Respiratory syncytial virus (RSV) causes significant morbidity and mortality in children and the elderly. There are no vaccines for RSV in use. Due to immunosenescence, the immunologic requirements for a successful RSV vaccine in the elderly might differ from a RSV vaccine for young children. Using an aged mouse model of RSV pathogenesis, we found that aged mice had impaired antigen-specific CD8+ T cell responses and delayed RSV clearance compared to young mice. In order to study vaccine-elicited RSV-specific CD8+ T cells in aged mice, we used a peptide vaccine approach. TriVax is a co-mixture of a peptide representing immunodominant RSV CD8+ T cell epitope M282–90, a Toll-like receptor agonist (polyI:C), and a costimulatory anti-CD40 antibody. TriVax vaccination generated robust, polyfunctional, and protective CD8+ T cell responses in young BALB/c mice but not in 18 month old (aged) BALB/c mice. We hypothesized that treatment of aged mice with agonistic anti (α)-CD137 (41BB) monoclonal antibody will partially restore T cell responses and TriVax efficacy in aged mice. We immunized 18-month old BALB/c mice twice with TriVax + α-41BB mAb or TriVax + isotype control Ab. Co-administration of α-41BB mAb with TriVax enhanced RSV-specific CD8+ T cell responses and TriVax efficacy in challenge experiments. Triggering the 41BB costimulatory pathway may be a strategy for enhancing T cell responses to vaccines in the elderly.
Introduction
Respiratory syncytial virus (RSV) is a major cause of morbidity and mortality in the elderly (1, 2). In elderly patients, RSV caused 11% of hospitalizations for pneumonia (1, 3). Immunosenescence, the deterioration of the immune system caused by aging, contributes to vaccine failure, susceptibility to infectious diseases, and cancer in the elderly (4). As human life expectancy increases, it is important to understand what potential factors contribute to immunosenescence and how it can be overcome in order to improve the health of this population. Immune mechanisms leading to RSV susceptibility in the elderly are unclear and likely involve multiple innate, humoral, and cellular immune pathways (5, 6). T cells play an important role in the control of RSV infection (7, 8). T cell responses correlate with protection and clearance in RSV-infected children (9). T cell immunodeficiencies lead to lethal RSV infections in adults (7). Immunosenescence has been associated with the decline in cell-mediated immunity (CMI) (10, 11). Improved vaccination strategies that target the aged immune system may overcome the limits of immunosenescence and provide better protection to the elderly population.
Optimal activation of T cells for clonal expansion requires TCR-MHC interaction and a costimulatory pathway (12, 13). The costimulatory molecules are largely divided into two groups, the B7 superfamily and the tumor necrosis factor (TNF) superfamily (12, 13). 41BB is an inducible receptor of the TNF superfamily found on activated T cells, NK cells, and dendritic cells (14). Signaling via 41BB leads to cytokine production, increased T cell proliferation, prolonged CD8+ T cell survival, and memory CD8+ T cells in vitro (12, 15). 41BB costimulation is crucial for the reversal of established T cell tolerance and anergy in vivo (16). Also, it has been shown that agonistic α-41BB mAb can participate to induce optimal T cell immune responses during virus infection and tumor progression in animal models (16–18).
We previously demonstrated that CD8+ T cells generated by the TriVax vaccination strategy provide complete protection to RSV A2-line19F challenge in young BALB/c mice (19). TriVax is a co-mixture of a peptide representing immunodominant RSV CD8+ T cell epitope M282–90, a Toll-like receptor agonist (polyI:C), and a costimulatory anti-CD40 antibody. Administration of peptide in combination with a Toll-like receptor 3 (TLR3) ligand (polyI:C) and agonistic α-CD40 antibody (previously termed TriVax by other groups) results in the generation of robust CD8+ T cell responses compared to other peptide vaccination strategies (20, 21).
In this study, we found that TriVax vaccination had no effect in aged BALB/c mice. Prior to this study, Bansal-Pakala et al demonstrated that α-4-1BB mAb rescued defective CD4+ T cell responses in aged mice (22). We then hypothesized that the 41BB co-stimulatory pathway is critical in immunosenecence. TriVax vaccination with addition of agonistic α-41BB monoclonal Ab (mAb) resulted in enhanced CD8+ T cell responses and protection against challenge with the A2-line19F RSV strain previously shown to be relatively pathogenic in BALB/c mice (23). Our results suggest that stimulation of the 41BB pathway in RSV vaccination for the elderly may partially restore T cell defects associated with aging.
Materials and Methods
Mice and Virus
Pathogen-free, 6–8-week and 18–24 month-old, female BALB/c mice were purchased from the National Institute of Aging (NIA, Bethesda, MD) (4). All animal procedures were conducted according to the guidelines of the Emory University Institutional Animal Care and Use Committee. RSV A2-line19F virus stocks were generated as described previously (23).
Peptide, antibody, and tetramers
The synthetic peptide SYIGSINNI from RSV M2 (M282–90) defined as an immunodominant H-2Kd-restricted CD8+ T cell epitope was purchased as > 95 % pure from EZBiolab Inc (Carmel, IN) or NeoBioLab Inc (Cambridge, MA), and 20 mg/ml stock solutions were made in DMSO. α-41BB (clone;3H3) was purified using BD CELLine Disposable Bioreactor (BD Biosciences, Bedford, MA) following manufacturer’s protocol. Control rat IgG and α-CD40 mAb (clone FGK4.5) were purchased from Bio X cell (West Lebanon, NH) (19, 21). Biotinylated H-2Kd-M282–90 monomer was provided by the National Institute of Allergy and Infectious Diseases Tetramer Facility (Emory University, Atlanta, GA). M282–90 tetramers were prepared as described previously (19).
Vaccination and infection
Mice were injected intravenously (i.v) with a mixture of 200 μg peptide, 50 μg α-CD40 mAb, and 50 μg poly (I:C) (Invivogen, San Diego, CA). Mice were given a second identical TriVax treatment (boost) two weeks following the primary treatment (19). Either 100 μg of α-41BB or control IgG was administered intraperitoneally (i.p.) on days 0, 2, 14, and 16. A detailed schedule of α-41BB injection and challenge was illustrated in Fig. 4. Mice were anesthetized by intramuscular injection of a ketamine-xylazine solution and infected intranasally with 3 × 105 PFU RSV A2-line19F in 100 μl at day 6 following the last TriVax treatment, as described (19).
Figure 4.
