Abstract
Reactive Oxygen Species (ROS) are known to cause oxidative damage to DNA, proteins and lipids. In addition, recent evidence suggests that ROS can also initiate signaling cascades that respond to stress and modify specific redox-sensitive moieties as a regulatory mechanism. This suggests that ROS are physiologically-relevant signaling molecules. However, these sensor/effector molecules are not uniformly distributed throughout the cell. Moreover, localized ROS damage may elicit site-specific compensatory measures. Thus, the impact of ROS can be likened to that of calcium, a ubiquitous second messenger, leading to the prediction that their effects are exquisitely dependent upon their location, quantity and even the timing of generation. Despite this prediction, ROS signaling is most commonly intuited through the global administration of chemicals that produce ROS or by ROS quenching through global application of antioxidants. Optogenetics, which uses light to control the activity of genetically-encoded effector proteins, provides a means of circumventing this limitation. Photo-inducible genetically-encoded ROS-generating proteins (RGPs) were originally employed for their phototoxic effects and cell ablation. However, reducing irradiance and/or fluence can achieve sub-lethal levels of ROS that may mediate subtle signaling effects. Hence, transgenic expression of RGPs as fusions to native proteins gives researchers a new tool to exert spatial and temporal control over ROS production. This review will focus on the new frontier defined by the experimental use of RGPs to study ROS signaling.
Abbreviations: KR, KillerRed; miniSOG, mini Singlet Oxygen Generator; ROS, Reactive Oxygen Species; PDT, photodynamic therapy; CALI, chromophore-assisted light inactivation; RGP, ROS generating protein
Keywords: ROS signaling, Photodynamic therapy, miniSOG, KillerRed, Optogenetics, Phototoxicity
Graphical abstract
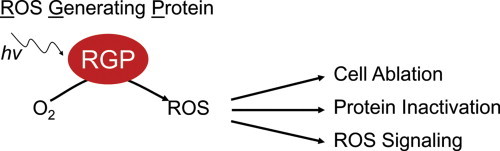
Highlights
-
•
ROS signaling is implicated in numerous cellular functions.
-
•
Genetically encoded proteins are capable light-induced ROS production.
-
•
Cell ablation, protein inactivation, and ROS signaling applications are described.
Introduction
Reactive Oxygen Species (ROS) play diverse roles in organism physiology and pathophysiology. ROS can cause damage in the cell through oxidative reactions, and excessive levels of ROS are associated with numerous pathologies [1], [2]. The destructive power of large-scale ROS production is highlighted by the fact that photodynamic therapy (PDT) uses photo-activation of chemicals that produce ROS, primarily but not exclusively singlet oxygen (1O2), to kill cancer cells and to treat local infections [3]. Similarly, the importance of protecting against such damage is highlighted by the significant resources that biology devotes to ROS detoxification, including a plethora of complex antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase, thioredoxin, peroxiredoxin etc.).
In addition to their ability to cause damage, ROS can also operate as signaling molecules and signal cell survival [4], [5], [6]. ROS can lead to changes in enzyme activity, gene transcription, metabolism and signal transduction, and physiologic ROS production may be necessary for adaptation to stress, normal development and regulation of lifespan [2], [7], [8], [9]. A thorough review of the signaling roles of ROS is beyond the scope of this manuscript, and readers are directed to several excellent reviews [10], [11], [12], [13], [14]. Notably however, despite the well-known signaling properties of ROS, studying them is limited by an inability to experimentally control local ROS levels.
Though widely utilized as an experimental approach, it is unclear that the global application of primary ROS or ROS-generating reagents (e.g. potassium superoxide, H2O2, and xanthine/xanthine oxidase) recapitulates endogenous ROS signaling. Furthermore, extrapolating the relatively non-specific effects of antioxidants such as N-acetylcysteine can be difficult. In fact, ROS may be conceptually similar to other second messengers such as Ca2+, whose signaling properties are highly dependent upon timing, magnitude, and the formation of signaling microdomains. With respect to Ca2+ signaling, the advent of “caged” Ca2+, where a light-sensitive protective moiety is used to prevent calcium׳s biologic activity, drove the field forward by allowing researchers to directly ask whether a localized, constrained elevation in Ca2+ was sufficient to elicit a particular outcome.
Optogenetics refers to an emerging field where light-sensitive proteins are used to manipulate cell signaling (for review, see [15]). Some of the more widely recognized optogenetic reagents include such proteins as channel-rhodoposins (ChR2), halorhodopsins (HR) and OptoXRs, which allow cell membrane potential (ChR2 and HR) or G protein-coupled second messenger signaling (OptoXR) to be controlled through the application of light [15]. The optogenetic toolbox is expanding rapidly, and one class of newly developed proteins photo-generates ROS. Much like PDT, ROS generating proteins (RGPs) were originally employed in cell ablation experiments for their toxic effects [16], [17], [18]. However, these RGPs have more recently been shown to generate sub-lethal amounts of ROS with both spatial and temporal precision. The use of RGPs to control ROS production is potentially transformative, and this review highlights their development and the initial studies that underlie their potential. Despite the ability of these RGPs to transform the field of free radical biology, there has to date been little adoption of these new methods within the field.
Reactive Oxygen Species (ROS)
There are a variety of reactive oxygen (and nitrogen) species within cells (for review see [11], [14]). The focus of this review will be on superoxide (O2•−), and singlet oxygen (1O2), two species of ROS that can be generated from photosensitizers. The following section will provide a brief overview of these molecules and their physical properties.
The main sources of O2•− within the cell include NAPDH oxidases (NOX family enzymes) xanthine/xanthine oxidase and mitochondria. Cells have developed mechanisms to cope with O2•− production, such as conversion to hydrogen peroxide (H2O2) by superoxide dismutase (SOD). H2O2 can then be removed through enzymatic reactions (e.g. catalase) and thiol-systems (e.g. glutathione) [19] to avoid the Fenton reaction formation of hydroxyl radical (HO•) [20] (Fig. 1). The generation of O2•− can result in a cascade of different ROS, each with unique properties and preferred biological targets. For example, O2•− is charged and has limited permeability while H2O2 is freely diffusible through biomembranes. The protonated form of O2•− (pKa~4.8) is the hydroperoxyl radical (HO2•), which has high reactivity and since it is uncharged may cross membranes [21]. Furthermore, O2•− preferentially reacts with iron sulfur centers and with nitric oxide, while H2O2 is mildly reactive with cysteine and methionine residues in proteins [8].
Fig. 1.
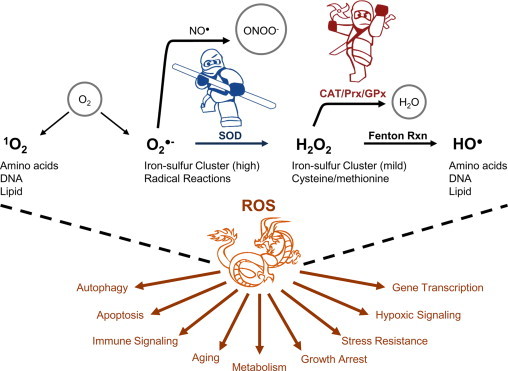
ROS and their biological impact. Each ROS has intrinsic chemical properties that will render it more likely to interact with a particular biological target. The exact target of a particular ROS will depend in part on the local environment in which it was produced. The cell presents a diverse range of environments and subsequent physiologic responses to ROS production. Abbreviations: SOD, superoxide dismutase; CAT, Catalase; Prx, Peroxiredoxin; and GPx, Glutathione peroxidase.
