Abstract
Dyskeratosis congenita is a telomere-mediated syndrome defined by mucocutaneous features. The X-linked mode of inheritance accounts for half the cases, and is thought to predominantly manifest in childhood as bone marrow failure. We identified two male probands who presented in the fifth decade with idiopathic pulmonary fibrosis and cancer. Their pedigrees displayed consecutively affected generations. Five of six females (83%) manifested mucocutaneous features of dyskeratosis congenita, and two had wound-healing complications. No mutations in autosomal dominant telomere genes were present, but exome sequencing revealed novel variants in the X-chromosome DKC1 gene that predicted missense mutations in conserved residues, p.Thr49Ser and p.Pro409Arg. Variants segregated with the telomere phenotype, and affected females were heterozygotes showing skewed X-inactivation. Telomerase RNA levels were compromised in cells from DKC1 mutation carriers, consistent with their pathogenic role. These findings indicate that females with heterozygous DKC1 mutations may be at increased risk for developing telomere phenotypes that, at times, may be associated with clinical morbidity.
Keywords: DKC1, dyskeratosis congenita, telomerase, pulmonary fibrosis, myelodysplastic syndrome
Dyskeratosis congenita (DC) is a syndrome of telomere shortening [Armanios and Blackburn, 2012]. It has been historically defined by a triad of mucocutaneous features in male children who have reticular skin hyperpigmentation, oral leukoplakia and nail dystrophy [Dokal, 2000]. DC patients suffer premature morbidity most commonly from bone marrow failure which affects 85% of cases before the age of 20 [Dokal, 2000]. Pulmonary fibrosis and cancer account for the majority of the remaining life-threatening complications [Dokal, 2000; Parry, et al., 2011]. DC falls on the severe end of a spectrum of syndromes characterized by inherited defects in telomere maintenance [Armanios and Blackburn, 2012]. In contrast to affected children, older adults with telomere-mediated disease usually manifest with pulmonary fibrosis, and in the absence of the classic DC features [Armanios and Blackburn, 2012]. Although DC was initially recognized as an X-linked disorder [Devriendt, et al., 1997; Knight, et al., 1998; Vulliamy, et al., 1997], it is appreciated now that autosomal dominant telomere syndromes are most prevalent [Armanios and Blackburn, 2012]. Loss-of-function mutations in the genes encoding telomerase holoenzyme components account for the majority of DC and related disorders cases. Mutations in the telomerase reverse transcriptase, TERT (MIM# 187270), and the telomerase RNA, TR (also TERC, MIM# 187270), cause haploinsufficiency and manifest in an autosomal dominant telomere syndromes [Armanios, et al., 2005; Vulliamy, et al., 2001]. Mutations in the DKC1 gene (MIM# 300126), which encodes the telomerase component dyskerin, cause X-linked disease [Heiss, et al., 1998; Mitchell, et al., 1999]. Dyskerin functions to stabilize TR, and interacts with TR through an essential Box H/ACA RNA domain in its 3’end [Alder, et al., 2011b; Mitchell, et al., 1999]. Approximately one-third of DC cases remain genetically uncharacterized.
Although DC and telomere syndromes follow Mendelian patterns of inheritance, several unique features distinguish their genetics. Disease phenotypes show variable penetrance that is age-dependent and autosomal dominant families display genetic anticipation, an earlier and more severe onset in successive generation [Armanios and Blackburn, 2012]. These features can confound recognizing the hereditary nature as well as the mode of inheritance in a given family [Armanios and Blackburn, 2012]. Moreover, autosomal dominant families display gender differences in disease penetrance with males developing complications at a younger age than their female siblings [Basel-Vanagaite, et al., 2008]. We sought here to characterize the genetic basis of telomere phenotypes in two unrelated families that had consecutively affected generations. The details of the subject recruitment, consent and the methods for the molecular studies are included in the Supporting Information’s Supp. Methods section.