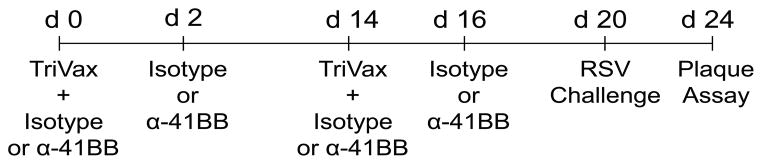
Schedule for vaccination, α-41BB treatment, and RSV challenge. For the priming, young and aged mice were vaccinated with TriVax on day 0 and treated with 100 μg α-41BB mAb or 100 μg control isotype mAb on day 0 and day 2. For the boost, mice were vaccinated and treated with identical materials on day 14 and day 16. Mice were challenged with RSV A2-line19F on day 20, and viral load and T cell assay were quantified on day 24.
Preparation of lung lymphocytes
Lung lymphocytes were purified as described previously (19). Briefly, right lungs were removed into complete RPMI supplemented with 10% FBS. The lung tissues were minced and ground through a sterile mesh to obtain a single-cell suspension. Cells were layered onto Fico/Lite-LM (mouse) (Atlanta Biologicals, Lawrenceville, GA), and lung mononuclear cells were isolated by centrifugation at 2,700 rpm.
Tetramer staining and intracellular cytokine staining (ICS)
Tetramer staining and intracellular cytokine staining (ICS) were performed as described previously (19). Briefly, peripheral blood lymphocytes (PBLs) or lung lymphocytes were counted and stained. For tetramer analysis, PBLs or lung lymphocytes in FACS buffer (10 ml FBS and 0.5 g sodium azide / 500 ml PBS) were incubated with H-2Kd M282–90 tetramer for 30 min at 4°C followed by surface staining for PerCP-Cy5.5-anti-CD3, PE-Cy7-anti-CD4, and APC-Cy7-anti-CD8. To enumerate cytokine producing cells, lung lymphocytes (5 × 105) were left untreated or stimulated with individual peptides (1 μg/sample) for 6 h at 37°C in 5% CO2 in the presence of Golgi-plug (BD Pharmingen, San Diego, CA). As a positive control, lung lymphocytes were stimulated in vitro with 50 ng/ml PMA (Sigma-Aldrich, St. Louis, MO), 500 ng/ml ionomycin (Sigma Aldrich), and 0.5 μl Golgi Plug (BD Biosciences). Cell surface staining was performed and followed by ICS using Cytofix/Cytoperm (BD Pharmingen, San Diego, CA) as described previously (19). FITC-anti-IFN-γ (XMG1.2) was used. All antibodies listed were purchased from BD Biosciences (Franklin Lakes, NJ) or eBioscience (San Diego, CA). Fluorescence was measured using an LSRII cytometer (BD Immunocytometry Systems) and analyzed using FlowJo software (Tree Star, Ashlan, OR). Singlet discrimination was performed by using plots for forward scatter height [FSC-H] versus forward scatter width [FSC-W]) and side scatter (SSC) (SSC-W versus SSC-H), and dead cells were excluded by scatter characteristics.
Plaque assay
Mice were euthanized days 1, 2, 4, 6, 8, 10, 12, 16 and 20 post-infection (p.i). We used a Beadbeater (Biospec Products, Bartlesville, OK) to homogenize the lungs as described (19, 24). Lung homogenates were serially diluted and inoculated subconfluent HEp-2 cells in 24-well plates. After 1 hr adsorption at room temperature on a rocking platform, the cells were overlayed with complete EMEM/10% FBS/penicillin G/streptomycin sulfate/amphotericin B solution/0.75% methylcellulose. After six days, the overlay media was removed and the cells fixed with methanol. Plaques were visualized by immunodetection as previously described (19, 24).
In vivo cytotoxic-T-lymphocyte (CTL) assay
In vivo CTL assays were performed as described previously (19). Naïve splenocytes were unpulsed or pulsed with 1μg M282–90 peptide for 3 h at 37°C. Splenocytes were labeled with low (0.5 μM, unpulsed) or high (5 μM, pulsed with peptide) concentrations of carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes, Carlsbad, CA). Labeled splenocytes were co-injected by the i.v. route in a 1:1 ratio (1 × 107 total cells in 100 μl of PBS) into naïve and TriVax + α-41BB treated aged mice. CFSEHi and CFSELo cells in splenocytes were quantified 18hr later by flow cytometry.
Statistical analyses
P values were determined by either a two-tailed t test or one-way ANOVA and Tukey multiple comparison test, using GraphPad Prism software (La Jolla, CA). Data values below limits of detection were assigned a value of half the limit of detection. Data are representative of at least three replicate experiments having consistent results.
Results
Delayed RSV clearance and impaired RSV-specific CD8+ T cells in aged mice
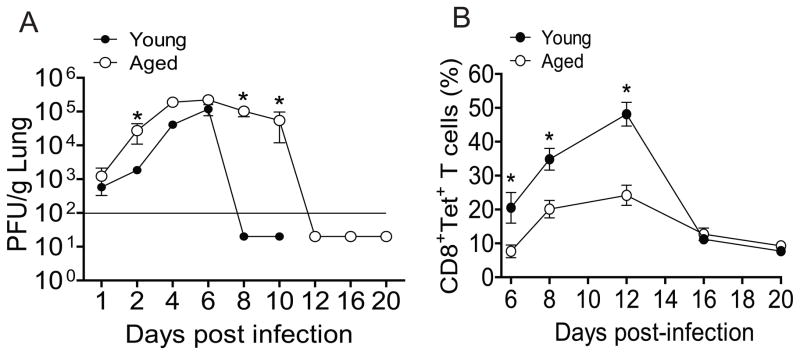
To determine the ability of the aged mice to control RSV infection compared to young mice, we measured viral load in lungs at varying time points after A2-line19F RSV challenge. Similar virus titers were observed on days 1, 4 and 6 p.i in the lungs of young and aged mice (Fig. 1A). However, viral load at days 2, 8, and 10 p.i. in aged mice was significantly higher than in young mice (Fig. 1A). Clearance of RSV to levels below the limit of detection of the plaque assay occurred at day 8 p.i. in young mice. In contrast, 105 PFU/g lung were still present at days 8 and 10 p.i. in aged mice. We observed RSV clearance in aged mice at day 12 p.i. Thus, in line with previous observation, there was delayed RSV clearance in aged mice (25). Next, we investigated RSV-specific CD8+ T cells in the lungs of young and aged mice. RSV-specific CD8+ T cells increased from 21% at day 6 p.i. to 47% at day 12 p.i. in young mice (Fig. 1B). In aged mice, RSV specific CD8+ T cells increased from 8% at day 6 p.i. to 25% at day 12 p.i., levels below those observed in young mice at both time points (Fig. 1B). The peak of RSV-specific CD8+ T cell responses in both young and aged mice was day 12 p.i. We found that CD8+ T cell responses in both young and aged mice were decreased at day 16 p.i. Interestingly, there was no difference between young and aged mice at day 16 and 20 p.i. (Fig. 1B). Taken together, impaired RSV-specific CD8+ T cells correlated with prolonged RSV infection in aged mice.