1O2 is the lowest lying electronic excited state of molecular oxygen produced in photosystem II of chloroplasts via photoexcitation of chlorophyll, and is commonly generated using light-sensitive molecules called photosensitizers. 1O2 is highly reactive, such that its reactions are diffusion limited and lack selectively. Indeed, because of its high reactivity in biological systems, 1O2 is believed to react in the immediate vicinity of its generation [22]. Furthermore, 1O2 differs from O2•− in that it cannot directly convert to other ROS endogenously generated by the mitochondrion. For example, 1O2 cannot directly dismutate to H2O2 like O2•−. However, 1O2 can generate intermediate products that are able to initiate ROS reactions that overlap with other ROS. One example is lipid peroxidation. Both 1O2 and HO• can generate lipid hydroperoxides (LOOH) but via distinct mechanisms. In the presence of transition metal ions these can give rise to the generation of free radicals, which can then re-initiate lipid peroxidation chain reactions [23], [24], [25]. While the formation of LOOH will most likely occur in the vicinity of ROS production due to the highly reactive nature of 1O2 and HO•, LOOH can propagate ROS reactions at sites distant from their formation [24], [25]. Plants have evolved a highly effective antioxidant system centered on plastoquinone (analogous to ubiquinone in mammalian mitochondria) and efficient 1O2 quenchers such as carotenoids, to circumvent the toxic effects of 1O2 reaction with biomolecules.
The characteristics of an individual ROS will influence its capabilities as an effective damaging and/or signaling molecule [26]. In general, reactivity and signaling ability are mutually exclusive. For example, HO• is highly reactive and displays no preference among reaction with different biological molecules, thus lacking the selectivity needed for a signaling molecule. It is important to note that while some ROS may possess signaling capabilities in a particular amount, the large scale overproduction of ROS may also result in damage. For the purposes of this review, the photogeneration of O2•− and 1O2 will be explored as they relate to ROS signaling and damage.
ROS are implicated in many signaling processes (Fig. 1), yet understanding their biological role is hampered by their short lifetime and indirect indicators. Many studies rely on the global application of ROS or antioxidants and indirect measurements with fluorescent indicators. Genetic approaches target antioxidant defenses by modulating expression of ROS scavenging enzymes [27], [28], [29]. Similarly in the field of reactive nitrogen species, the effects of over-expressing various isoforms of nitric oxide synthase (NOS) have been well studied, although such overexpression studies have not been extensively applied to ROS generating enzymes such as the NOXs.
Similar to other signaling molecules, the effects of ROS may be largely determined by their concentration in the local environment. The reactivity of many ROS and their rapid conversion by detoxifying enzymes support the likelihood that their signaling capacity is greatly limited by distance. Recent evidence has certainly suggested that this is the case, and advances in mitochondrial targeting of ROS probes (e.g. mitoB [30]) and antioxidants (e.g. mitoTEMPO [31] and mitoQ [32]) confirm these ideas. The use of these agents has allowed researchers to demonstrate the specific need for mitochondrial ROS in physiological responses ranging from hypertension to aging [31], [33], [34]. However, just because ROS are necessary does not indicate that they are also sufficient to exert a physiologic response. A method to induce the localized de novo production of ROS would pioneer experimental approaches that address the flip-side of the “necessary and sufficient” coin – that is, sufficiency – as well as allowing potential spatial and temporal constraints that may influence signaling output to be tested.
Photodynamic therapy (PDT)
The idea that ROS can be generated on demand using light is not novel. In fact, photodynamic therapy (PDT) is a clinical technique where chemical photosensitizers are triggered to generate ROS in a target cell (e.g. tumor cell) by illuminating them. This results in a killing field restricted by selective exposure to light. PDT has been approved by health regulatory agencies around the world for the treatment of a variety of cancers and pre-cancers including those of the skin, esophagus, lung, and head and neck. Clinical trials continue to expand the role of PDT in cancer and in the treatment of localized microbial infections, as reviewed in [3], [35]. The predominant type of ROS generated by the photosensitizer depends on the type of reaction and local oxygen concentrations, such that Type I reaction produces O2•− while Type II produces 1O2 [36]. Chemicals such as malachite, fluorescein, eosin, Rose Bengal and methylene blue have all been used as photosensitizers in PDT. Most of the photosensitizers approved for clinical use to date have been porphyrins, chlorins, or chemically related species [35], [37]. While these chemicals have a high efficiency to produce 1O2, improvement in the targeting and delivery of exogenous photosensitizers may facilitate PDT treatments.
An initial approach to restrict targeting of a chemical photosensitizer used malachite green conjugated to an antibody [38]. While this method capitalized on the large scale ROS production of a chemical sensitizer and specificity of immunological approaches it was limited by the necessity to generate a target antibody, conjugate it to the photosensitizer and apply it at selective concentrations. The development of biarsenical fluorophore methods bypassed immunological obstacles by utilizing a genetic –Cys–Cys–X–X–Cys–Cys– tag [39], [40]. Biarsenical derivatives of fluorescent molecules (e.g. fluorescein, FlAsH; resorufin, ReAsH) would bind with high affinity and specificity to the motif and upon illumination, generate ROS [39], [40]. These techniques advanced the targeting specificity of photosensitizers; however, they require the addition of exogenous chemicals, which may result in untagged sensitizers yielding nonspecific side reactions.
Genetically encoded ROS generating proteins (RGPs)
More recently, genetically-encoded ROS generators have been developed that circumvent the need for exogenous cofactors. The ability to target these proteins to various cellular locations (e.g. nucleus or lysosome) and cell types (e.g. intestine or neuronal) using transgenic technologies allows for temporal and spatial control of ROS production. Fluorescent proteins such as GFP have been used in numerous applications as cell, organelle and protein labels [41], [42]. Such widespread use required these fluorescent proteins to act as photochemically inert labels. Indeed, most GFP-related proteins are inefficient at producing ROS (Fig. 2); however, photochemically active versions have been discovered, and these reagents have the potential to open new avenues of research. The following sections will focus on genetically-encoded ROS generating proteins (RGPs), their application for cell ablation and protein inactivation, and their potential to study ROS signaling.
Fig. 2.