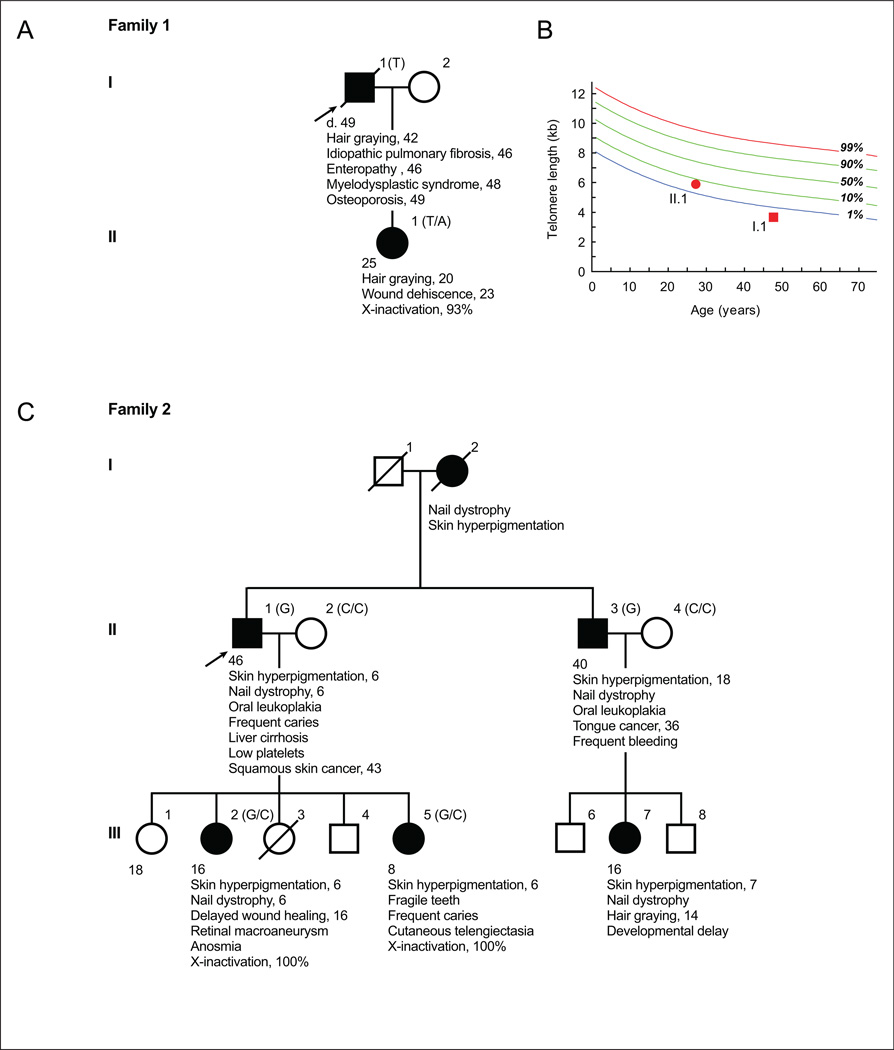
Family 1
The first proband was evaluated as part of the Johns Hopkins Telomere Syndrome Registry [Jonassaint, et al., 2013]. He was a previously healthy male who was diagnosed with idiopathic pulmonary fibrosis at age 46. He showed no features of DC. His family history was significant for a daughter with premature hair graying since the age 20 and who had a history of wound dehiscence after abdominal surgery. During the proband’s pulmonary fibrosis treatment course on a clinical trial with pirfenidone, he developed progressive thrombocytopenia and hematologic evaluation revealed aplastic anemia. The hematologic abnormalities persisted despite discontinuation of the drug, and he became transfusion dependent by age 48. He was subsequently diagnosed with myelodysplastic syndrome with complex cytogenetics including deletions of chromosomes 5q, 7, 17 and 20. He was treated with a demethylating agent, but continued to have progressive dyspnea and died at age 49 from end stage lung disease and marrow failure. Because the co-occurrence of pulmonary fibrosis and bone marrow failure is specific for telomere syndromes [Armanios, 2009], telomere length was measured and confirmed a very short age-adjusted length in lymphocytes, below the first percentile. His daughter’s telomere length was near the 5th percentile (Figure 1B). The clinical history is summarized in Figures 1A and 1B.
