Abstract
Catalases are efficient scavengers of H2O2 and protect cells against H2O2 stress. Examination of the H2O2 stimulon in Saccharomyces cerevisiae revealed that the cytosolic catalase T (Ctt1) protein level increases 15-fold on H2O2 challenge in synthetic complete media although previous work revealed that deletion of the CCT1 or CTA1 genes (encoding peroxisomal/mitochondrial catalase A) does not increase the H2O2 sensitivity of yeast challenged in phosphate buffer (pH 7.4). This we attributed to our observation that catalase activity is depressed when yeast are challenged with H2O2 in nutrient-poor media. Hence, we performed a systematic comparison of catalase activity and cell viability of wild-type yeast and of the single catalase knockouts, ctt1∆ and cta1∆, following H2O2 challenge in nutrient-rich medium (YPD) and in phosphate buffer (pH 7.4). Ctt1 but not Cta1 activity is strongly induced by H2O2 when cells are challenged in YPD but suppressed when cells are challenged in buffer. Consistent with the activity results, exponentially growing ctt1∆ cells in YPD are more sensitive to H2O2 than wild-type or cta1∆ cells, whereas in buffer all three strains exhibit comparable H2O2 hypersensitivity. Furthermore, catalase activity is increased during adaptation to sublethal H2O2 concentrations in YPD but not in buffer. We conclude that induction of cytosolic Ctt1 activity is vital in protecting yeast against exogenous H2O2 but this activity is inhibited by H2O2 when cells are challenged in nutrient-free media.
Keywords: Catalase activity, Yeast, Peroxide challenge, Cell viability, Culture medium
Graphical abstract
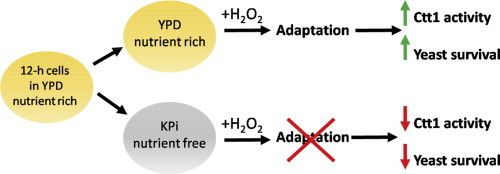
Highlights
-
•
Ctt1 activity increases on H2O2 challenge in nutrient-rich medium (YPD) but not in nutrient-free buffer.
-
•
Upregulation of Ctt1 is critical for yeast survival on H2O2 challenge.
-
•
The role of inducible Ctt1 activity in stress response is masked in nutrient-free medium.
-
•
To confirm their role in stress response, antioxidant enzyme activities should be compared for wild-type and knockout cells.
Introduction
Hydrogen peroxide is an inorganic messenger produced by cells during aerobic respiration [1]. At low levels, this hormone triggers adaptive responses that increase cell resistance to oxidants by augmenting the expression of antioxidant enzymes and stress related proteins [2–4]. In contrast, H2O2 is toxic at high concentration, triggering programmed cell death [5] and generating hydroxyl radicals in the presence of redox-active transition metals [6]. Thus, intracellular H2O2 levels are tightly regulated by the activity of H2O2 metabolizing enzymes.
Catalases efficiently disproportionate H2O2 to water and dioxygen (kcat/Km 106–107 M−1 s−1) without consuming additional electron-donor substrates [7]. Thus, these enzymes can metabolize high levels of H2O2 and the intracellular reducing environment is preserved [8,9]. Nonetheless, conflicting results have been published on the importance of catalase activity in protecting yeast cells against H2O2. Analysis of the yeast H2O2 stimulon revealed that the protein levels of Cta1, the catalase isoform found in yeast peroxisomes and mitochondria, remained unchanged [10] but production of Ctt1, the cytosolic catalase, increased 15-fold after challenge with a bolus of H2O2 [10]. Although this might suggest a role for Ctt1 in combating H2O2 stress, deletion of CTT1 or both CTT1 and CTA1 did not render growth-phase yeast hypersensitive to bolus H2O2 but impaired the H2O2 adaptive response [11]. Also, a questionable linear correlation between catalase activity and yeast survival on H2O2 challenge has been reported [12].
Differences in yeast phenotype can be frequently attributed to variation in genetic background [12]. However, nutrient availability during H2O2 challenge was dramatically different in the study that examined H2O2-induced protein expression [10] vs. those that recorded cell viability [11,12]. For instance, Godon et al. [10] looked at changes in protein expression after challenging exponentially growing wild-type YPH98 cells in synthetic complete dextrose (SCD). Izawa et al. [11] and Bayliak et al. [12] grew YPH250 cells in yeast extract peptone dextrose (YPD) but switched them to 100 mM potassium phosphate buffer, pH 7.4 (KPi) for the H2O2 challenge. Since nutrients are required for protein synthesis and repair [13], the comparable H2O2 hypersensitivity of wild-type, ctt1∆, and acatalasemic yeast [11] may be a consequence of starvation when cells are challenged in KPi.