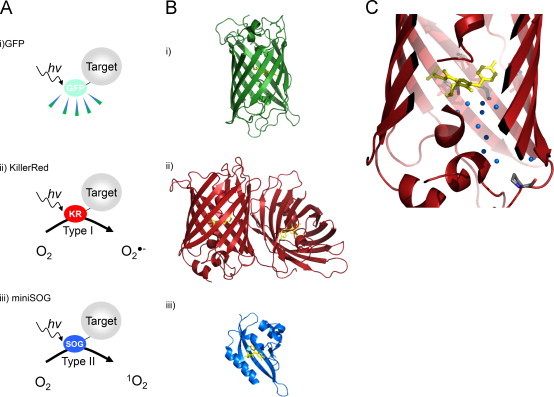
Optogenetic approaches and ROS generating proteins (RGPs). (A) Unique properties of fluorescent proteins. In general, fluorescent proteins such as GFP (i) are considered inert markers. Mutations in fluorescent proteins have yielded RGPs such as KillerRed (ii) and miniSOG (iii) capable of producing ROS via a type I or type II mechanism resulting in superoxide (O2•−) or singlet oxygen (1O2), respectively. 1O2 is a highly reactive ROS and its characteristics make it attractive use in cell ablation and chromophore assisted light inactivation (CALI) approaches. The RGP KillerRed (KR) produces O2•− upon illumination which can be decomposed through endogenous ROS scavenging pathways. (B) Structural differences in the RGPs. Structural consideration must be taken in to account when targeting a protein, for example GFP can be a monomer (i) while functional KillerRed is a dimer (ii). The size of the protein may also affect trafficking making the small size of miniSOG (iii) ideal. (C) Mechanism of Phototoxicity. Although similar in structure to other relatively inert fluorescent proteins, the mechanism of ROS production by KillerRed is currently unknown. Recent advances demonstrate a water (blue spheres) filled channel that reaches the chromophore (yellow) in KillerRed. In conjunction with other introduced mutations that may help maintain the excited state of the chromophore, it is suggested to partake in KillerRed׳s phototoxicity. Images of PDB IDs 1EMA [92], 2WIQ [56], 4EEP [93] were generated using PyMOL. The structure of miniSOG is currently not defined. Thus, the parent protein, LOV2 domain of Arabidopsis thaliana phototropin 2, was used in its place. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Variables that control the suitability of an RGP to exert a specific biological effect will depend on (i) the type of ROS produced and (ii) the location of ROS production. Discerning the dominant ROS produced by an RGP is best achieved by combining a variety of techniques. 1O2 can be detected by the time-dependent photobleaching of anthracenedipropionic acid (ADPA), monitoring the phosphorescence of 1O2, or by using chemiluminescent probes such as trans-1-(2׳-methoxyvinyl)pyrene [16], [43], [44]. O2•− phototoxicity can be followed using free radial probes (e.g. TEMPO) [45], [46] and fluorescent probes (e.g. DHE; reviewed in [47]), or alternatively in genetic model systems by assessing the consequences of manipulating levels of O2•− detoxifying enzymes [48]. Differentiating between 1O2 and O2•− derived damage involves comparing the phototoxic effect in H2O and deuterium oxide (D2O) based media. The lifetime of 1O2 is greater in D2O (~68 µs) [49] than H2O (~3.5 µs) [50], therefore 1O2 phototoxicity is suggested to increase in D2O based systems [45]. However, 1O2 is very reactive, such that the lifetime in a fluid solution may not translate into a complex cellular milieu. Thus, the lack of an effect in D2O may not necessarily exclude 1O2 in the mechanism of phototoxicity.
A distinct advantage of RGPs is their ability to be targeted to precise, defined locations in specific cells using transgenic technology, an advantage that is not shared with chemical photosensitizers. For example, the expression can be targeted to specific areas using commonly used signal sequences (e.g. mitochondrial targeting using the TOMM-20 targeting sequence or nuclear targeting using the SV40 nuclear localization signal). The initial fluorescence of the RGP preceding photobleaching can be used to confirm protein targeting. However, low copy RGP expression coupled with rapid photobleaching may not yield sufficient fluorescence to determine localization [16], [51] and immunodetection may be necessary. These parameters can in theory be optimized to regulate an RGP׳s impact. For example, the targeting of RGPs has been shown to cause varied responses to illumination: nuclear localization can prevent cell division [52] while plasma membrane targeting leads to cell death [17], as discussed in more detail below.
KillerRed
Although the yield of ROS from GFP is lower than that produced by chemical sensitizers [53], the ROS generated is sufficient to oxidize 3,3׳-diaminobenzidine (DAB) into a precipitate thereby allowing ultrastructural visualization using electron microscopy [54]. The low phototoxic effect of GFP is attributed to the structure of GFP, which shields the chromophore (Fig. 2). KillerRed, the first phototoxic fluorescent protein, was derived from a homolog of GFP, anm2CP, and produces ROS upon illumination with red light (excitation maximum of 585-nm; (Table 1)) [16]. The structure of KillerRed has a unique water-filled channel reaching the chromophore (Fig. 2), which may be responsible for its phototoxic nature [55], [56]. KillerRed is generally acknowledged to produce O2•− via a type I reaction [18], [55]. 1O2 is not detected or is negligible since KillerRed is unable to degrade ADPA upon illumination [18], [57], [58]. Furthermore, D2O does not increase KillerRed phototoxicity [51]. Finally, a recent study using Caenorhabditis elegans observed that manipulating expression of SOD-1, a O2•− detoxifying enzyme, affected phototoxicity [48]. Together, these observations support a type I reaction for KillerRed.
Table 1.
Light-induced ROS production can be through a variety of chemical and genetic photosensitizers. Each photosensitizer has unique properties and abilities to generate ROS. Quantum yield of 1O2 is defined as the number of photosensitized 1O2 molecules per absorbed photon.
| ROS producer | Monomer or dimer | Size (kDa) | Excitation (max) | Emission | Quantum yield of 1O2 |
|---|---|---|---|---|---|
| Malachite green | 628 | <0.003 [79] | |||
| GFP | Monomer | 27 | 395 | 475 | b |
| HBDI | 0.004 [80] | ||||
| FMN | 450 | 535 | 0.051 [18] | ||
| KillerRed [16] | Dimer | 27a | 585 | 610 | 0.000 [18] |
| SuperNova [45] | Monomer | 29 | 579 | 610 | |
| miniSOG | Monomer | 15.3 | 448 | 500/528 | 0.47 [18] |
| ReAsH [36] | 593 | 608 | 0.024 [18] | ||
| FlAsH [35] | 508 | 528 | |||
| Rose Bengal | 540 | 550–600 | 0.75 [18] |
Abbreviations as follows: HBDI, 4-hydroxybenzylidene-1,2-dimethylimidazoline (EGFP fluorophore); FMN, flavin mononucleotide.
Indicates the size of monomer.
Unquantifiable.
Active KillerRed is a dimer of two monomers, and the tendency to dimerize can affect localization, function and folding of fusion proteins [59]. For example, when fused to fibrillarin, KillerRed can cause improper localization resulting in fluorescence in the cytosol as opposed to the nuclear fluorescence observed with a fibrillarin::EGFP fusion protein [59]. Dimerization is particularly problematic for membrane bound proteins. One approach that circumvents dimerization is to use “tandem KillerRed” [52]. Tandem KillerRed is a pseudo-monomeric genetic fusion of two KillerRed coding sequences allowing for intramolecular dimerization and maturation of the protein [48], [52]. This approach has been successfully exploited to block cell division using tandem KillerRed fused to histone H2B [52]. Recently, SuperNova, a monomeric ROS generating protein (RGP), was generated using random mutagenesis of KillerRed [59]. SuperNova fusion proteins to fibrillarin, keratin, and connexin 43 demonstrated proper localization [59]. SuperNova has been used successfully as a photosensitizer [59]. The predominant ROS responsible for the phototoxic effects in SuperNova remains to be elucidated, since SuperNova has been postulated to produce both O2•− and 1O2, as measured using DHE and ADPA bleaching, respectively [59].
miniSOG
Recently, a new RGP was introduced as mini Singlet Oxygen Generator (miniSOG) [18]. miniSOG is a 106 amino acid green fluorescent flavoprotein generated from Arabidopsis phototropin 2 (Fig. 2) with an excitation maximum of 448-nm (Table 1) [18]. The small size of miniSOG facilitates protein tagging and is less likely to influence protein targeting than larger tags. MiniSOG requires a flavin mononucleuotide cofactor (Fig. 2), but unlike other types of protein tags where the cofactor must be added exogenously, the flavin mononucleotide is endogenously present in cells. Like SuperNova, miniSOG monomers are capable of generating 1O2 as determined by photobleaching of ADPA [18], but they have not been shown to generate O2•−. However, the ability to generate localized, reactive 1O2 in high yield has made this RGP an effective tool for both electron microscopy imaging and for inducing cell death [18], [60].