Figure 1.
Delayed RSV clearance and impaired M282–90 epitope-specific CD8+ T cell responses in aged mice. Young (n=5) and aged (n=5) BALB/c mice were infected with 3 × 105 PFU of A2-line19F virus. (A) RSV titers in lungs of infected young and aged BALB/c mice at days 1, 2, 4, 6, 8, 10, 12, 16, and 20. The lungs were harvested at indicated time points, and infectious RSV was titrated by plaque assay. Significant differences were observed between young and aged mice marked by an asterisk at day 2, 8 and 10 p.i. The solid line represents the limit of detection (* = P × 0.05, ANOVA). (B) The lung lymphocytes were harvested at indicated time points from right lungs of the same mice. M282–90-specific tetramer positive cells were quantified as a percentage of CD3+CD8+ T cells by flow cytometry. Data show an average of three separate experiments with equivalent results.
TriVax vaccination protects young mice but not aged mice against RSV infection
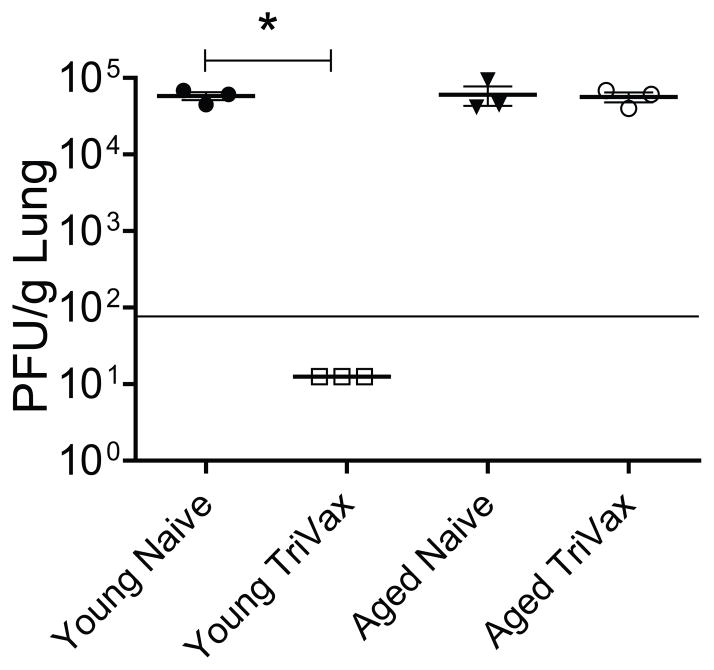
We recently showed that TriVax vaccine-elicited CD8+ T cells can protect against RSV A2-line19F challenge (19). As infection of BALB/c mice with A2-line19F results in greater viral load than laboratory A2 RSV strain, A2-line19F strain provides robust RSV challenge model (23). To assess RSV TriVax efficacy in aged mice, young and aged mice vaccinated twice with TriVax were challenged with 3 × 105 RSV A2-line19F strain. As previously reported, TriVax vaccination completely protected young mice from RSV infection (Fig. 2) (19). However, TriVax had no effect in aged mice (Fig. 2). We first hypothesized that the defect would be in the generation of vaccine-elicited RSV-specific CD8+ T cells in aged mice because we previously demonstrated dramatic induction of RSV-specific CD8+ T cells by TriVax vaccination in young mice (19). We measured M282–90-specific CD8+ T cell responses in young and aged mice after TriVax vaccination. There were 3-fold more tetramer positive CD8+ T cells in young mice than aged mice following TriVax vaccination (Fig. 3A and B). TriVax vaccination was less immunogenic in aged mice.
Figure 2.
Efficacy of TriVax in young and aged mice. Young (n=3) and aged (n=3) BALB/c mice were vaccinated with TriVax at d 0 and d 14. Mice were challenged with 3 × 105 PFU of A2-line19F virus at day 20. The lungs were harvested at day 24 (day 4 p.i), and infectious RSV was titrated by plaque assay. Significant difference was observed between young mice as marked by an asterisk. Each symbol represents one mouse. The horizontal line depicts the limit of detection. Data shown represent one of three experiments with similar results.
Figure 3.
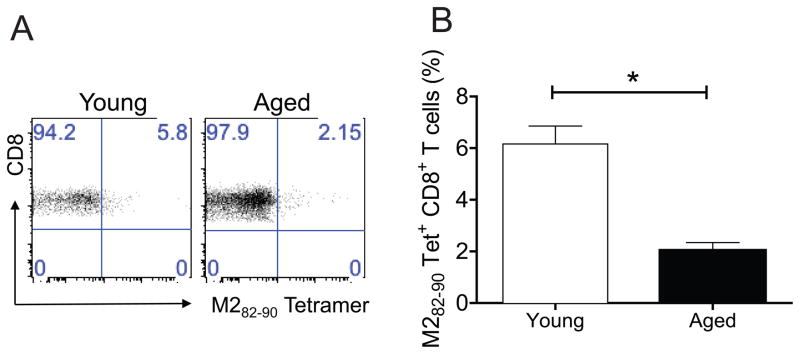
Comparison of M282–90 epitope-specific CD8+ T cells in young and aged mice after TriVax vaccination. Young BALB/c mice (n=5) and aged BALB/c mice (n=5) were used. Groups of mice were vaccinated once with TriVax via i.v route. PBLs were isolated at day 6 post prime. (A) Representative dot plots are shown where the numbers in upper right quadrant gate represent the % of CD3+CD8+ cells that were tetramer positive. (B) The % tetramer positive CD3+CD8+ T cells in PBLs ± SEM (right) (* = P < 0.05, t-test). Data shown represent one of three experiments with similar results.