Figure 1. Clinical features of male probands with telomere-mediated disease and their families.
A&B. Clinical history of Family 1 and plot showing the adjacent age-adjusted telomere length of the proband and his daughter, respectively. C. Pedigree and history of Family 2. Shaded squares and circles represent males and females who have features of telomere-mediated disease, respectively. DKC1 genotypes at the variant nucleotide are included in parentheses in the pedigrees.
Family 2
Proband 2 and his family were evaluated at Memorial Sloan Kettering Hospital for a genomic instability syndrome in 1979 (Figure 1C). Based on the clinical phenotype, the family was enrolled in the International Fanconi Anemia Registry [Auerbach, et al., 1979]. The proband had the mucocutaneous triad of DC and reported a history of skin cancer as well as liver cirrhosis. His family history was remarkable for three consecutive generations with classic DC features that were documented in his mother, a brother, two daughters and a niece (Figure 1C). Female relatives had a history of delayed wound healing and brittle teeth, both complications reported in DC, and one daughter was found to have anosmia, a loss of the sense of smell (Figure 1C). Phytohemagglutinin (PHA) stimulation of peripheral blood did not show spontaneous chromosome instability, and challenge with diepoxybutane (DEB), an alkyling agent, showed no abnormal breaks or sister chromatid exchange events relative to controls, and in contrast to Fanconi anemia cells [Auerbach, 2009].
Genetic and Molecular Studies
Given recent progress in the genetics of human telomere-mediated disease, we sought to identify the inherited gene(s) in these families. Several factors led us to prefer an autosomal dominant model. The probands had adult-onset disease, in contrast to nearly 90% of reported X-linked DC cases [Dokal, 2000], and the first presenting symptoms in both individuals were not bone marrow-related. Furthermore, the high penetrance of disease in females (5 of 6 affected) made X-linked inheritance less likely given the reported low estimate of penetrance in DC females [Knight, et al., 1998]. Sequencing of TERT and TR revealed no variants, and genotyping of flanking microsatellite ruled out involvement of these loci in Family 2. We thus performed exome sequencing on the two probands using genomic DNA extracted from blood or fibroblasts. We used the SureSelect Human Exome 38 Mb Kit (Agilent, Santa Clara, CA), and the ABI SOLiD sequencing platform (Applied Biosystems, Carlsbad, CA). Manual inspection of candidate telomere gene sequences using the Integrative Genome Viewer identified a novel DKC1 exon 3 variant in proband 1: c.145A>T that predicted a missense substitution p.Thr49Ser near the nuclear localization signal of dyskerin (Figure 2A). This variant was initially missed by the calling software because of low depth of coverage (2X, Figure 2B). In the second proband, an exon 12 variant c.1226C>G predicted a p.Pro409Arg substitution distal to the pseudouridine synthase (PUA) domain of dyskerin (Figures 2A–2B). The single nucleotide variants identified in the probands segregated with the telomere phenotypes, with affected males showing hemizygous status and females were heterozygous showing extremely skewed X-inactivation patterns (Figure 1A, 1C). These data suggested that females with DKC1 mutations can show highly penetrant telomere phenotypes.
Figure 2. Molecular characterization of DKC1 mutations identified in two pedigrees.
A&B. Integrative Genome Viewer images showing read sequences that diverge from reference in DKC1 for families 1 and 2 respectively (highlighted inside the rectangle). There are significantly fewer reads in panel A compared to panel B. C. Schema showing evolutionary conservation of mutated residues in the dyskerin protein. The S. cerevisiae alignment refers to the dyskerin homolog, NAP57. D&E. Quantitative real time PCR of telomerase RNA (TR) levels in male-derived cells from probands and controls. RNA was extracted from early passage lymphoblasts (D) and fibroblasts (E). The mean for controls represents independent cells with the number noted below. Replicate runs from the same individual are plotted for mutation carriers. Error bars represent standard error of the mean. F. Exome read coverage of coding and exon-flanking sequences of telomere syndrome genes in the two probands.