Applications
Photoablation
In a recent study, KillerRed was fused to an antibody to target tumor cells [51]. The resulting photoinduction of ROS resulted in specific tumor cell death [51]. This suggests that genetically-encoded photosensitizers may be useful for PDT [51], but the more obvious applicability is to basic research. This is especially true in model organisms such as the nematode C. elegans and mice where transgenesis, or the expression of recombinant transgenes, is routine. For example, optogenetic reagents such as ChR2 and HR are routinely used in these models as promoter-driven molecular switches to turn specific neurons on and off and to define their role in complex behaviors [15].
So what is the virtue of a genetic photosensitizer? One advantage is throughput: laser ablation has classically been used in C. elegans to define a cell׳s role in development or behavior. This approach is labor intensive and can only be performed on one animal at a time. However, more recent approaches have adopted RGPs for cell ablation in C. elegans, in which transgenic populations of worms can be exposed to light, and both KillerRed and miniSOG have been used for this purpose [48], [60].
With respect to cell specificity, some cell types may be predisposed to handle ROS and have an increased ROS scavenging mechanism. A recent study expressed plasma membrane-targeted tandem-KillerRed in different classes of C. elegans neurons and found individual neurons, such as the AVM mechanosensory neuron or the AWB amphid sensory neuron, that are more resistant to ROS than other neurons [48]. One suggested mechanism is a higher expression of ROS detoxifying enzymes such as SOD [48]. Similarly, the intracellular targeting of an RGP such as miniSOG may impact the efficiency of photoablation. For example, aconitase isoforms ACO-1 and ACO-2 are expressed in the cytoplasm and the mitochondrial matrix, respectively. Cytosol targeting of miniSOG through fusion to ACO-1 resulted in a weak phototoxic effect, while mitochondrial targeting through fusion to ACO-2 yielded more cell death [60]. Similarly, while the mitochondrion plays a role in mediating cell death, targeting different regions of the organelle with an RGP can result in variations in photoablation efficiency. For instance, a fusion between miniSOG and the N-terminus of a complex IV subunit (COX8a), which is found in the mitochondrial matrix, was less potent than outer membrane targeting using the N-terminus of TOMM-20 [60] (Fig. 3). Again, this may represent the localized ROS buffering capacity. Mitochondrial-specific isoforms of SOD may play a role, or perhaps the effect is related to the role of outer membrane permeabilization in the formation of the apoptosome [61].
Fig. 3.
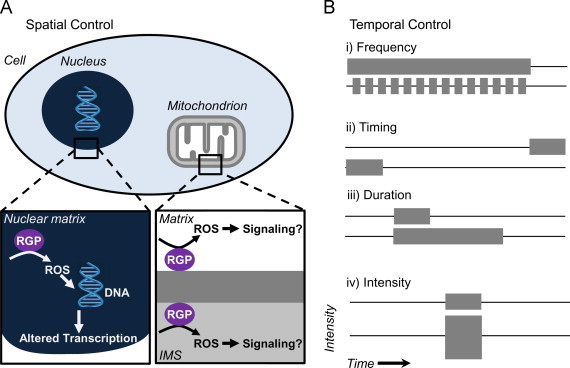
Spatial and temporal control of ROS production using Optogenetics. (A) Genetically encoded RGPs allow for spatial control. An RGP can be targeted to specific sites throughout the cell such as the nucleus or mitochondrion. The spatial control can induce ROS-mediated DNA modifications, subsequently, resulting in altered transcription. The characteristics of an individual ROS can restrict their distribution. For example, O2•− is a charged ROS that has limited permeability. Targeting a RGP to each side of a membrane can determine the relevant signaling environment. (B) Temporal control of ROS production. In addition to the site of formation, the temporal control of ROS production can affect the physiologic output. Illumination protocols which alter the frequency (i), timing (ii), duration (iii) or intensity (iv) can affect the propagation of ROS levels through particular environments, each with a particular ROS detoxifying mechanism. Abbreviation: IMS, intermembrane space.
The illumination parameters are also important (Fig. 3). As one would predict, phototoxic effects can be graded by modulating the duration and intensity of light [48], [60]. However, changing the light exposure from continuous to pulsed has also been shown to increase the effectiveness of cell ablation in C. elegans expressing outer membrane targeted miniSOG in motor neurons [60]. Pulsed light may allow for oxygen to diffuse into the RGP active site and thus produce more ROS. In applications for treating tumors in vivo, the transparency of the tissue and size of the tumor will become important aspects of the treatment protocol since light will need to penetrate deeper into the tissue to elicit ROS generation [62]. The penetration of light through the tissue will depend on the wavelength, such that 600–1200 nm is the most effective [3]. As such, the development of far-red-shifted variants of RGPs would greatly facilitate their clinical use. These and other observations suggest that the frequency, timing, duration, and intensity all contribute to the cytotoxicity of ROS, and this theme becomes even more important when considering ROS as a signaling molecule, as discussed below.
Chromophore-assisted light inactivation (CALI)
Chromophore-assisted light inactivation (CALI) uses ROS to selectively inactivate a protein of interest. Ideally, the type of ROS produced would be highly reactive and have a short diffusion distance (e.g. HO• or 1O2). In this regard, malachite green and FlAsH are effective agents [38], [39], [40]. However, RGPs increase the specificity of CALI by keeping protein of interest in close proximity to the photosensitizer in the form of a fusion protein [16], [17], [63]. Upon illumination, ROS sensitive residues in the target protein will be impacted by ROS, potentially resulting in altered protein function. CALI is a particularly powerful approach to investigate the acute loss- or gain-of-function of a protein in a living organism when knockout or over-expression of the protein is lethal. CALI requires exposing a chromophore to light with an optimized light dose and increased spatial precision to result in the acute inactivation of a target protein [64]. The specificity of CALI is paramount: ROS generated must react with the protein to which the photosensitizer is attached and titered to avoid collateral damage. Both KillerRed and miniSOG fusion proteins have been used successfully for CALI [16], [17], [63]. Published approaches for miniSOG fusion proteins utilize pulses on the order of minutes and light intensities in the 5–20 mW/mm2 range [63], [65]. Interestingly, protocols for photoablation incorporate reduced power (57 mW/cm2, or roughly ~25× less) for extended periods [60] which may reflect the intracellular targeting of RGP fusion proteins or, potentially, activation of cell death pathways by ROS. For example, RGP targeting to the outer mitochondrial membrane, which is a critical to apoptosis, was most efficient at inactivating neuronal cell function in C. elegans [60]. CALI requires selectivity, and recent advances demonstrate that miniSOG fusions to synaptic proteins (VAMP2 and SYP1) can inhibit neurotransmitter release while maintaining spatial specificity [63].