Thus, here we compare catalase activity and cell viability of wild-type, ctt1∆ and cta1∆ cells after H2O2 challenge in YPD and KPi. We find that although the H2O2 sensitivity of wild-type cells depends on their genetic background, resistance requires stimulation of catalase activity. Ctt1 activity is upregulated by H2O2 challenge in nutrient-rich YPD and ctt1∆ cells exhibit lower survival than wild-type or cta1∆ cells. However, the three strains are equally hypersensitive to H2O2 when challenged in KPi since catalase activity falls below basal levels. Furthermore, wild-type and cta1∆ cells adapt to pre-challenge with a sublethal H2O2 dose in YPD [11] but not in KPi. Thus, we conclude that Ctt1 is stimulated by H2O2 and this activity is essential in protecting yeast cells against H2O2 challenge. Starvation impairs the H2O2-adaptive response by preventing induction of Ctt1 activity and masks its contribution to H2O2 resistance.
Materials and methods
Yeast strains, media, and growth conditions
The Saccharomyces cerevisiae strains used in this study are listed in Table 1. Wild-type BY4741 was purchased from the European Saccharomyces cerevisiae Archive for Functional Analysis (EUROSCARF, Frankfurt, Germany). Catalase knockouts (cta1∆ and ctt1∆) in the BY47471 genetic background were kindly provided by Prof. Christopher Brett (Department of Biology, Concordia University), and are also available from EUROSCARF. Profs Philip Hieter (Department of Medical Genetics, University of British Columbia) and Shingo Izawa (Department of Applied Biology, Kyoto Institute of Technology) generously supplied wild-type YPH250 cells, and cta1∆ and ctt1∆ cells in the YPH250 background, respectively. All strains were inoculated at initial OD600=0.01 in liquid YPD medium (1% yeast extract, 2% peptone and 2% glucose) and incubated at 30 °C with shaking at 225 rpm and a flask-to-medium volume ratio of 1:5.
Table 1.
S. cerevisiae strains used in this study.
| Strain | Description | Reference |
|---|---|---|
| Wild-type YPH250 | MATa ade2Δ101 his3Δ200 leu2Δ1 lys2Δ801 trpΔ1 ura3Δ52 | [11] |
| cta1∆ Strain | YPH250 cells with cta1::TRP1 | [11] |
| ctt1∆ Strain | YPH250 cells with ctt1::URA3 | [11] |
| Wild-type BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | EUROSCARF |
| cta1∆ Strain | BY4741 cells with cta1::KAN4MX | EUROSCARF |
| ctt1∆ Strain | BY4741 cells with ctt1::KAN4MX | EUROSCARF |
Challenge of yeast cells with exogenous H2O2
Cultures were grown to mid-exponential phase (12 h; OD600=0.5) under the conditions described above. Cells were washed twice with 100 mM potassium phosphate buffer, pH 7.4 (KPi), collected by centrifugation at 2000g for 10 min at 25 °C and divided in two fractions. The fractions were resuspended in the YPD from the original culture or in KPi, and challenged with H2O2 at 30 °C for 1.5 h with shaking at 225 rpm and a flask-to-medium volume ratio of 1:5. Following the challenge, the cultures were serially diluted, plated on YPD agar and incubated at 30 °C for 2 days [14]. Cell viability was determined by counting the total colony forming units (cfu) in H2O2-challenged and untreated cultures.
To examine adaptation, cells grown for 12 h in YPD as described above, were pre-challenged with 0.2 mM H2O2 at 30 °C for 1 h, switched to KPi and challenged with 2 mM H2O2 at 30 °C for 1.5 h (YPD/KPi column, Scheme 1). Alternatively, cells grown for 12 h in YPD were switched to KPi and both pre-adapted and challenged in this medium without buffer exchange (KPi column, Scheme 1). The viability of pre-adapted cells (Fig. 4B) is expressed as the ratio of the cfu following the lethal H2O2 challenge divided by the cfu following the 1 h pre-adaptation. Average viabilities ±SD of three independent cultures (n=3) are presented.
Scheme 1.
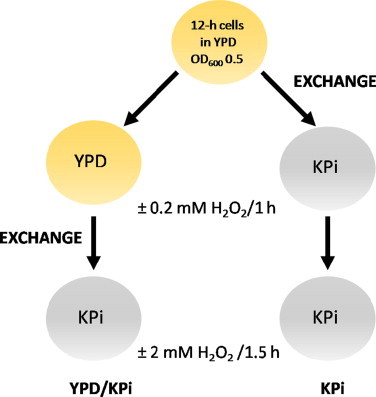
Pre-adaptation of YPH250 yeast cells to H2O2. Left row (YPD/KPi): Cells grown in YPD for 12 h at 30 °C were pre-challenged with 0.2 mM H2O2 at 30 °C for 1 h, switched to KPi and challenged with 2 mM H2O2 at 30 °C for 1.5 h. Right row (KPi): cells grown in YPD for 12 h at 30 °C were switched to KPi, pre-challenged with 0.2 mM H2O2 at 30 °C for 1 h, and challenged with 2 mM H2O2 at 30 °C for 1.5 h. Following challenge, both cultures were assayed for cell viability and catalase activity as described under Materials and methods.