Agonistic α-41BB increases RSV-specific CD8+ T cell responses in aged mice
One of many aspects of defective T cell responses associated with aging is the lack of sufficient costimulation (26). Since agonistic α-41BB mAb augments T cell responses in viral and tumor models, we tested whether 4-1BB costimulation could be also useful for augmenting defective T cell responses in aged mice (22). Aged mice were vaccinated with TriVax + agonistic α-41BB mAb or TriVax + isotype control Ab following the schedule as depicted in Fig. 4. We compared percent of CD3+, CD3+CD4+, CD3+CD8+ T cells as well as Tet+CD8+ T cells in PBLs of aged mice following TriVax + α-41BB mAb or TriVax + isotype vaccination. Dot plots presented in Fig. 5 are representative of the percentage of CD3+, CD3+CD4+, CD3+CD8+, and CD8+Tet+ T cells. The percentage of CD3+ cells was 10.9 ± 7.2 % in TriVax + isotype treated group and 44 ± 27 % in TriVax + α-41BB treated group. In addition, we found that the % of CD3+CD8+ T cells was 41 ± 12 % in TriVax + isotype treated group and 60 ± 15.3 % in TriVax + α-41BB treated group. Similarly, we found that the % Tet+CD8+ was 24 ± 10.2 % in TriVax + isotype treated mice and 60 ± 12 % in TriVax + α-41BB treated group. Notably, treatment of α-41BB mAb increased the percentage of CD3+, CD3+CD8+, and Tet+ CD8+ T cells in aged mice compared to TriVax + isotype control group (Fig. 5). However, there was no significant difference in the percentage of CD8+Tet+ T cells between young mice with or without α-41BB treatment since TriVax alone enhanced high level of RSV-specific CD8+ T cell responses at effector phase (data not shown). These data demonstrate that co-administration of α-41BB in conjunction with TriVax significantly enhanced RSV-specific CD8+ T cells in aged mice.
Figure 5.
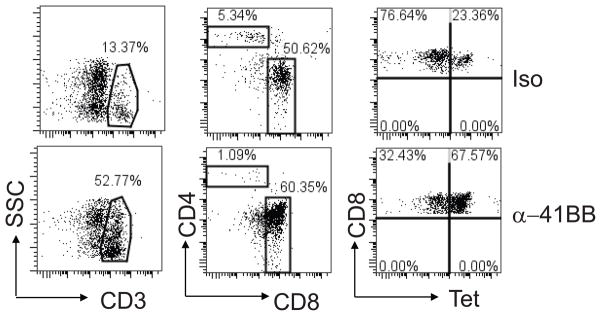
Enumeration of CD3+, CD3+CD4+, CD3+CD8+ , Tet+CD8+ T lymphocytes in blood. Groups of aged BALB/c mice (n=5) were vaccinated with TriVax + isotype control (iso, upper plots) or TriVax + α-41BB mAb (α-41BB, bottom plots) as illustrated in Fig. 4. PBLs were isolated at day 20. Polygon gates showed the % CD3+ T cells in blood. % CD3+CD4+ or % CD3+CD8+ T cells was shown in a rectangular gate in the middle plot. Upper right quadrant gates represented the % of CD3+CD8+ T cells that were M282–90 tetramer positive. Dot plots presented in Fig. 5 are representative of the percentage of CD3+, CD3+CD4+, CD3+CD8+, and CD8+Tet+ T cells.
Co-administration of α-41BB enhances TriVax vaccine protection against RSV challenge
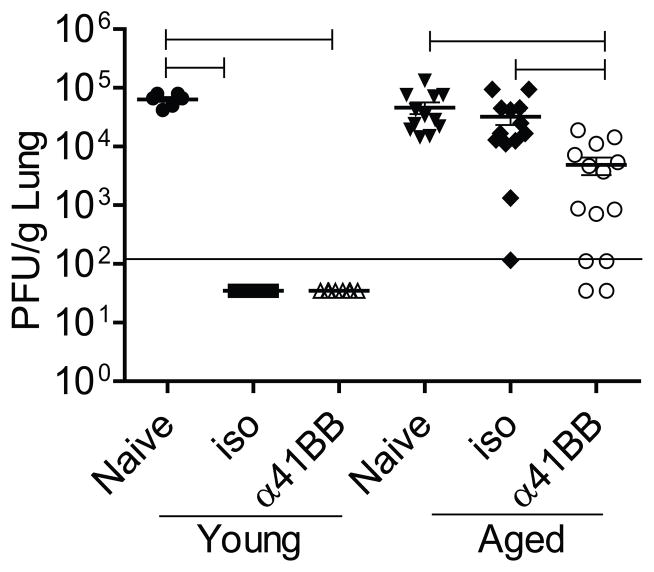
We sought to investigate whether co-administration of α-41BB along with TriVax vaccination would enhance TriVax vaccine efficacy in aged mice in a RSV challenge model. Young and aged mice were challenged with RSV A2-line19F following TriVax + isotype or TriVax + α-41BB mAb treatment. No difference was observed between isotype treated group and α-41BB treated group in young mice because both groups of young mice were protected completely against RSV challenge. However, we observed that aged mice co-administered with α-41BB mAb exhibited reduced viral load at day 4 p.i compared to TriVax + iso treated group (Fig. 6). The protection was partial in aged mice that received TriVax + α-41BB mAb treatment. Collectively, we demonstrated that α-41BB treatment enhanced protection against RSV challenge more than 1 log (Fig. 6).
Figure 6.