ROS signaling – a new frontier
An unexploited aspect of RGPs is their unique suitability to study ROS signaling. The following section speculates on future approaches enabled by this application. Global redox status is an important component of cell homeostasis, but transient, localized ROS production is increasingly being recognized as physiologically significant in its own right. Mechanistically, ROS can interact with redox-sensitive residues (e.g. thiols) and change shape or charge of the target protein resulting in modified activity. For example, ROS modification of thiols can activate matrix metalloproteinases [66], [67], and there are a variety of postulated redox sensors involved in metabolic plasticity [68], [69], [70], [71]. ROS can also activate or stabilize transcription factors such as Nrf2 or hypoxia-inducible factor-1α resulting in adaptation to stress conditions or increasing antioxidant defenses (for review, see [72], [73], [74], [75]). Depending on the type of reaction, these modifications may be reversible, as well [76].
There are various sources of ROS within the cell and an important point source for ROS is the mitochondrion. In addition to being the powerhouse of the cell, mitochondria also feature prominently in ROS signaling. Mitochondria can couple metabolism to ROS through modifications in enzymes [77], [78] and Krebs cycle intermediates [79], [80]. Different types of ROS have different diffusion capabilities and reactivity towards targets. O2•− and H2O2 are produced in a number of cellular reactions; however, the initial ROS generated by the mitochondrion is O2•− which is rapidly converted by superoxide dismutase (SOD) to freely diffusible H2O2 (Fig. 1). ROS generated in the mitochondrion can impact the cytosol and act as a second messenger though the process of “ROS-induced ROS release” [81]. During this process, ROS generated within a mitochondrion can surpass a threshold resulting in a transient increase in electron transport chain generated ROS which is ultimately released in the cytosol [81]. This process can foster ROS production and contribute to pathologies such as ischemia-reperfusion injury [81], [82].
The reactive nature of ROS makes studying their role in cellular responses difficult. The ability to target KillerRed to highly-localized domains makes it an attractive approach to mimic biological ROS production. More importantly, the site of generation or cellular compartment will also determine the physiological output of the ROS signal (Fig. 3). For example, the mitochondrial matrix contains unique ROS scavenging capabilities that are unique to the cellular cytosol [12]. Furthermore, the formation and scavenging of ROS in a local environment can contribute to levels of ROS that are dynamic (Fig. 4). The ability of generate ROS with RGPs could help understand ROS dynamics.
Fig. 4.
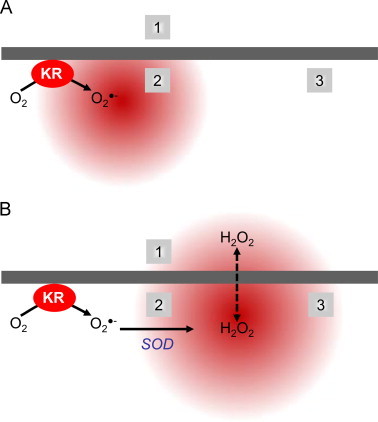
ROS specific signaling microdomains. (A) Superoxide׳s sphere of influence. Upon illumination KillerRed (KR) generates superoxide (O2•−). The concentration of O2•− can be decreased by spontaneous dismutation or SOD activity, such that the local levels of O2•− available to modify/signal are affected. Areas residing close to the site of O2•− generation (2) may see a higher local concentration of areas than those further away (3). Since O2•− is a charged molecule, membrane permeability is limited and sites separated by a membrane (1) may not be affected. (B) Hydrogen peroxide׳s sphere of influence. O2•− can be rapidly converted to hydrogen peroxide (H2O2). The mitochondrial matrix lacks catalase and combined with the ability to cross membranes, H2O2 concentrations may be able to diffuse to affect a greater area and have a larger sphere of influence such that all areas may be affected.
As an example, there are several sites of ROS production in the mitochondrial respiratory chain. A reasonable question to ask is whether ROS produced at each of these sites is equal in its impact on mitochondrial physiology or signaling capacity (Fig. 3). Similarly, mitochondria contain a matrix and an intermembrane space, and the concentration/expression of antioxidants and ROS scavengers may not be uniform between them [83]. Does it matter to the mitochondrion on which side of a membrane ROS is produced? The effect of mitochondrial ROS can extend beyond the mitochondrion. Recently, ROS were shown to modify DNA sequences in the hypoxia response element resulting in altered transcription [9]. What is the origin of these ROS and how does the nucleus handle them? Presumably, as our ability to reduce the sphere of ROS׳s influence through photoactivation of transgenic RGPs, our ability to answer these types of questions will increase.
Having a repertoire of RGPs that produce different types of ROS also diversifies the types of questions that we can ask. The different ROS have different signaling and damaging capabilities. For example, the type II mechanism of miniSOG is well-suited for CALI approaches and light-inducible loss-of-function models. Since 1O2 is highly reactive and cannot interconvert with endogenous ROS species, it is more likely to cause damage than to elicit signal transduction. However, the type I mechanism of KillerRed leads to the production of O2•− and should be amenable to endogenous detoxification mechanisms, resulting in diffusible ROS signaling molecules such as H2O2. Hence, the use of KillerRed will facilitate determining how a cell responds to “normal” local ROS production. In this respect, KillerRed fusions have been used to elicit photo-inducible changes in mitochondrial morphology [48]. Localized to the mitochondrial outer membrane via the TOMM-20 targeting, KillerRed was able to fragment the reticulated network of mitochondria in body wall muscle of C. elegans [48]. The fact that normal morphology was restored over time without significant organism deficits [48] suggests, like CALI approaches, that the amount of ROS is titratable on a sub-lethal scale. Interestingly, TOMM-20 targeting of miniSOG to the mitochondrial outer membrane caused efficient 1O2-mediated cell ablation [60]. Although these two experiments are not directly comparable due to differences in cell-specific RGP expression and illumination protocols, it would be intriguing to assess whether this difference in outcome is related to the specific type of ROS generated.
Other uses of KillerRed that may be related to ROS signaling include cell-specific membrane targeting in zebrafish, which revealed a dose-dependent relationship between damage and illumination [84]. When KillerRed expressed at the plasma membrane in the heart was illuminated for 5 min the larvae developed pericardial edema. When illumination time was increased to 8 min signs of apoptosis appeared in addition to edema [84]. Although ROS signaling was not directly assessed, it demonstrates that illumination of RGPs can be titrated to produce levels of ROS that can elicit a physiological output rather than overt cell death. Recent reports have used KillerRed-generated ROS to study mitophagy, which is the autophagic removal of mitochondria [85], [86]. Impaired mitochondria can induce Parkin translocation to the mitochondrion and initiate recruitment of autophagy machinery [87]. The optogenetic approach allows for the induction of ROS at specific mitochondria rather than widespread activation and may further our understanding of mitophagy in vivo [85], [86].
RGPs may also allow us to address existing controversies regarding ROS generation and handling. As a specific example, in vivo measurements using a permutated yellow fluorescent protein targeted to the mitochondrial matrix (mt-cpYFP) have been suggested to reflect spontaneous bursts of O2•− at the single mitochondrion level [88]. This phenomenon was termed “superoxide flashes” and is postulated to reflect specific aspects of mitochondrial metabolism. Changes in mt-cpYFP fluorescence responded as predicted to ROS scavengers and O2•− mimetics [88]. However, the nature of the mt-cpYFP flashes has been hotly debated [89], [90]. In particular, the high degree specificity of mt-cpYFP for O2•− has been questioned. One alternative proposes that the mt-cpYFP flashes are instead reflecting oscillations in mitochondrial pH [90]. An opportunity to challenge the controversy would be to use an RGP (e.g. KillerRed) and determine its effect on mt-cpYFP fluorescence. Modulating the frequency of light (e.g. continuous or pulsatile) would alter O2•− production, which, if the current theory is correct, would allow cause and effect to be assigned to mt-cpYFP flashes.