Fig. 4.
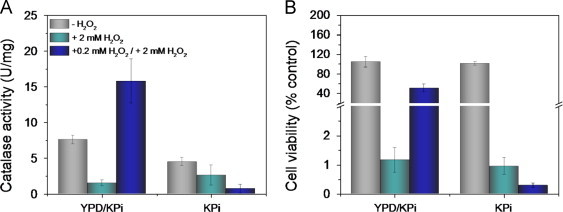
Adaptation of wild-type YPH250 cells to H2O2 challenge requires nutrient-rich medium to stimulate catalase activity. Catalase activity (A) and cell viability (B) without (control) and with pre-adaptation of cells to H2O2 followed by challenge with a lethal H2O2 dose. Exponentially growing cells in YPD medium for 12 h (OD600=0.5) were pre-challenged with 0.2 mM H2O2 for 1 h in YPD or in KPi followed by challenge with 2 mM H2O2 in KPi for 1.5 h (see Scheme 1). Note that control cells were incubated for 1.5 h (YPD/KPi) and 2.5 h (KPi) in the absence of glucose and had likely started to respire, which may explain their higher catalase activity relative to control cells in Fig. 2A. Additionally, the switch of cells from YPD to KPi increases catalase activity of control cells as shown in Fig. 3A. Cell viability and catalase activity were measured as described under Materials and methods and results are the average ±SD for three independent cultures (n=3).
Preparation of soluble protein extracts
These were prepared as previously described [14,15] with slight modification. Cells were collected at 2000g, washed twice with KPi containing 0.1 mM PMSF, the pellets were suspended in the same buffer, and mixed with an equal volume of acid-washed glass beads. Cell suspensions were vortexed (4×15 s cycles), cell debris was removed by centrifugation at 13000g for 10 min at 4 °C, and the total protein concentration in the supernatants (i.e., the soluble protein extracts) was determined by the Bradford assay with bovine serum albumin as a standard [16].
Catalase activity assay
The catalase activity of the soluble protein extracts was assayed as described previously [17]. Briefly, 40–100 μL of extract was added to 1.0 mL of 20 mM H2O2 in 50 mM KPi buffer (pH 7.0), and H2O2 decomposition was monitored at 240 nm (ε240=43.6 M−1 cm−1) [17]. One unit of catalase activity catalyzed the degradation of 1 µmol of H2O2 per min and results are presented as the average ±SD catalase activity from three independent cultures (n=3).
Results
Ctt1 activity is stimulated on H2O2 challenge in YPD and increases cell survival
We first compared catalase activity and cell viability after exposing wild-type YPH250 and wild-type BY4741 cells to increasing doses of H2O2 in nutrient-rich YPD media. Challenge of YPH250 cells with 0.2 and 0.4 mM H2O2 stimulates their catalase activity by 8- and 10-fold, respectively (Fig. 1A), and the cells remain 100% viable (Fig. 1B). Increasing the H2O2 dose to 1 mM and 2 mM results in weak catalase simulation and inhibition, respectively (Fig. 1 A) and a 50–80% drop in cell viability (Fig. 1B). Notably, H2O2 induces loss of viability in YPH250 cells with ~7 U of catalase activity per mg protein (Fig. 1 A) which, significantly, is also observed for wild-type BY4741 cells (Fig. 1C,D). In this strain the maximum stimulated catalase activity is close to 10 U per mg protein (Fig. 1C) and cells become sensitive to H2O2 at 0.4 mM (Fig. 1D). Interestingly, both strains require ~10 U of catalase activity per mg protein for protection against a bolus of H2O2 in the low millimolar range and, in the absence of catalase stimulation, the observed viability drops below 25% (Figs. 1 and 2). In sum, stimulation of catalase activity is required for protection of exponentially growing yeast against exogenous H2O2 and their enhanced catalase stimulation renders YPH250 cells more resistant to H2O2 than BY4741 cells (Fig. 1A,B vs. Fig. 1C,D).
Fig. 1.