Co-administration of α-41BB partially enhanced TriVax efficacy against RSV challenge in aged mice. Young and aged BALB/c mice were vaccinated with either TriVax + isotype control (iso) or TriVax + α-41BB mAb (α-41BB) following the schedule depicted in Fig 4. At day 20, mice were challenged with 3 × 105 PFU of RSV A2-line19F. Lungs were harvested at d 4 p.i. and infectious RSV was titrated by plaque assay. Each symbol represents one mouse. The horizontal solid line depicts the limit of detection. Data are from four independent experiments. (Brackets, P < 0.05, ANOVA)
α-41BB treatment promotes RSV-specific CD8+ T cells in lung
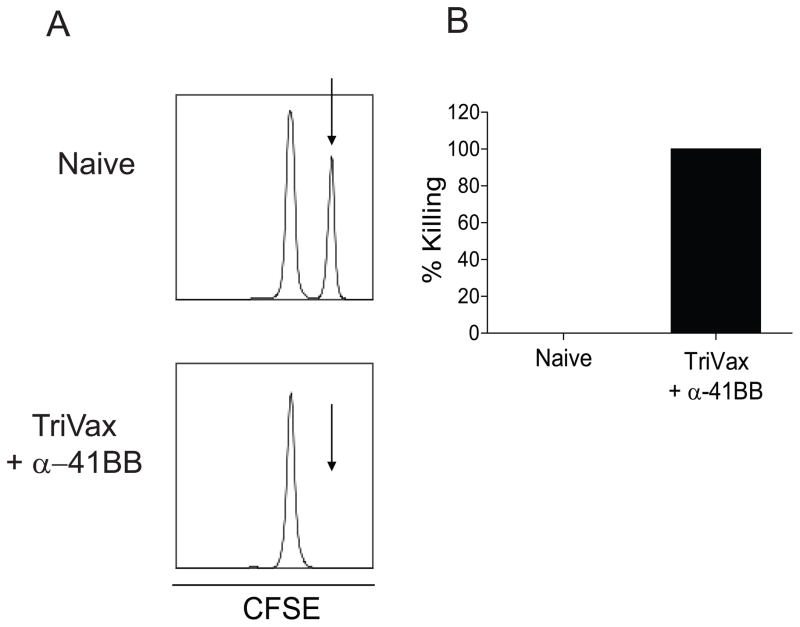
Next, we sought to determine whether increased vaccine efficacy in α-41BB treated aged mice depends on the frequency of CD8+ T cells initially induced in the lungs. Recently, Salek-Ardakani et al demonstrated that α-OX40 induced vaccinia virus specific CD8+ T cell in lungs before virus challenge, and the protection level correlated with the T cell frequency initially induced (27). To determine if the frequency of T cells initially induced in our model is different between the isotype treated group and α-41BB mAb treated group, we measured % Tet+CD8+ T cells and % IFNγ+CD8+ T cells in the lungs of aged mice that received either iso or α-41BB mAb treatment. When we compared the RSV-specific CD8+ T cell frequency in the lungs before RSV challenge, we found that % Tet+CD8+ T cells and % IFNγ+CD8+ T cells were different between iso treated and α-41BB treated aged mice (Fig. 7). While the % Tet+CD8+ T cells was 17.4 ± 2.4 and the % IFN+CD8+ T cells was in 6.7 ± 1 in iso treated group, the % Tet+CD8+ T cells was 36.4 ± 6.5 and the % IFN+CD8+ T cells was in 29.9 ± 5.9 in α-41BB treated group. The ratio of % IFNγ+ T cells to % Tet+ T cells was 26 vs. 84 (Fig. 7A). The total number of Tet+CD8+ T cells in α-41BB treated group was 13 fold higher than the iso treated group (Fig. 7B). Notably, the total number of IFN+CD8+ T cells was 29 fold higher than iso treated group (Fig. 7C). Lastly, an in vivo CTL assay was performed to quantify cytotoxic function mediated by TriVax + α-41BB treatment. While M282–90 pulsed CFSEHI target cells (Fig. 8A, arrow) were present in unvaccinated naïve mice, CFSEHI target cells were undetectable 18h after injection into aged mice received TriVax + α-41BB treatment. In order to determine RSV-specific CD8+ T cell frequency after RSV infection, we performed identical experiments following RSV challenge (Fig. 9). While the % Tet+CD8+ T cells were 2 fold higher after RSV challenge compared to before RSV challenge in iso treated group, no difference of % Tet+CD8+ T cells in aged mice treated with α-41BB was observed between before and after challenge (Figs. 7 and 9). Collectively, the results suggest that TriVax vaccine efficacy with α-41BB treatment is dependent upon the frequency of RSV-specific CD8+ T cells initially in the lungs before RSV challenge.
Figure 7.
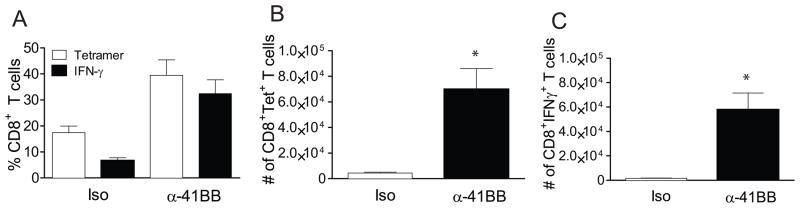
Significant induction of RSV-specific CD8+ T cell responses in α-41BB mAb treated aged mice. Groups of aged BALB/c mice (n=5) were vaccinated with TriVax + isotype control or TriVax + α-41BB mAb as illustrated in Fig. 4. Lung lymphocytes were isolated at day 20 (day 6 post boost without RSV challenge) and incubated in the presence of M282–90 peptide (1 μg/sample) for ICS assay. (A) Ratio of % IFN-γ producing CD8+ T cells to % Tet+ CD8+ T cells. (B) Frequency of tetramer positive CD8+ T cells in lungs isolated from TriVax + isotype control (iso) or TriVax + α-41BB (α-41BB). The number of tetramer positive CD8+ T cells in lung lymphocytes ± SEM was presented. (* P < 0.05, t-test) (C) Frequency of IFN-γ producing CD8+ T cells in lungs isolated from TriVax + isotype control (iso) or TriVax + α-41BB (α-41BB) following re-stimulation with M282–90 peptide. The number of IFN-γ producing CD8+ T cells in lung lymphocytes ± SEM was presented. (* P < 0.05, t-test) Data are means and SEM for each group from two separate experiments.
Figure 8.
RSV-specific CD8+ T cells from the spleen are highly cytolytic in aged mice treated with TriVax + α-41BB. (A) In vivo CTL assays were performed to investigate the cytotoxic functionality of RSV-specific CD8+ T cells after TriVax + α-41BB vaccination in aged mice. Representative data comparing specific killing in a naïve mouse to that in an immunized mouse are shown. (B) Quantification of the results from all TriVax + α-41BB treated mice (n=3 per group) is shown in a graphical format. All depicted data are representative of two independent in vivo CTL assays.
Figure 9.
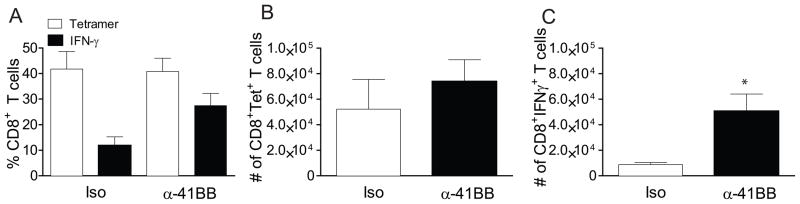
Tetramer responses and cytokine production following RSV challenge in aged mice that were treated with TriVax and α-41BB. Aged BALB/c mice (n=5 per group) were vaccinated with either TriVax + isotype control (iso) or TriVax + α-41BB mAb (α-41BB) and challenged with 3 × 105 PFU of A2-line19F. Lung lymphocytes were isolated on day 4 p.i. (A) Ratio of % IFN-γ producing CD8+ T cells to % Tet+ CD8+ T cells. (B) Frequency of tetramer positive CD8+ T cells in lungs isolated from RSV challenged aged mice treated with either TriVax + isotype control (iso) or TriVax + α-41BB (α-41BB). The number of tetramer positive CD8+ T cells in lung lymphocytes ± SEM was presented. (C) Frequency of IFN-γ producing CD8+ T cells in lungs isolated from RSV challenged aged mice treated with either TriVax + isotype control (iso) or TriVax + α-41BB (α-41BB). Lung lymphocytes were re-stimulated with M282–90 peptide. The number of IFN-γ producing CD8+ T cells in lung lymphocytes ± SEM is presented. (* P < 0.05, t-test) Data are an average of three separate experiments with similar results.