Conclusion
ROS play an important role in normal organism physiology and disease states [1]. ROS have two capabilities: (1) to cause damage through oxidative modifications and (2) to initiate signaling through modification of specific redox-sensitive moieties. Currently, it is difficult to distinguish between these capabilities independently. Most studies use the global administration of antioxidants or genetic ablation of ROS scavengers (e.g. SOD) to determine a role for ROS in a pathway or response. The advent of genetically encoded RGPs allows for the temporal and spatial control of ROS production. RGPs can be used to tag proteins of interest and expressed at physiologic levels [91]. Improvement in light titrations and delivery may allow for the use of RGPs to studying ROS with unmatched precision in live organisms, such as the genetically-amenable and optically transparent C. elegans model. Ultimately these probes may help define redox signaling pathways and help determine how their dysfunction relates to disease.
Acknowledgments
We thank Keith Nehrke and Paul S Brookes (University of Rochester Medical Center) for their valuable discussions and for critically reading the manuscript. We thank Chris Callan for her figure artwork and Nicholas Leioatts for generating the structure figures for KillerRed, GFP and the LOV2 domain. This work was funded by grants from the US National Institutes of Health R01CA068409 (THF), R01GM082483 (KN and PSB), R01NS064945 (KN), and the American Heart Association, Founder׳s Affiliate Postdoctoral Fellowship award to APW (11POST7290028).
References
- 1.Pieczenik S.R., Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007;83:84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Wojtovich A.P., Nadtochiy S.M., Brookes P.S., Nehrke K. Ischemic preconditioning: the role of mitochondria and aging. Exp. Gerontol. 2012;47:1–7. doi: 10.1016/j.exger.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agostinis P., Berg K., Cengel K.A., Foster T.H., Girotti A.W., Gollnick S.O., Hahn S.M., Hamblin M.R., Juzeniene A., Kessel D., Korbelik M., Moan J., Mroz P., Nowis D., Piette J., Wilson B.C., Golab J. Photodynamic therapy of cancer: an update. CA Cancer J. Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burwell L.S., Nadtochiy S.M., Brookes P.S. Cardioprotection by metabolic shut-down and gradual wake-up. J. Mol. Cell. Cardiol. 2009;46:804–810. doi: 10.1016/j.yjmcc.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagliaro P., Moro F., Tullio F., Perrelli M.G., Penna C. Cardioprotective pathways during reperfusion: focus on redox signaling and other modalities of cell signaling. Antioxid. Redox Signal. 2011;14:833–850. doi: 10.1089/ars.2010.3245. [DOI] [PubMed] [Google Scholar]
- 6.Penna C., Mancardi D., Rastaldo R., Pagliaro P. Cardioprotection: a radical view free radicals in pre and postconditioning. Biochim. Biophys. Acta. 2009;1787:781–793. doi: 10.1016/j.bbabio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Van Raamsdonk J.M., Hekimi S. Reactive oxygen species and aging in Caenorhabditis elegans: causal or casual relationship? Antioxid. Redox Signal. 2010;13:1911–1953. doi: 10.1089/ars.2010.3215. [DOI] [PubMed] [Google Scholar]
- 8.D׳Autreaux B., Toledano M.B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell. Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 9.Pastukh V.M., Swiger B.M., Reed D.J., Patel M.R., Bardwell G.C., Pastukh V.V., Alexeyev M.F., Gillespie M.N. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci. Signal. 2012;5:ra47. doi: 10.1126/scisignal.2002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins Y., Chouchani E.T., James A.M., Menger K.E., Cocheme H.M., Murphy M.P. Mitochondrial redox signalling at a glance. J. Cell Sci. 2012;125:801–806. doi: 10.1242/jcs.098475. [DOI] [PubMed] [Google Scholar]
- 11.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy M.P. Mitochondrial thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications. Antioxid. Redox Signal. 2012;16:476–495. doi: 10.1089/ars.2011.4289. [DOI] [PubMed] [Google Scholar]
- 13.Buettner G.R., Schafer F.Q. Free radicals, oxidants, and antioxidants. Teratology. 2000;62:234. doi: 10.1002/1096-9926(200010)62:4<234::AID-TERA10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Buettner G.R. Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide. Anticancer Agents Med. Chem. 2011;11:341–346. doi: 10.2174/187152011795677544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenno L., Yizhar O., Deisseroth K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bulina M.E., Chudakov D.M., Britanova O.V., Yanushevich Y.G., Staroverov D.B., Chepurnykh T.V., Merzlyak E.M., Shkrob M.A., Lukyanov S., Lukyanov K.A. A genetically encoded photosensitizer. Nat. Biotechnol. 2006;24:95–99. doi: 10.1038/nbt1175. [DOI] [PubMed] [Google Scholar]
- 17.Bulina M.E., Lukyanov K.A., Britanova O.V., Onichtchouk D., Lukyanov S., Chudakov D.M. Chromophore-assisted light inactivation (CALI) using the phototoxic fluorescent protein KillerRed. Nat. Protoc. 2006;1:947–953. doi: 10.1038/nprot.2006.89. [DOI] [PubMed] [Google Scholar]
- 18.Shu X., Lev-Ram V., Deerinck T.J., Qi Y., Ramko E.B., Davidson M.W., Jin Y., Ellisman M.H., Tsien R.Y. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011;9:e1001041. doi: 10.1371/journal.pbio.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schafer F.Q., Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd R.V., Hanna P.M., Mason R.P. The origin of the hydroxyl radical oxygen in the Fenton reaction. Free Radic. Biol. Med. 1997;22:885–888. doi: 10.1016/s0891-5849(96)00432-7. [DOI] [PubMed] [Google Scholar]
- 21.De Grey A.D. HO2•: the forgotten radical. DNA Cell Biol. 2002;21:251–257. doi: 10.1089/104454902753759672. [DOI] [PubMed] [Google Scholar]
- 22.Kessel D. Correlation between subcellular localization and photodynamic efficacy. J. Porphyr. Phthalocyanines. 2004;8:1009–1014. [Google Scholar]
- 23.Sevanian A., Hochstein P. Mechanisms and consequences of lipid peroxidation in biological systems. Annu. Rev. Nutr. 1985;5:365–390. doi: 10.1146/annurev.nu.05.070185.002053. [DOI] [PubMed] [Google Scholar]
