Abstract
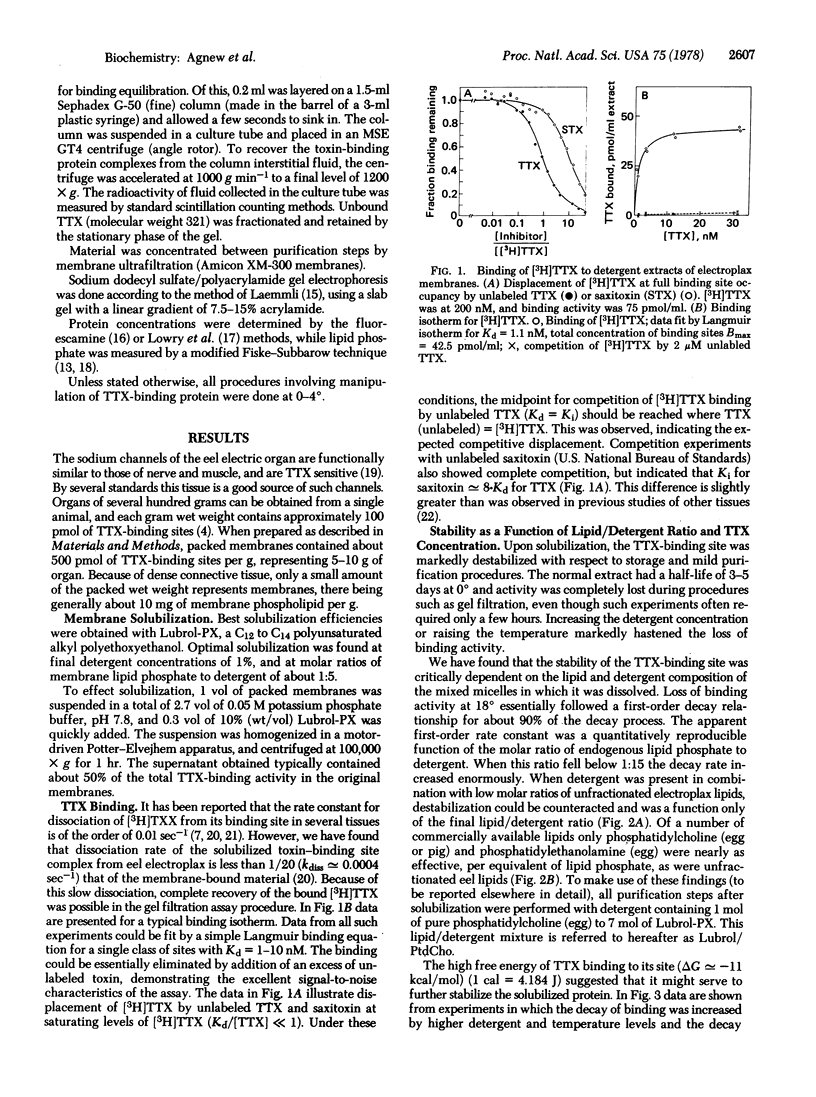
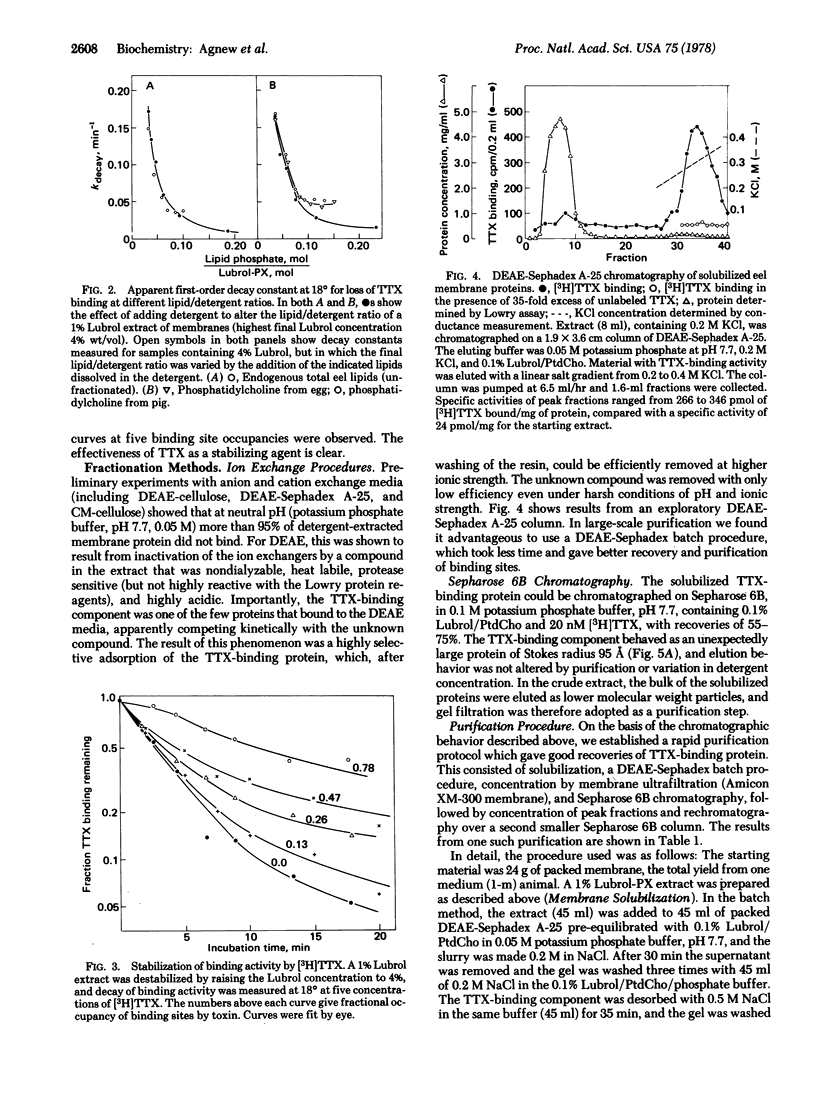
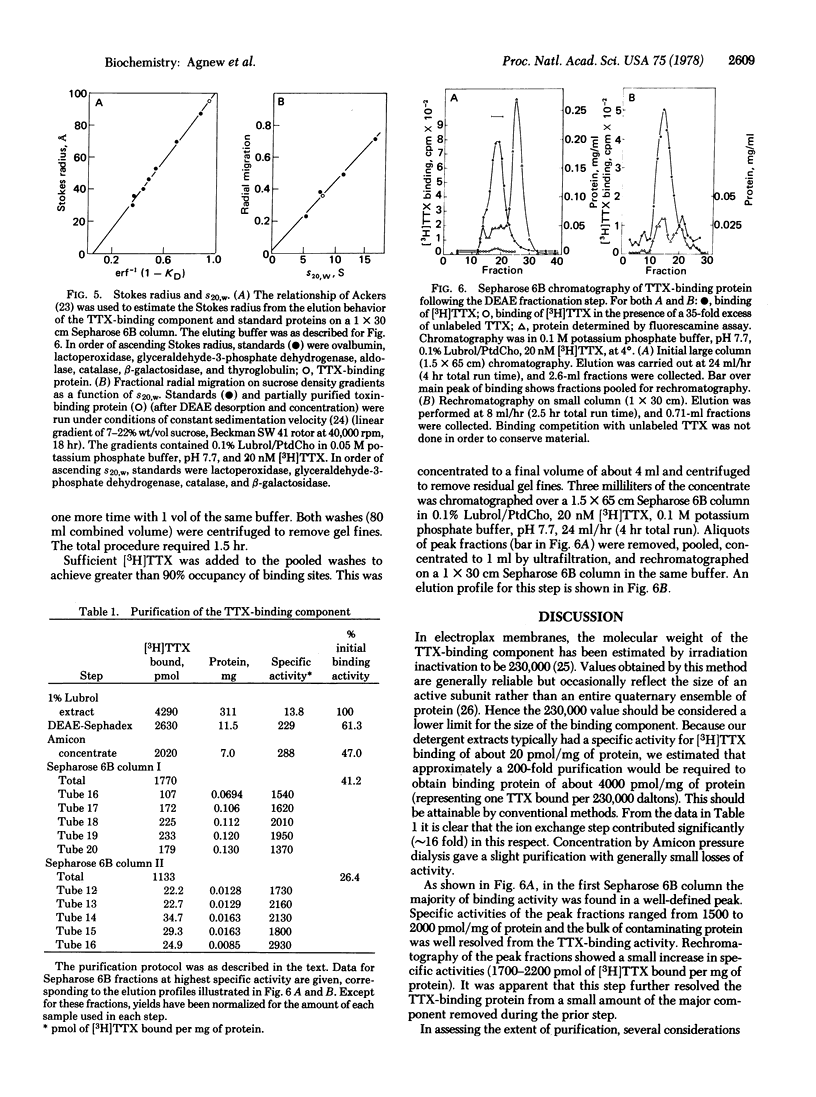
The tetrodotoxin-binding component associated with the voltage-sensitive sodium channel from electroplax membranes of Electrophorus electricus has been purified. The toxin-binding site could be efficiently solubilized with Lubrol-PX, resulting in an extract of high initial specific activity. Purification was facilitated by the development of a rapid, quantitative binding assay. The binding component was stabilized during purification by the use of mixed lipid/detergent micelles of defined composition, and by the saturation of the site with tetrodotoxin. The purification was achieved by means of a highly selective adsorption of the toxin-binding component to DEASE-Sephadex A-25, followed by desorption at high ionic strength and chromatography over Sepharose 6B. Final peak specific activities were at least 50% of the specific activity expected for a pure, undenatured toxin-binding componenet of 230,000 molecular weight. The purified material exhibited a sedimentation coefficient of approximately 8 S and an unusual Stokes radius of 95 A. Purified material showed a relatively simple pattern on sodium dodecyl sulfate/polyacrylamide gel electrophoresis, being comprised of only three polypeptides.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Levinson S. R. Tetrodotoxin binding to normal depolarized frog muscle and the conductance of a single sodium channel. J Physiol. 