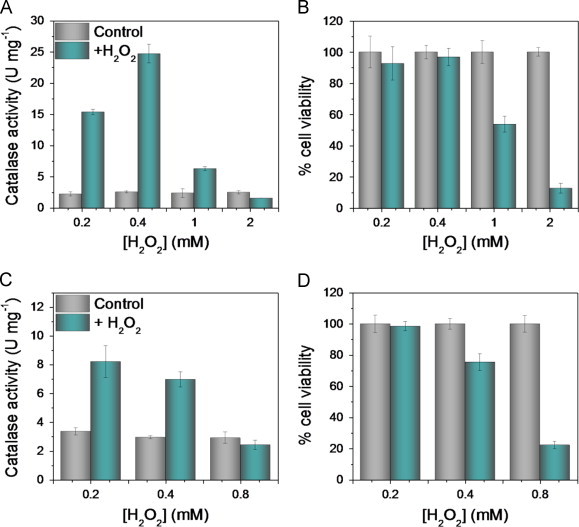
Induction of catalase activity correlates with survival of exponentially growing yeast on H2O2 challenge in YPD medium. Total catalase activity (left panels) and cell viability (right panels) of wild-type yeast cells in the YPH250 (A and B, respectively) and BY4741 (C and D, respectively) genetic backgrounds following H2O2 challenge. Exponentially growing cells (12 h, OD600=0.5) in YPD medium were exposed to KPi (control, grey bars) or challenged with 0.2–2 mM H2O2 (green bars) for 1.5 h at 30 °C and 225 rpm. Catalase activity and cell viability were measured as described under Materials and methods and results are the average viability ±SD for three independent cultures (n=3).
Fig. 2.
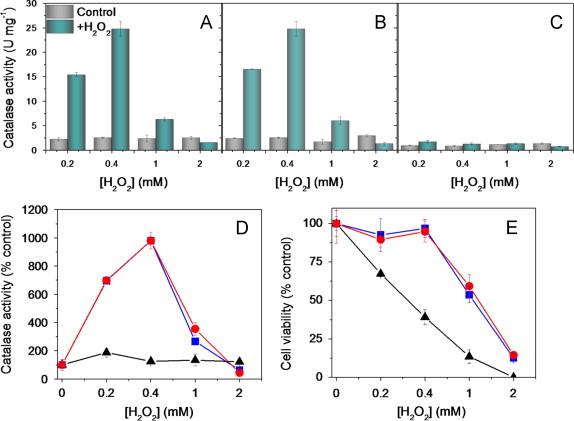
Deletion of cytosolic catalase T (CTT1) results in hypersensitivity of exponentially growing YPH250 cells to H2O2 challenge in YPD medium. Catalase activity before and after H2O2 challenge of: (A) wild-type cells producing both Ctt1 and Cta1; (B) cta1∆ cells producing cytosolic Ctt1 only; and (C) ctt1∆ cells producing peroxisomal/mitochondrial Cta1 only. (D) Catalase activity and (E) cell viability following H2O2 exposure relative to the control (no H2O2) for wild-type ( ), cta1∆ (
), cta1∆ ( ) and ctt1∆ (▴) cells. Exponentially growing cells in YPD medium were challenged after 12 h (OD600=0.5) with 0.2–2 mM H2O2 for 1.5 h at 30 °C and 225 rpm. Catalase activity and cell viability were measured as described under Materials and methods. Results are the average ±SD for three independent cultures (n=3). Lines between the data points in panels D and E were added as a visualization aid.
) and ctt1∆ (▴) cells. Exponentially growing cells in YPD medium were challenged after 12 h (OD600=0.5) with 0.2–2 mM H2O2 for 1.5 h at 30 °C and 225 rpm. Catalase activity and cell viability were measured as described under Materials and methods. Results are the average ±SD for three independent cultures (n=3). Lines between the data points in panels D and E were added as a visualization aid.
S. cerevisiae possess two catalase isoforms, a peroxisomal/mitochondrial Cta1 [18] and an atypical cytosolic Ctt1 [19]. Hence, we next addressed the relative importance of each isoform in protecting cells against H2O2 challenge using the single catalase knockouts, ctt1∆ and cta1∆. Comparable catalase activity is induced in wild-type and cta1∆ YPH250 cells after H2O2 exposure (Fig. 2A,B,D) but negligible catalase induction occurs in the ctt1∆ mutant strain (Fig. 2C,D). Similar results were observed for the catalase knockouts in the BY4741 genetic background (data not shown). Combined, our data indicate that cytosolic Ctt1 activity is stimulated ~10-fold by exogenous H2O2 in YPD, consistent with the 15-fold increase in production of the Ctt1 protein on H2O2 challenge in SCD [10]. Furthermore, cta1∆ cells exhibit wild-type resistance to H2O2 (Fig. 2E), whereas the ctt1∆ mutant exhibits H2O2 hypersensitivity with cell viability dropping by 30% on challenge with 0.2 mM H2O2 (Fig. 2E). H2O2 challenge in YPD does not upregulate Cta1 activity (Fig. 3C) and no increase in Cta1 protein levels were detected on H2O2 challenge in SCD [10]. Thus, we conclude that stimulation of cytosolic Ctt1 activity on H2O2 challenge in YPD is a critical factor in protecting cells against this ROS.