- 24.Girotti A.W. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- 25.Girotti A.W. Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J. Photochem. Photobiol. B. 2001;63:103–113. doi: 10.1016/s1011-1344(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 26.Winterbourn C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 27.Doonan R., McElwee J.J., Matthijssens F., Walker G.A., Houthoofd K., Back P., Matscheski A., Vanfleteren J.R., Gems D. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev. 2008;22:3236–3241. doi: 10.1101/gad.504808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai D.F., Santana L.F., Vermulst M., Tomazela D.M., Emond M.J., MacCoss M.J., Gollahon K., Martin G.M., Loeb L.A., Ladiges W.C., Rabinovitch P.S. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cabreiro F., Ackerman D., Doonan R., Araiz C., Back P., Papp D., Braeckman B.P., Gems D. Increased life span from overexpression of superoxide dismutase in Caenorhabditis elegans is not caused by decreased oxidative damage. Free Radic. Biol. Med. 2011;51:1575–1582. doi: 10.1016/j.freeradbiomed.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cocheme H.M., Quin C., McQuaker S.J., Cabreiro F., Logan A., Prime T.A., Abakumova I., Patel J.V., Fearnley I.M., James A.M., Porteous C.M., Smith R.A., Saeed S., Carre J.E., Singer M., Gems D., Hartley R.C., Partridge L., Murphy M.P. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 2011;13:340–350. doi: 10.1016/j.cmet.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dikalova A.E., Bikineyeva A.T., Budzyn K., Nazarewicz R.R., McCann L., Lewis W., Harrison D.G., Dikalov S.I. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelso G.F., Porteous C.M., Coulter C.V., Hughes G., Porteous W.K., Ledgerwood E.C., Smith R.A., Murphy M.P. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J. Biol. Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 33.Abadir P.M., Foster D.B., Crow M., Cooke C.A., Rucker J.J., Jain A., Smith B.J., Burks T.N., Cohn R.D., Fedarko N.S., Carey R.M., O׳Rourke B., Walston J.D. Identification and characterization of a functional mitochondrial angiotensin system. Proc. Natl. Acad. Sci. USA. 2011;108:14849–14854. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith R.A., Hartley R.C., Murphy M.P. Mitochondria-targeted small molecule therapeutics and probes. Antioxid. Redox Signal. 2011;15:3021–3038. doi: 10.1089/ars.2011.3969. [DOI] [PubMed] [Google Scholar]
- 35.Brown S.B., Brown E.A., Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- 36.Ogilby P.R. Singlet oxygen: there is indeed something new under the sun. Chem. Soc. Rev. 2010;39:3181–3209. doi: 10.1039/b926014p. [DOI] [PubMed] [Google Scholar]
- 37.O׳Connor A.E., Gallagher W.M., Byrne A.T. Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009;85:1053–1074. doi: 10.1111/j.1751-1097.2009.00585.x. [DOI] [PubMed] [Google Scholar]
- 38.Liao J.C., Roider J., Jay D.G. Chromophore-assisted laser inactivation of proteins is mediated by the photogeneration of free radicals. Proc. Natl. Acad. Sci. USA. 1994;91:2659–2663. doi: 10.1073/pnas.91.7.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffin B.A., Adams S.R., Tsien R.Y. Specific covalent labeling of recombinant protein molecules inside live cells. Science. 1998;281:269–272. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- 40.Adams S.R., Campbell R.E., Gross L.A., Martin B.R., Walkup G.K., Yao Y., Llopis J., Tsien R.Y. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J. Am. Chem. Soc. 2002;124:6063–6076. doi: 10.1021/ja017687n. [DOI] [PubMed] [Google Scholar]
- 41.Chudakov D.M., Lukyanov S., Lukyanov K.A. Fluorescent proteins as a toolkit for in vivo imaging. Trends Biotechnol. 2005;23:605–613. doi: 10.1016/j.tibtech.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Giepmans B.N., Adams S.R., Ellisman M.H., Tsien R.Y. The fluorescent toolbox for assessing protein location and function. Science. 2006;312:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- 43.Lindig B., Rodgers M.A., Schaap A.P. Determination of the lifetime of singlet oxygen in D2O using 9,10-anthracenedipropionic acid, a water-soluble probe. J. Am. Chem. Soc. 1980;102:5590–5593. [Google Scholar]
- 44.Redmond R.W., Heihoff K., Braslavsky S.E., Truscott T.G. Thermal-lensing and phosphorescence studies of the quantum yield and lifetime of singlet molecular oxygen (1 delta g) sensitized by hematoporphyrin and related porphyrins in deuterated and non-deuterated ethanols. Photochem. Photobiol. 1987;45:209–213. doi: 10.1111/j.1751-1097.1987.tb05365.x. [DOI] [PubMed] [Google Scholar]
- 45.Vegh R.B., Solntsev K.M., Kuimova M.K., Cho S., Liang Y., Loo B.L., Tolbert L.M., Bommarius A.S. Reactive oxygen species in photochemistry of the red fluorescent protein “KillerRed”. Chem. Commun. (Camb.) 2011;47:4887–4889. doi: 10.1039/c0cc05713d. [DOI] [PubMed] [Google Scholar]
- 46.Buettner G.R., Mason R.P. Spin-trapping methods for detecting superoxide and hydroxyl free radicals in vitro and in vivo. Methods Enzymol. 1990;186:127–133. doi: 10.1016/0076-6879(90)86101-z. [DOI] [PubMed] [Google Scholar]
- 47.Kalyanaraman B., Darley-Usmar V., Davies K.J., Dennery P.A., Forman H.J., Grisham M.B., Mann G.E., Moore K., Roberts L.J., Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic. Biol. Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams D.C., El B.R., Ramirez P.M., Coakley S., Kim S.A., Lee H., Wen Q., Samuel A., Lu H., Hilliard M.A., Hammarlund M. Rapid and permanent neuronal inactivation in vivo via subcellular generation of reactive oxygen with the use of KillerRed. Cell Rep. 2013 doi: 10.1016/j.celrep.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogilby P.R., Foote C.S. Chemistry of singlet oxygen. 36. Singlet molecular oxygen luminescence in solution following pulsed laser excitation. Solvent deuterium isotope effects on the lifetime of singlet oxygen. J. Am. Chem. Soc. 1981;104:2069–2070. [Google Scholar]
- 50.Egorov S.Y., Kamalov V.F., Koroteev N.I., Kransnovsky A.A., Toleutaev B.N., Zinukov S.V. Rise and decay of kinetics of photosensitized singlet oxygen luminescence in water. Measurements with nanosecond time-correlated single photon counting technique. Chem. Phys. Lett. 1989;163:421–424. [Google Scholar]
- 51.Serebrovskaya E.O., Edelweiss E.F., Stremovskiy O.A., Lukyanov K.A., Chudakov D.M., Deyev S.M. Targeting cancer cells by using an antireceptor antibody-photosensitizer fusion protein. Proc. Natl. Acad. Sci. USA. 2009;106:9221–9225. doi: 10.1073/pnas.0904140106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serebrovskaya E.O., Gorodnicheva T.V., Ermakova G.V., Solovieva E.A., Sharonov G.V., Zagaynova E.V., Chudakov D.M., Lukyanov S., Zaraisky A.G., Lukyanov K.A. Light-induced blockage of cell division with a chromatin-targeted phototoxic fluorescent protein. Biochem. J. 2011;435:65–71. doi: 10.1042/BJ20101217. [DOI] [PubMed] [Google Scholar]
- 53.Dixit R., Cyr R. Cell damage and reactive oxygen species production induced by fluorescence microscopy: effect on mitosis and guidelines for non-invasive fluorescence microscopy. Plant J. 2003;36:280–290. doi: 10.1046/j.1365-313x.2003.01868.x. [DOI] [PubMed] [Google Scholar]
- 54.Grabenbauer M., Geerts W.J., Fernadez-Rodriguez J., Hoenger A., Koster A.J., Nilsson T. Correlative microscopy and electron tomography of GFP through photooxidation. Nat. Methods. 2005;2:857–862. doi: 10.1038/nmeth806. [DOI] [PubMed] [Google Scholar]