1975 May;247(2):483–509. doi: 10.1113/jphysiol.1975.sp010943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Ionic pores, gates, and gating currents. Q Rev Biophys. 1974 May;7(2):179–210. doi: 10.1017/s0033583500001402. [DOI] [PubMed] [Google Scholar]
- Benzer T. I., Raftery M. A. Partial characterization of a tetrodotoxin-binding component from nerve membrane. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3634–3637. doi: 10.1073/pnas.69.12.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer T. I., Raftery M. A. Solubilization and partial characterization of the tetrodotoxin binding component from nerve axons. Biochem Biophys Res Commun. 1973 Apr 16;51(4):939–944. doi: 10.1016/0006-291x(73)90017-x. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Henderson R., Ritchie J. M. The binding of labelled tetrodotoxin to non-myelinated nerve fibres. J Physiol. 1972 Dec;227(1):95–126. doi: 10.1113/jphysiol.1972.sp010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Ritchie J. M., Strichartz G. R. The binding of labelled saxitoxin to the sodium channels in nerve membranes. J Physiol. 1973 Dec;235(3):783–804. doi: 10.1113/jphysiol.1973.sp010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Wang J. H. Solubilization of a specific tetrodotoxin-binding component from garfish olfactory nerve membrane. Biochemistry. 1972 Nov 21;11(24):4565–4569. doi: 10.1021/bi00774a022. [DOI] [PubMed] [Google Scholar]