Fig. 3.
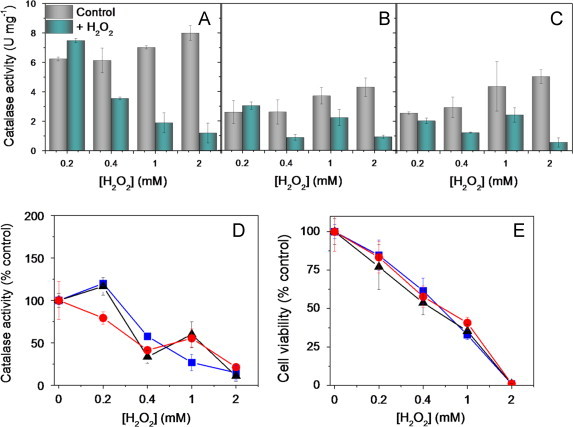
H2O2 challenge in KPi impairs catalase activation in YPH250 cells. Catalase activity before and after H2O2 challenge relative to the control (no H2O2) of: (A) wild-type cells producing both Ctt1 and Cta1; (B) cta1∆ cells producing cytosolic Ctt1 only; and (C) ctt1∆ cells producing peroxisomal/mitochondrial Cta1 only. (D) Catalase activity and (E) cell viability following H2O2 exposure relative to the control (no H2O2) for wild-type ( ), cta1∆ (
), cta1∆ ( ) and ctt1∆ (▴) cells. Exponentially growing cells in YPD medium were washed twice after 12 h (OD600=0.5), suspended to the same OD600 in KPi, and challenged with 0.2–2 mM H2O2 for 1.5 h at 30 °C and 225 rpm. Catalase activity and cell viability were measured as described under Materials and methods. Results are the average ±SD for three independent cultures (n=3). Lines between the data points in panels D and E were added as a visualization aid.
) and ctt1∆ (▴) cells. Exponentially growing cells in YPD medium were washed twice after 12 h (OD600=0.5), suspended to the same OD600 in KPi, and challenged with 0.2–2 mM H2O2 for 1.5 h at 30 °C and 225 rpm. Catalase activity and cell viability were measured as described under Materials and methods. Results are the average ±SD for three independent cultures (n=3). Lines between the data points in panels D and E were added as a visualization aid.
Ctt1 upregulation is abolished on H2O2 challenge in KPi and cell viability is lower
Izawa et al. [11] reported that single or double deletion of the catalase isoforms does not alter sensitivity to H2O2 when YPH250 cells are challenged in KPi. We hypothesized that H2O2 challenge of starving wild-type cells in KPi might lead to negligible stimulation of Ctt1 activity since this involves de novo Ctt1 protein synthesis [10] and in addition NADPH is required to prevent catalase inactivation during turnover [20]. Hence, we switched YPH250 cells from YPD to KPi and found that challenge in buffer with >0.2 mM H2O2 significantly inhibits catalase activity in wild-type and cta1∆ cells (Fig. 3A,B,D) in contrast to the stimulation observed in YPD (Fig. 2A,B,D). Thus, starvation prevents induction of Ctt1 activity by attenuating de novo Ctt1 synthesis and/or by promoting NADPH depletion. Furthermore, we speculate that variation in NAPDH depletion rates following the YPD–KPi switch may contribute to the relatively large variation in catalase activity of unchallenged cells in KPi (Fig. 3A–C). Nevertheless, all three strains exhibit comparable H2O2 hypersensitivity in KPi (Fig. 3E) and the protective effect of Ctt1 activity seen in YPD (Fig. 2E) is obliterated on nutrient withdrawal.
Of note, Ctt1 activity accounts for 60–80% of the total catalase activity of unchallenged wild-type cells in YPD (Fig. 2A–C). In contrast, when cells are switched to KPi both Cta1 and Ctt1 contribute equally to the total catalase activity of wild-type cells (Fig. 3A–C). This may be a consequence of relieving the tighter glucose repression of Cta1 vs. Ctt1 synthesis [21].
Wild-type cells become H2O2-adapted on pre-challenge with 0.2 mM H2O2 in YPD but not in KPi
Challenge of yeast cells with sublethal doses of a stressor activates stress-response signaling pathways to make cells more resistant to the stressor, a phenomenon known as adaptation [11,22–24]. Izawa et al. pre-challenged wild-type YPH250 cells in YPD with 0.2 mM H2O2, a sublethal concentration, and observed increased resistance to higher H2O2 levels [11]. This is consistent with the results in Fig. 2A, which demonstrates that exposure of wild-type YPH250 cells to 0.2 mM H2O2 in YPD results in an 8-fold increase in catalase activity. However, catalase upregulation is negligible in KPi (Fig. 3A) so pre-challenge with a low dose of H2O2 in KPi should not protect cells against a subsequent challenge with a lethal H2O2 dose whereas pre-challenge in YPD should be protective.