To test the functional significance of the dyskerin variants, we examined their evolutionary conservation and found the mutated amino acids fell in highly invariant residues in vertebrate dyskerins as well as in the yeast homolog NAP57 (Figure 2C). Alternate substitutions at these residues, Thr49Met and Pro409Leu, have been described previously in DC [Ding, et al., 2004; Knight, et al., 1999], suggesting that mutations in these amino acids enrich for telomere phenotypes. Cells derived from the probands showed severely compromised TR levels (20% and 50% of control levels in families 1 and 2, respectively, Figures 2D–2E). TR levels were similarly decreased in cells from known DKC1 mutation carriers, as well as deleterious TR mutations in the Box H motif (Figure 2D). These data established that the dyskerin missense mutations identified are functionally deleterious.
We report here that mutations in the telomerase holoenzyme component DKC1 can cause telomere-related phenotypes in heterozygous females. Five of 6 females examined (83%) displayed at least two telomere phenotypes in this study. There was morbidity associated with this heterozygous state evidenced by post-operative wound dehiscence. Females also had non-classic DC features including fragile teeth, and one had anosmia. The olfactory bulb has a high turnover rate and telomerase null mice have been suggested to have olfaction defects [Jaskelioff, et al., 2011]. The anosmia in this female may thus be telomere-mediated and may represent a first association in humans. The high degree of penetrance seen in our families (83%) lies in contrast to a prior estimate of 15% [Knight, et al., 1998], which predated elucidation of the genetic basis of DC and appreciation for its full phenotypic spectrum. Vigilance for telomere-related complications in heterozygous females with DKC1 mutations may therefore be warranted in certain clinical settings, and the possibility of telomere-dependent phenotypes should inform genetic counseling discussions. X-linked inheritance should in turn be considered as a potential mode of inheritance in families with consecutively affected generations where male-to-male transmission is not evident, and even when the telomere phenotype manifests in adulthood.
Two mechanisms may contribute to telomere-mediated disease in female carriers. Telomere length is heritable, and X-linked DC females have shorter telomere lengths relative to age-matched controls [Mitchell, et al., 1999; Parry, et al., 2011]. In the families we studied, the male-to-female pattern of transmission of the mutant gene may therefore have contributed to the penetrance of the telomere phenotypes in female carriers in contrast to families where female-to-female transmission may have predominated. Short telomere length is sufficient to cause degenerative phenotypes in wildtype mice that inherit their telomere defects from telomerase mutant parents [Armanios, et al., 2009; Hao, et al., 2005]. Similarly, in autosomal dominant families, telomerase wildtype offspring of mutation carriers also have short telomere length, and lung disease has been described in these individuals, so far exclusively in the setting of cigarette smoke exposure history [Alder, et al., 2011a; Diaz de Leon, et al., 2011]. It may also be that the dyskerin mutant cells in females contribute directly to disease risk in parenchymal organs. Although bone-marrow derived cells from heterozygous DKC1 mutation carriers show skewed X-inactivation patterns consistent with an advantage for cells that have intact telomerase levels, such a competitive advantage is not possible in parenchymal organs. It is therefore possible that female carriers of DKC1 mutations may be at risk for developing life-threatening telomere-mediated organ failure especially when they are exposed certain environmental toxins, albeit at low penetrance. In support of this model, none of the female carriers from X-linked families who have been studied in the Johns Hopkins Telomere Syndrome Registry have had evidence of hematologic abnormalities to date (0 of 10 total). However, one female smoker developed symptomatic lung disease by 45 years of age, and was diagnosed with emphysema. It is therefore possible that DKC1 heterozygous mutation carriers may be at risk for developing degenerative parenchymal disease, such as emphysema, fibrosis or cancer, especially in the setting of certain environmental insults.