- Hille B. An essential ionized acid group in sodium channels. Fed Proc. 1975 Apr;34(5):1318–1321. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Haber E., O'Hara D. Identification of the cardiac beta-adrenergic receptor protein: solubilization and purification by affinity chromatography. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2828–2832. doi: 10.1073/pnas.69.10.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson S. R., Ellory J. C. Molecular size of the tetrodotoxin binding site estimated by irradiation inactivation. Nat New Biol. 1973 Sep 26;245(143):122–123. doi: 10.1038/newbio245122a0. [DOI] [PubMed] [Google Scholar]
- Levinson S. R. The purity of tritiated tetrodotoxin as determined by bioassay. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):337–348. doi: 10.1098/rstb.1975.0013. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Reed J. K., Raftery M. A. Properties of the tetrodotoxin binding component in plasma membranes isolated from Electrophorus electricus. Biochemistry. 1976 Mar 9;15(5):944–953. doi: 10.1021/bi00650a002. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M. Binding of tetrodotoxin and saxitoxin to sodium channels. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):319–336. doi: 10.1098/rstb.1975.0012. [DOI] [PubMed] [Google Scholar]
- Schwarz J. R., Ulbricht W., Wagner H. H. The rate of action of tetrodotoxin on myelinated nerve fibres of Xenopus laevis and Rana esculenta. J Physiol. 1973 Aug;233(1):167–194. doi: 10.1113/jphysiol.1973.sp010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]