To test this, wild-type cells were pre-adapted with 0.2 mM H2O2 in both YPD and KPi and challenged with 2 mM H2O2 in KPi (Scheme 1). Cells pre-adapted in YPD exhibit ~10-fold higher catalase activity and ~60-fold higher viability than non-adapted cells (Fig. 4A,B). In contrast, pre-challenge in KPi abrogates the adaptive response to H2O2 and exposure of starving cells to 0.2 mM H2O2 prior to challenge with 2 mM H2O2 actually lowers their viability compared to challenge with a single high dose of H2O2 (Fig. 4A).
The dramatic increase in catalase activity and cell survival on pre-adaptation in YPD (Fig. 4) further demonstrates the strong link between stimulation of catalase activity and cell viability on H2O2 challenge. Because of their experimental design, Izawa et al. [11] concluded that catalase is important in H2O2 adaptation but not in direct H2O2 challenge. But since H2O2 challenge of non-adapted cells was performed in KPi, cells were unable to increase their catalase activity and respond to the stress [11,12]. In contrast, cells were pre-adapted in YPD so the induced catalase activity protected them against the subsequent H2O2 challenge [11]. If they had also pre-adapted the cells in KPi, the protective effects of catalase would have been lost (Fig. 4). Notably, Wieser et al. observed that mild heat shock of cells in YPD induced H2O2 adaptation via increased CTT1 expression, consistent with the reported protective effects of heat shock against oxidative stress [23].
Discussion
Here we demonstrate that cytosolic Ctt1 activity is induced on H2O2 challenge of yeast cells in nutrient-rich YPD medium. Catalase provides protection against H2O2 stress, consistent with its being a highly efficient H2O2 scavenger. Critically, H2O2 challenge of starving cells expressing the CTT1 gene in KPi prevents upregulation of Ctt1 activity and masks the hypersensitivity of ctt1Δ cells to this ROS.
Although catalases have been studied over many years, there are conflicting reports regarding their protective role on H2O2 challenge [10–12]. Yeast strains with different genetic backgrounds were examined but the nutrient status of the growth medium is likely a more critical parameter controlling the outcome of H2O2 challenge. For example, a dramatic H2O2-induced catalase upregulation was observed in cells challenged in SCD [10] but not in cells challenged in KPi [11,12]. H2O2 adaptation experiments yielded more consistent results but cells were pre-challenged only in nutrient-rich media [11,23]. Since catalases are synthesized de novo in response to endogenous or exogenous H2O2 challenge or pre-challenge [10,19,23], nutrients are necessary for their induction and few studies report on catalase activity pre- and post-H2O2 challenge [12].
Glutathione production protects yeast cells on H2O2 challenge in nutrient-free media [25] but this is a highly abundant antioxidant, reaching levels of 10 mM in yeast cytosol [26]. Also, H2O2 challenge in nutrient-free media may reveal the importance of highly abundant, constitutively produced antioxidant enzymes such as glutathione and thioredoxin peroxidases, whereas information on H2O2-induced antioxidant defenses such as catalases is lost. Furthermore, some peroxidases perform roles in addition to H2O2 removal. For example, thioredoxin peroxidase 1 (Tsa1), the most abundant cytosolic peroxiredoxin in yeast, is converted to a dodecameric chaperone on H2O2 stress [27], and is likely critical in protecting other proteins against H2O2-induced misfolding. Therefore, the striking H2O2 hypersensitivity and poor growth on TSA1 deletion [28] may be due mainly to loss of its chaperone rather than H2O2-scavenging function. Deletion of glutathione peroxidase 3 (Gpx3) also results in a dramatic increase in H2O2 sensitivity but not knockout of the Gpx1 and Gpx2 isoforms [29]. This may be attributed to the non-redundant sensor function of Gpx3 [30–32] in activation of the Yap1-dependent oxidative stress response [30,32]. In essence, the importance of highly abundant antioxidant enzymes, especially those with chaperone or signaling functions, may be apparent on challenge in nutrient-free media whereas the contribution of less abundant, inducible enzymes such as catalases can be masked in starving cells due to the lack of de novo protein synthesis and/or repair.