Correlation of the degree of protection and IFN-γ production after RSV challenge
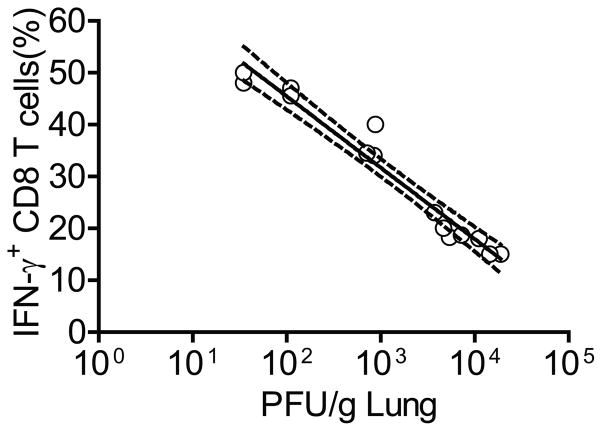
As there was a discrepancy between induced CD8+ T cell responses in the lungs and suppression of RSV replication in aged mice that received α-41BB (Figs. 6 and 7), we tried to address why the effect on RSV replication was partial with different degrees of protection in aged mice co-administered with α-41BB although % Tet+CD8+ T cells and % IFNγ+CD8+ T cells are similar compared to those elicited in young mice. We further analyzed RSV-specific CD8+ T cell responses after RSV challenge. To determine if % IFNγ+CD8+ T cells and the degree of protection is truly correlated, nonlinear regression analysis was used. As shown in Fig. 10, % IFNγ+CD8+ T cells were clearly correlated with the degree of protection (R2 = 0.9574).
Figure 10.
Correlation of IFN-γ+CD8+ T cells and protection against RSV challenge in aged mice treated with TriVax + α-41BB. Aged BALB/c mice (n=5 per group) were vaccinated with TriVax + α-41BB mAb (α-41BB) and challenged with 3 × 105 PFU of A2-line19F. % IFN-γ producing CD8+ T cells in lungs isolated from RSV challenged aged mice treated with TriVax + α-41BB (α-41BB). Confidence intervals (CI) of 95% are shown with the dotted line. Data show combined results of four independent experiments.
Discussion
In this study, we examined the role of 41BB costimulation in RSV-specific CD8+ T cell immunity in aged mice. Compared to young mice, aged mice had impaired RSV-specific CD8+ T cell responses and delayed RSV clearance in primary infection. While TriVax vaccination showed complete protection against RSV challenge at the effector phase in young mice, there was no TriVax-induced protection against RSV challenge in aged mice. We demonstrate that co-administraion of α-41BB mAb in conjunction with TriVax enhanced RSV-specific CD8+ T cells expressing IFN-γ. The % IFN-γ+CD8+ T cells in the lung after RSV challenge correlated with the degree of protection against RSV challenge. Our results suggest that targeting costimulatory signals including 41BB pathway may be a promising strategy as an adjuvant for the elderly in order to restore the function of CD8+ T cell.
RSV is a major cause of morbidity and mortality in the elderly, but the role of RSV-specific CD8+ T cell immunity in the elderly is largely unexplored. However, some studies have investigated the correlation between RSV severity and CD8+ T cell immunity. First, aging is associated with a defect in the T cell responses to RSV since older subjects produced significantly lower IFN-γ levels than younger subject (28). Second, the numbers of RSV-specific memory CD8+ T cells were found to be lower in the elderly (29). Third, diminished RSV-specific CTL activity in the spleen was reported in a RSV aged mouse model (30). In line with previous observations, impaired RSV-specific CD8+ T cells during RSV infection and the reduction of cytokine production by CD8+ T cells were observed in our aged mouse model system. It is likely that anti-RSV CD8+ T cell immunity is a key mechanism in immunosenescence to explain RSV susceptibility in the elderly.
Costimulation is critical for generating robust antigen-specific CD8+ T cell responses (31). Thus, we hypothesized that triggering a costimulatory signal might improve the generation of optimal RSV-specific CD8+ T cell immunity to protect aged mice from RSV infection. In this study, we chose the TNF superfamily over the B7 family because CD8+ T cells in the elderly lack expression of CD28, a B7 family member (32). Lustgarten et al. showed that aged mice successfully induced protective antitumor immune responses with proper costimulation, using agonist α-OX40 mAb, a TNF superfamily member (26). 41BB, also a member of the TNF superfamily, is a costimulatory molecule for CD8+ T cells and is known to enhance cytokine production and cytolytic activity (33, 34). 41BB also increases CD8+ T cell survival by inhibition of apoptosis (35). As only activated T cells, not resting or naïve T cells, express 41BB on their surface, 41BB triggering depends on prior up-regulation of the 41BB receptor by antigen recognition (33). Based on this observation, we administered agonist α-41BB on the day of TriVax vaccination, and again 2 days later to trigger 41BB expressed on activated CD8+ T cells, as described previously (16).
For optimal T-cell response, signal 1 (T cell receptor and antigen) is not sufficient. Signal 2 provided by costimatory molecules is required for optimal T-cell activation. Besides CD28-B7 interaction, TNF superfamily can promote T cell survival or induce effector functions of T cells (14). Signaling via 41BB increased T cell proliferation, prolonged CD8+ T cell survival, and memory CD8+ T cells in vitro (12, 15). When 41BB ligand is absent, fewer Ag-reactive CD8+ T cells and memory T cells develop (31). Thus, previous studies indicated that 41BB signal also play an important role for the generation and persistence of memory CD8+ T cells.