Our study highlights some of the limitations of current exome sequencing technology for clinical use. Although the variant calling software detected one of the mutated variants, the other was missed due to low coverage and it was only detected after manual inspection of the candidate gene and confirmation by direct Sanger sequencing. To date, nine genes have been linked to the telomere syndrome phenotypes [Armanios and Blackburn, 2012; Ballew, et al., 2013; Le Guen, et al., 2013; Walne, et al., 2013], and of these, several had extremely poor coverage including TERT (24–41% at 8X coverage) and TR (0% at 8X) relative to the entire exome (mean 70%, Figure 2F). Although exome capture and sequencing technologies continue to rapidly evolve, this caveat (i.e. missed calls in low coverage regions) may present one limitation of current platforms for clinical use because the coverage depth at individual genes is not yet routinely assessed or reported. These limitations should be considered in ongoing efforts to integrate new exome technology in clinical practice. Advances in capture and sequencing technologies along with whole genome approaches may minimize these limitations in the future.
In summary, mosaic females with DKC1 mutations may be at risk for developing cutaneous and parenchymal telomere phenotypes. Longitudinal studies of carrier females will be important to fully define disease penetrance in these females.
Supplementary Material
Acknowledgments
We are grateful to the subjects who participated in these studies and to their families. We acknowledge Jennifer Meyers and Timothy Mosbruger for their assistance with exome sequencing and data analysis. This work was supported by funds from the United States National Institutes of Health (NIH) RO1CA160433 (MA) and K99HL113105 (JKA), and the Doris Duke Charitable Foundation (MA). The Next Generation Sequencing Core at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center is supported by NIH P30CA006973. Dr. Alder is a Parker B. Francis Foundation Fellow.
Footnotes
Conflict of Interest Statement
The authors have no conflict of interest to declare.
References
- Alder JK, Cogan JD, Brown AF, Anderson CJ, Lawson WE, Lansdorp PM, Phillips JA, 3rd, Loyd JE, Chen JJ, Armanios M. Ancestral mutation in telomerase causes defects in repeat addition processivity and manifests as familial pulmonary fibrosis. PLoS genetics. 2011a;7:e1001352. doi: 10.1371/journal.pgen.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder JK, Guo N, Kembou F, Parry EM, Anderson CJ, Gorgy AI, Walsh MF, Sussan T, Biswal S, Mitzner W, et al. Telomere Length is a Determinant of Emphysema Susceptibility. Am J Respir Crit Care Med. 2011b;184:904–912. doi: 10.1164/rccm.201103-0520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992;51:1229–1239. [PMC free article] [PubMed] [Google Scholar]
- Armanios M. Syndromes of telomere shortening. Annu Rev Genomics Hum Genet. 2009;10:45–61. doi: 10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios M, Alder JK, Parry EM, Karim B, Strong MA, Greider CW. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am J Hum Genet. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, Eshleman JR, Cohen AR, Chakravarti A, Hamosh A, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci U S A. 2005;102:15960–15964. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, 3rd, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4–10. doi: 10.1016/j.mrfmmm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach AD, Lieblich LM, Ehrenbard L, Auerbach R, Chaganti RSK. Dyskeratosis congenita: Cytogenetic studies in a family with an unusual pattern of inheritance. American Journal of Human Genetics. 1979;31 [Google Scholar]