We also observed that when the catalase activity drops below a threshold level of ~10 U per mg protein, YPH250 and BY4741 cells become sensitive to exogenous H2O2. In fact, based on loss of cell viability, H2O2 becomes lethal to S. cerevisiae when it overwhelms the cell's inducible Ctt1 activity. For example, on challenge of YHP250 cells with ~2 mM H2O2 the catalase activity drops to the basal level and cell viability plummets (Fig. 1A,B). BY4741 cells respond in a similar manner on challenge with only 0.8 mM H2O2 (Fig. 1C,D). We speculate that turnover inactivation by excess H2O2 lowers catalase activity and the induction of apoptosis-like death [5] may play a role in the resulting high cell death rates.
Conclusions
We identified Ctt1 activity as essential in protecting yeast cells against exogenous H2O2 and unmasked the H2O2-hypersensitive phenotype of ctt1Δ cells. More generally, our results reveal that investigations of the relative importance of an antioxidant enzyme should consider: (1) its relative abundance, (2) its inducible vs. constitutive expression, (3) nutrient availability for triggering its induction, and (4) the stressor dose that causes its inactivation. Specifically, when analyzing the role of yeast catalases we found that it is critical to provide nutrients for the cell to respond to the H2O2 insult by de novo Ctt1 synthesis and likely enhanced NADPH generation. Furthermore, catalase deletion by genetic intervention had to be complemented by activity measurements before and after H2O2 challenge to uncover the importance of Ctt1 activity in defending cells. Thus, our work underscores the necessity of directly measuring the enzyme activity of interest in wild-type and knockout cells before and after applying a stress. Equally important, inducible antioxidant defences should be examined in the presence of nutrients so cells can adapt to the challenge.
Acknowledgments
This work was supported by funding from the Natural Sciences and Engineering Research Council of Canada (NSERC) and Concordia University. A.M.E holds a Concordia University Research Chair. D.M. acknowledges a doctoral scholarship from FRQ-NT (Quebec) and additional awards from Concordia University and PROTEO, the FRQ-NT Network for Research on Protein Function, Structure, and Engineering.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Veal E.A., Day A.M., Morgan B.A. Hydrogen peroxide sensing and signaling. Mol. Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Ristow M., Schmeisser S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011;51:327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Mesquita A., Weinberger M., Silva A., Sampaio-Marques B., Almeida B., Leao C., Costa V., Rodrigues F., Burhans W.C., Ludovico P. Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc. Natl. Acad. Sci. USA. 2010;107:15123–15128. doi: 10.1073/pnas.1004432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg A.A., Bourque S.D., Kyryakov P., Gregg C., Boukh-Viner T., Beach A., Burstein M.T., Machkalyan G., Richard V., Rampersad S., Cyr D., Milijevic S., Titorenko V.I. Effect of calorie restriction on the metabolic history of chronologically aging yeast. Exp. Gerontol. 2009;44:555–571. doi: 10.1016/j.exger.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Madeo F., Frohlich E., Ligr M., Grey M., Sigrist S.J., Wolf D.H., Frohlich K.U. Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 1999;145:757–767. doi: 10.1083/jcb.145.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koppenol W.H. The haber-weiss cycle—70 years later. Redox Rep. 2001;6:229–234. doi: 10.1179/135100001101536373. [DOI] [PubMed] [Google Scholar]
- 7.Switala J., Loewen P.C. Diversity of properties among catalases. Arch. Biochem. Biophys. 2002;401:145–154. doi: 10.1016/S0003-9861(02)00049-8. [DOI] [PubMed] [Google Scholar]
- 8.Kuhajda F.P. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66:5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 9.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godon C., Lagniel G., Lee J., Buhler J.M., Kieffer S., Perroti M., Boucheriei H., Toledano M.B., Labarre J. The H2O2 stimulon in Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:22480–22489. doi: 10.1074/jbc.273.35.22480. [DOI] [PubMed] [Google Scholar]
- 11.Izawa S., Inoue Y., Kimura A. Importance of catalase in the adaptive response to hydrogen peroxide: analysis of acatalasaemic Saccharomyces cerevisiae. Biochem. J. 1996;320:61–67. doi: 10.1042/bj3200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayliak M., Semchyshyn H., Lushchak V. Effect of hydrogen peroxide on antioxidant enzyme activities in Saccharomyces cerevisiae is strain-specific. Biochemistry (Mosc) 2006;71:1013–1020. doi: 10.1134/s0006297906090100. [DOI] [PubMed] [Google Scholar]