As TriVax platform was a potent tool for the induction of robust RSV-specific CD8+ T cell immune responses at the effector phase, as shown previously, we were unable to detect an additional effect by targeting 41BB at the effector phase in young mice (19). Although we were unable to detect an additional effect by α-41BB in young BALB/c mouse since TriVax vaccination was sufficient to protect against RSV infection by enhancing functional RSV-specific CD8+ T cell responses, several studies suggest that α-41BB plays a key role in the survival of memory CD8+ T cells as well (12, 36). We are currently investigating this possibility in a young BALB/c mouse model.
The role of costimulation by triggering 41BB has been studied previously in viral models. Tan et al demonstrated that CD8+ T cells were diminished in 4-1BBL−/−mice after LCMV infection (31). However, no effect was observed in CD4+ T cell immunity and B cell immune responses in the LCMV model system (31). The addition of α-41BB mAb treatment increased CD8+ T cell immune responses during influenza secondary responses (37). Recently, mice treated with α-41BB mAb enhanced poxvirus-specific effector CD8+ T cells during infection with attenuated vaccinia virus vectors or with an immunization strategy with vaccinia virus peptides (38). Although the requirement and stage of response for 41BB activity was variable across these studies, the data provided a good rational for targeting 41BB in vaccination strategies for viruses as they demonstrate that 41BB stimulation can act as an adjuvant. To our knowledge, the present work represents the first attempt to evaluate the role of 41BB triggering on CD8+ T cell immunity in viral infection in an aged setting.
Although we demonstrated that TriVax + α-41BB vaccination increased vaccine efficacy in aged mice, it is still unclear why individual mice responded differently to α-41BB treatment. We can speculate several possibilities. First, initially induced IFN-γ+CD8+ T cells in the lung after the boost are a factor that determines the level of protection. Indeed, we observed different levels of IFN-γ+CD8+ T cells in lungs of aged mice that received TriVax + α-41BB treatment. Second, it could be the result of inefficient IFN-γ secretion by RSV-specific CD8+ T cells. Previous studies have shown that RSV infection altered function of CD8+ T cell in the lungs (19, 39). Notably, we observed a similar pattern of IFN-γ secretion in α-41BB treated aged mice before and after challenge (Figs. 7 and 9). Lastly, there is a possibility that a high dose of α-41BB mAb used in this study was detrimental to T cell responses. We administered 100 μg α-41BB mAb for prime and boost. The effect of a lower dose of α-41BB (25 μg) was superior to a higher dose of α-41BB (100 μg or 150 μg) in the study of attenuated vaccinia virus vector (38). As other cell types such as CD4+ T cells, NK cells, NKT cells, and dendritic cells also express 41BB on their surface besides activated CD8+ T cells, we could not rule out the possibility that excessive amounts of α-41BB would be harmful by triggering other cell types (38, 40). An alternative and likely reason that coadministraion of α-41BB failed to completely rescue TriVax efficacy in aged mice is that other immune mechanisms such as innate and humoral responses would be involved in RSV susceptibility in the aged mouse model.
Although using aged mice is one model for RSV studies in the setting of immunosenescence, there are important caveats. Here, we used RSV naïve aged mice. There are several studies clearly demonstrating the existence of RSV-specific memory B cells and T cells in the elderly (41–43). It is largely unknown why the elderly are more susceptible to RSV infection although they have pre-existing memory B cells and T cells. It might be that both B and T memory cells induced by natural RSV infection in the elderly would be not enough to prevent RSV reinfection. It is currently unknown whether targeting the 41BB pathway will enhance in the context of repeat exposure and memory.
Taken together, we demonstrated that CD8+ T cell immune function in aged mice was improved by using a TriVax vaccination approach combined with α-41BB mAb. This study provides information on how costimulation can contribute to improve CD8+ T cell function in the setting of immunusenescence. Our results also suggest that the induction of robust RSV-specific CD8+ T cells would be an approach to overcome some barriers to RSV vaccine development in the elderly.
Acknowledgments
This work was supported by the following grants: NIH 1R01AI087798 (Moore) and NIH 1U19AI095227 (Moore), an Emory Egleston Children Research Center (EECRC) grant (Lee), and an American Federation of Aging Research (AFAR) research grant (Lee).
We thank the Emory Children’s Pediatric Research Center flow cytometry core, which is supported by Children’s Healthcare of Atlanta (CHOA). We also thank the NIAID Tetramer Facility for providing valuable monomers.
Abbreviations
- RSV
Respiratory Syncytial Virus
- CMI
Cell mediated immunity
- TNF
Tumor necrosis factor
Footnotes
Disclosures: The authors have no financial conflicts of interest.
References
- 1.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 2.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 3.Falsey AR, Walsh EE. Respiratory syncytial virus infection in elderly adults. Drugs Aging. 2005;22:577–587. doi: 10.2165/00002512-200522070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherukuri A, Stokes KL, Patton K, Kuo H, Sakamoto K, Lambert S, Stillman E, Moore ML, Lee S. An adjuvanted respiratory syncytial virus fusion protein induces protection in aged BALB/c mice. Immunity & ageing : I & A. 2012;9:21. doi: 10.1186/1742-4933-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherukuri A, Patton K, Gasser RA, Jr, Zuo F, Woo J, Esser MT, Tang RS. Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin Vaccine Immunol. 2013;20:239–247. doi: 10.1128/CVI.00580-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falsey AR. Respiratory syncytial virus infection in older persons. Vaccine. 1998;16:1775–1778. doi: 10.1016/s0264-410x(98)00142-x. [DOI] [PubMed] [Google Scholar]
- 7.Graham BS. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol Rev. 2011;239:149–166. doi: 10.1111/j.1600-065X.2010.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall CB, Powell KR, MacDonald NE, Gala CL, Menegus ME, Suffin SC, Cohen HJ. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315:77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 9.Lukens MV, van de Pol AC, Coenjaerts FE, Jansen NJ, Kamp VM, Kimpen JL, Rossen JW, Ulfman LH, Tacke CE, Viveen MC, Koenderman L, Wolfs TF, van Bleek GM. A systemic neutrophil response precedes robust CD8(+) T-cell activation during natural respiratory syncytial virus infection in infants. J Virol. 2010;84:2374–2383. doi: 10.1128/JVI.01807-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 11.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 12.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 13.Vinay DS, Cha K, Kwon BS. Dual immunoregulatory pathways of 4-1BB signaling. J Mol Med. 2006;84:726–736. doi: 10.1007/s00109-006-0072-2. [DOI] [PubMed] [Google Scholar]