- Ballew BJ, Yeager M, Jacobs K, Giri N, Boland J, Burdett L, Alter BP, Savage SA. Germline mutations of regulator of telomere elongation helicase 1, RTEL1, in Dyskeratosis congenita. Hum Genet. 2013;132:473–480. doi: 10.1007/s00439-013-1265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basel-Vanagaite L, Dokal I, Tamary H, Avigdor A, Garty BZ, Volkov A, Vulliamy T. Expanding the clinical phenotype of autosomal dominant dyskeratosis congenita caused by TERT mutations. Haematologica. 2008;93:943–944. doi: 10.3324/haematol.12317. [DOI] [PubMed] [Google Scholar]
- Devriendt K, Matthijs G, Legius E, Schollen E, Blockmans D, van Geet C, Degreef H, Cassiman JJ, Fryns JP. Skewed X-chromosome inactivation in female carriers of dyskeratosis congenita. Am J Hum Genet. 1997;60:581–587. [PMC free article] [PubMed] [Google Scholar]
- Diaz de Leon A, Cronkhite JT, Yilmaz C, Brewington C, Wang R, Xing C, Hsia CC, Garcia CK. Subclinical lung disease, macrocytosis, and premature graying in kindreds with telomerase (TERT) mutations. Chest. 2011;140:753–763. doi: 10.1378/chest.10-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YG, Zhu TS, Jiang W, Yang Y, Bu DF, Tu P, Zhu XJ, Wang BX. Identification of a novel mutation and a de novo mutation in DKC1 in two Chinese pedigrees with Dyskeratosis congenita. J Invest Dermatol. 2004;123:470–473. doi: 10.1111/j.0022-202X.2004.23228.x. [DOI] [PubMed] [Google Scholar]
- Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–779. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- Hao LY, Armanios M, Strong MA, Karim B, Feldser DM, Huso D, Greider CW. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, Poustka A, Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadinanos J, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassaint NL, Guo N, Califano JA, Montgomery EA, Armanios M. The gastrointestinal manifestations of telomere-mediated disease. Aging Cell. 2013;12:319–323. doi: 10.1111/acel.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight S, Vulliamy T, Copplestone A, Gluckman E, Mason P, Dokal I. Dyskeratosis Congenita (DC) Registry: identification of new features of DC. Br J Haematol. 1998;103:990–996. doi: 10.1046/j.1365-2141.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- Knight SW, Heiss NS, Vulliamy TJ, Aalfs CM, McMahon C, Richmond P, Jones A, Hennekam RC, Poustka A, Mason PJ, et al. Unexplained aplastic anaemia, immunodeficiency, and cerebellar hypoplasia (Hoyeraal-Hreidarsson syndrome) due to mutations in the dyskeratosis congenita gene, DKC1. Br J Haematol. 1999;107:335–339. doi: 10.1046/j.1365-2141.1999.01690.x. [DOI] [PubMed] [Google Scholar]
- Le Guen T, Jullien L, Touzot F, Schertzer M, Gaillard L, Perderiset M, Carpentier W, Nitschke P, Picard C, Couillault G, et al. Human RTEL1 deficiency causes Hoyeraal-Hreidarsson syndrome with short telomeres and genome instability. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt178. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- Parry EM, Alder JK, Lee SS, Phillips JA, 3rd, Loyd JE, Duggal P, Armanios M. Decreased dyskerin levels as a mechanism of telomere shortening in X-linked dyskeratosis congenita. Journal of Medical Genetics. 2011;48:327–333. doi: 10.1136/jmg.2010.085100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlevsky JD, Bley CJ, Omana RV, Qi X, Chen JJ. The telomerase database. Nucleic Acids Res. 2008;36:D339–D343. doi: 10.1093/nar/gkm700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- Vulliamy TJ, Marrone A, Knight SW, Walne A, Mason PJ, Dokal I. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107:2680–2685. doi: 10.1182/blood-2005-07-2622. [DOI] [PubMed] [Google Scholar]
- Vulliamy TJ, Knight SW, Dokal I, Mason PJ. Skewed X-inactivation in carriers of X-linked dyskeratosis congenita. Blood. 1997;90:2213–2216. [PubMed] [Google Scholar]
- Walne AJ, Vulliamy T, Kirwan M, Plagnol V, Dokal I. Constitutional Mutations in RTEL1 Cause Severe Dyskeratosis Congenita. Am J Hum Genet. 2013;92:448–453. doi: 10.1016/j.ajhg.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.