- 13.Mager W.H., Dekruijff A.J.J. Stress-induced transcriptional activation. Microbiol. Rev. 1995;59:506. doi: 10.1128/mr.59.3.506-531.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martins D., Kathiresan M., English A.M. Cytochrome c peroxidase is a mitochondrial heme-based H2O2 sensor that modulates antioxidant defense. Free Radic. Biol. Med. 2013;65:541–551. doi: 10.1016/j.freeradbiomed.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 15.Jiang H., English A.M. Phenotypic analysis of the ccp1Delta and ccp1Delta-ccp1W191F mutant strains of Saccharomyces cerevisiae indicates that cytochrome c peroxidase functions in oxidative-stress signaling. J. Inorg. Biochem. 2006;100:1996–2008. doi: 10.1016/j.jinorgbio.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Bradford M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.Beers R.F., Sizer I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 18.Thieringer R., Shio H., Han Y.S., Cohen G., Lazarow P.B. Peroxisomes in Saccharomyces cerevisiae: immunofluorescence analysis and import of catalase A into isolated peroxisomes. Mol. Cell. Biol. 1991;11:510–522. doi: 10.1128/mcb.11.1.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bissinger P.H., Wieser R., Hamilton B., Ruis H. Control of Saccharomyces cerevisiae catalase T gene (CTT1) expression by nutrient supply via the RAS-cyclic AMP pathway. Mol. Cell. Biol. 1989;9:1309–1315. doi: 10.1128/mcb.9.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirkman H.N., Gaetani G.F. Catalase: a tetrameric enzyme with four tightly bound molecules of NADPH. Proc. Natl. Acad. Sci. USA. 1984;81:4343–4347. doi: 10.1073/pnas.81.14.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cross H.S., Ruis H. Regulation of catalase synthesis in Saccharomyces cerevisiae by carbon catabolite repression. Mol. Gen. Genet. 1978;166:37–43. doi: 10.1007/BF00379727. [DOI] [PubMed] [Google Scholar]
- 22.Bilinski T., Kwolek M., Sas E., Krynicka M., Koziol S., Owsiak-Teleon A., Krzepilko A., Bartosz G. A novel test for identifying genes involved in aldehyde detoxification in the yeast. Increased sensitivity of superoxide-deficient yeast to aldehydes and their metabolic precursors. Biofactors. 2005;24:59–65. doi: 10.1002/biof.5520240107. [DOI] [PubMed] [Google Scholar]
- 23.Wieser R., Adam G., Wagner A., Schuller C., Marchler G., Ruis H., Krawiec Z., Bilinski T. Heat shock factor-independent heat control of transcription of the CTT1 gene encoding the cytosolic catalase T of Saccharomyces cerevisiae. J. Biol. Chem. 1991;266:12406–12411. [PubMed] [Google Scholar]
- 24.Davies J.M.S., Lowry C.V., Davies K.J.A. Transient adaptation to oxidative stress in yeast. Arch. Biochem. Biophys. 1995;327:1–6. doi: 10.1006/abbi.1995.1128. [DOI] [PubMed] [Google Scholar]
- 25.Izawa S., Inoue Y., Kimura A. Oxidative stress-response in yeast—effect of glutathione on adaptation to hydrogen-peroxide stress in Saccharomyces cerevisiae. FEBS Lett. 1995;368:73–76. doi: 10.1016/0014-5793(95)00603-7. [DOI] [PubMed] [Google Scholar]
- 26.Penninckx M.J. An overview on glutathione in Saccharomyces versus non-conventional yeasts. FEMS Yeast Res. 2002;2:295–305. doi: 10.1016/S1567-1356(02)00081-8. [DOI] [PubMed] [Google Scholar]
- 27.Trotter E.W., Rand J.D., Vickerstaff J., Grant C.M. The yeast Tsa1 peroxiredoxin is a ribosome-associated antioxidant. Biochem. J. 2008;412:73–80. doi: 10.1042/BJ20071634. [DOI] [PubMed] [Google Scholar]
- 28.Munhoz D.C., Netto L.E. Cytosolic thioredoxin peroxidase I and II are important defenses of yeast against organic hydroperoxide insult: catalases and peroxiredoxins cooperate in the decomposition of H2O2 by yeast. J. Biol. Chem. 2004;279:35219–35227. doi: 10.1074/jbc.M313773200. [DOI] [PubMed] [Google Scholar]
- 29.Inoue Y., Matsuda T., Sugiyama K., Izawa S., Kimura A. Genetic analysis of glutathione peroxidase in oxidative stress response of Saccharomyces cerevisiae. J. Biol. Chem. 1999;274:27002–27009. doi: 10.1074/jbc.274.38.27002. [DOI] [PubMed] [Google Scholar]
- 30.Delaunay A., Pflieger D., Barrault M.B., Vinh J., Toledano M.B. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell. 2002;111:471–481. doi: 10.1016/s0092-8674(02)01048-6. [DOI] [PubMed] [Google Scholar]
- 31.D’Autreaux B., Toledano M.B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell. Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 32.Paulsen C.E., Carroll K.S. Chemical dissection of an essential redox switch in yeast. Chem. Biol. 2009;16:217–225. doi: 10.1016/j.chembiol.2009.01.003. [DOI] [PubMed] [Google Scholar]