- 14.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 15.Laderach D, Movassagh M, Johnson A, Mittler RS, Galy A. 4-1BB co-stimulation enhances human CD8(+) T cell priming by augmenting the proliferation and survival of effector CD8(+) T cells. Int Immunol. 2002;14:1155–1167. doi: 10.1093/intimm/dxf080. [DOI] [PubMed] [Google Scholar]
- 16.Wilcox RA, Tamada K, Flies DB, Zhu G, Chapoval AI, Blazar BR, Kast WM, Chen L. Ligation of CD137 receptor prevents and reverses established anergy of CD8+ cytolytic T lymphocytes in vivo. Blood. 2004;103:177–184. doi: 10.1182/blood-2003-06-2184. [DOI] [PubMed] [Google Scholar]
- 17.Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat Immunol. 2002;3:536–541. doi: 10.1038/ni798. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Dominguez AL, Lustgarten J. Aging affect the anti-tumor potential of dendritic cell vaccination, but it can be overcome by co-stimulation with anti-OX40 or anti-4-1BB. Experimental gerontology. 2006;41:78–84. doi: 10.1016/j.exger.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Stokes KL, Currier MG, Sakamoto K, Lukacs NW, Celis E, Moore ML. Vaccine-elicited CD8+ T cells protect against respiratory syncytial virus strain A2-line19F-induced pathogenesis in BALB/c mice. J Virol. 2012;86:13016–13024. doi: 10.1128/JVI.01770-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assudani D, Cho HI, DeVito N, Bradley N, Celis E. In vivo expansion, persistence, and function of peptide vaccine-induced CD8 T cells occur independently of CD4 T cells. Cancer Res. 2008;68:9892–9899. doi: 10.1158/0008-5472.CAN-08-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bansal-Pakala P, Croft M. Defective T cell priming associated with aging can be rescued by signaling through 4-1BB (CD137) J Immunol. 2002;169:5005–5009. doi: 10.4049/jimmunol.169.9.5005. [DOI] [PubMed] [Google Scholar]
- 23.Moore ML, Chi MH, Luongo C, Lukacs NW, Polosukhin VV, Huckabee MM, Newcomb DC, Buchholz UJ, Crowe JE, Jr, Goleniewska K, Williams JV, Collins PL, Peebles RS., Jr A chimeric A2 strain of respiratory syncytial virus (RSV) with the fusion protein of RSV strain line 19 exhibits enhanced viral load, mucus, and airway dysfunction. J Virol. 2009;83:4185–4194. doi: 10.1128/JVI.01853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stokes KL, Chi MH, Sakamoto K, Newcomb DC, Currier MG, Huckabee MM, Lee S, Goleniewska K, Pretto C, Williams JV, Hotard A, Sherrill TP, Peebles RS, Jr, Moore ML. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J Virol. 2011;85:5782–5793. doi: 10.1128/JVI.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu B, Kimura Y. Local immune response to respiratory syncytial virus infection is diminished in senescence-accelerated mice. The Journal of general virology. 2007;88:2552–2558. doi: 10.1099/vir.0.83089-0. [DOI] [PubMed] [Google Scholar]
- 26.Lustgarten J, Dominguez AL, Thoman M. Aged mice develop protective antitumor immune responses with appropriate costimulation. J Immunol. 2004;173:4510–4515. doi: 10.4049/jimmunol.173.7.4510. [DOI] [PubMed] [Google Scholar]
- 27.Salek-Ardakani S, Moutaftsi M, Sette A, Croft M. Targeting OX40 promotes lung-resident memory CD8 T cell populations that protect against respiratory poxvirus infection. J Virol. 2011;85:9051–9059. doi: 10.1128/JVI.00619-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Looney RJ, Falsey AR, Walsh E, Campbell D. Effect of aging on cytokine production in response to respiratory syncytial virus infection. The Journal of infectious diseases. 2002;185:682–685. doi: 10.1086/339008. [DOI] [PubMed] [Google Scholar]
- 29.de Bree GJ, Heidema J, van Leeuwen EM, van Bleek GM, Jonkers RE, Jansen HM, van Lier RA, Out TA. Respiratory syncytial virus-specific CD8+ memory T cell responses in elderly persons. The Journal of infectious diseases. 2005;191:1710–1718. doi: 10.1086/429695. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Wang Y, Gilmore X, Xu K, Wyde PR, Mbawuike IN. An aged mouse model for RSV infection and diminished CD8(+) CTL responses. Exp Biol Med (Maywood) 2002;227:133–140. doi: 10.1177/153537020222700208. [DOI] [PubMed] [Google Scholar]
- 31.Tan JT, Whitmire JK, Ahmed R, Pearson TC, Larsen CP. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J Immunol. 1999;163:4859–4868. [PubMed] [Google Scholar]
- 32.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends in immunology. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurtado JC, Kim YJ, Kwon BS. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J Immunol. 1997;158:2600–2609. [PubMed] [Google Scholar]
- 34.Schulz M, Zinkernagel RM, Hengartner H. Peptide-induced antiviral protection by cytotoxic T cells. Proc Natl Acad Sci U S A. 1991;88:991–993. doi: 10.1073/pnas.88.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, Kwon BS. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169:4882–4888. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- 36.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev. 2009;229:192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 37.Bertram EM, Dawicki W, Sedgmen B, Bramson JL, Lynch DH, Watts TH. A switch in costimulation from CD28 to 4-1BB during primary versus secondary CD8 T cell response to influenza in vivo. J Immunol. 2004;172:981–988. doi: 10.4049/jimmunol.172.2.981. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, Tahiliani V, Salek-Ardakani S, Croft M. Targeting 4-1BB (CD137) to enhance CD8 T cell responses with poxviruses and viral antigens. Frontiers in immunology. 2012;3:332. doi: 10.3389/fimmu.2012.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang J, Braciale TJ. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat Med. 2002;8:54–60. doi: 10.1038/nm0102-54. [DOI] [PubMed] [Google Scholar]
- 40.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Bree GJ, Heidema J, van Leeuwen EM, van Bleek GM, Jonkers RE, Jansen HM, van Lier RA, Out TA. Respiratory syncytial virus-specific CD8+ memory T cell responses in elderly persons. J Infect Dis. 2005;191:1710–1718. doi: 10.1086/429695. [DOI] [PubMed] [Google Scholar]
- 42.Falsey AR, Walsh EE. Humoral immunity to respiratory syncytial virus infection in the elderly. Journal of medical virology. 1992;36:39–43. doi: 10.1002/jmv.1890360108. [DOI] [PubMed] [Google Scholar]
- 43.Walsh EE, Falsey AR. Respiratory syncytial virus infection in adult populations. Infectious disorders drug targets. 2012;12:98–102. doi: 10.2174/187152612800100116. [DOI] [PubMed] [Google Scholar]