- 55.Pletnev S., Gurskaya N.G., Pletneva N.V., Lukyanov K.A., Chudakov D.M., Martynov V.I., Popov V.O., Kovalchuk M.V., Wlodawer A., Dauter Z., Pletnev V. Structural basis for phototoxicity of the genetically encoded photosensitizer KillerRed. J. Biol. Chem. 2009;284:32028–32039. doi: 10.1074/jbc.M109.054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carpentier P., Violot S., Blanchoin L., Bourgeois D. Structural basis for the phototoxicity of the fluorescent protein KillerRed. FEBS Lett. 2009;583:2839–2842. doi: 10.1016/j.febslet.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 57.Serebrovskaya E.O., Edelweiss E.F., Stremovskiy O.A., Lukyanov K.A., Chudakov D.M., Deyev S.M. Targeting cancer cells by using an antireceptor antibody-photosensitizer fusion protein. Proc. Natl. Acad. Sci. USA. 2009;106:9221–9225. doi: 10.1073/pnas.0904140106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vegh R.B., Solntsev K.M., Kuimova M.K., Cho S., Liang Y., Loo B.L., Tolbert L.M., Bommarius A.S. Reactive oxygen species in photochemistry of the red fluorescent protein “KillerRed”. Chem. Commun. (Camb.) 2011;47:4887–4889. doi: 10.1039/c0cc05713d. [DOI] [PubMed] [Google Scholar]
- 59.Takemoto K., Matsuda T., Sakai N., Fu D., Noda M., Uchiyama S., Kotera I., Arai Y., Horiuchi M., Fukui K., Ayabe T., Inagaki F., Suzuki H., Nagai T. SuperNova, a monomeric photosensitizing fluorescent protein for chromophore-assisted light inactivation. Sci. Rep. 2013;3:2629. doi: 10.1038/srep02629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qi Y.B., Garren E.J., Shu X., Tsien R.Y., Jin Y. Photo-inducible cell ablation in Caenorhabditis elegans using the genetically encoded singlet oxygen generating protein miniSOG. Proc. Natl. Acad. Sci. USA. 2012;109:7499–7504. doi: 10.1073/pnas.1204096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riedl S.J., Salvesen G.S. The apoptosome: signalling platform of cell death. Nat. Rev. Mol. Cell. Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- 62.Ryumina A.P., Serebrovskaya E.O., Shirmanova M.V., Snopova L.B., Kuznetsova M.M., Turchin I.V., Ignatova N.I., Klementieva N.V., Fradkov A.F., Shakhov B.E., Zagaynova E.V., Lukyanov K.A., Lukyanov S.A. Flavoprotein miniSOG as a genetically encoded photosensitizer for cancer cells. Biochim. Biophys. Acta. 2013;1830:5059–5067. doi: 10.1016/j.bbagen.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 63.Lin J.Y., Sann S.B., Zhou K., Nabavi S., Proulx C.D., Malinow R., Jin Y., Tsien R.Y. Optogenetic inhibition of synaptic release with chromophore-assisted light inactivation (CALI) Neuron. 2013;79:241–253. doi: 10.1016/j.neuron.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacobson K., Rajfur Z., Vitriol E., Hahn K. Chromophore-assisted laser inactivation in cell biology. Trends Cell Biol. 2008;18:443–450. doi: 10.1016/j.tcb.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou K., Stawicki T.M., Goncharov A., Jin Y. Position of UNC-13 in the active zone regulates synaptic vesicle release probability and release kinetics. Elife. 2013:2. doi: 10.7554/eLife.01180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ra H.J., Parks W.C. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okamoto T., Akaike T., Nagano T., Miyajima S., Suga M., Ando M., Ichimori K., Maeda H. Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: a novel mechanism for procollagenase activation involving nitric oxide. Arch. Biochem. Biophys. 1997;342:261–274. doi: 10.1006/abbi.1997.0127. [DOI] [PubMed] [Google Scholar]
- 68.Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tretter L., dam-Vizi V. Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:2335–2345. doi: 10.1098/rstb.2005.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gardner P.R., Nguyen D.D., White C.W. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc. Natl. Acad. Sci. USA. 1994;91:12248–12252. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brookes P.S., Freeman R.S., Barone M.C. A shortcut to mitochondrial signaling and pathology: a commentary on “Nonenzymatic formation of succinate in mitochondria under oxidative stress”. Free Radic. Biol. Med. 2006;41:41–45. doi: 10.1016/j.freeradbiomed.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 72.Kang K.W., Lee S.J., Kim S.G. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid. Redox Signal. 2005;7:1664–1673. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- 73.Fourquet S., Guerois R., Biard D., Toledano M.B. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J. Biol. Chem. 2010;285:8463–8471. doi: 10.1074/jbc.M109.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guzy R.D., Sharma B., Bell E., Chandel N.S., Schumacker P.T. Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol. Cell Biol. 2008;28:718–731. doi: 10.1128/MCB.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kietzmann T., Gorlach A. Reactive oxygen species in the control of hypoxia-inducible factor-mediated gene expression. Semin. Cell Dev. Biol. 2005;16:474–486. doi: 10.1016/j.semcdb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 76.Hashemy S.I., Holmgren A. Regulation of the catalytic activity and structure of human thioredoxin 1 via oxidation and S-nitrosylation of cysteine residues. J. Biol. Chem. 2008;283:21890–21898. doi: 10.1074/jbc.M801047200. [DOI] [PubMed] [Google Scholar]
- 77.Patel M.S., Korotchkina L.G. Regulation of the pyruvate dehydrogenase complex. Biochem. Soc. Trans. 2006;34:217–222. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- 78.Wang S., Song P., Zou M.H. AMP-activated protein kinase, stress responses and cardiovascular diseases. Clin. Sci. (Lond.) 2012;122:555–573. doi: 10.1042/CS20110625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fedotcheva N.I., Sokolov A.P., Kondrashova M.N. Nonezymatic formation of succinate in mitochondria under oxidative stress. Free Radic. Biol. Med. 2006;41:56–64. doi: 10.1016/j.freeradbiomed.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 80.Wojtovich A.P., Brookes P.S. The endogenous mitochondrial complex II inhibitor malonate regulates mitochondrial ATP-sensitive potassium channels: implications for ischemic preconditioning. Biochim. Biophys. Acta. 2008;1777:882–889. doi: 10.1016/j.bbabio.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial ROS-induced ROS release: an update and review. Biochim. Biophys. Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 82.Zinkevich N.S., Gutterman D.D. ROS-induced ROS release in vascular biology: redox-redox signaling. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H647–H653. doi: 10.1152/ajpheart.01271.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herrmann J.M., Riemer J. The intermembrane space of mitochondria. Antioxid. Redox Signal. 2010;13:1341–1358. doi: 10.1089/ars.2009.3063. [DOI] [PubMed] [Google Scholar]
- 84.Teh C., Chudakov D.M., Poon K.L., Mamedov I.Z., Sek J.Y., Shidlovsky K., Lukyanov S., Korzh V. Optogenetic in vivo cell manipulation in KillerRed-expressing zebrafish transgenics. BMC Dev. Biol. 2010;10:110. doi: 10.1186/1471-213X-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choubey V., Safiulina D., Vaarmann A., Cagalinec M., Wareski P., Kuum M., Zharkovsky A., Kaasik A. Mutant A53T alpha-synuclein induces neuronal death by increasing mitochondrial autophagy. J. Biol. Chem. 2011;286:10814–10824. doi: 10.1074/jbc.M110.132514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang J.Y., Yang W.Y. Spatiotemporally controlled initiation of Parkin-mediated mitophagy within single cells. Autophagy. 2011;7:1230–1238. doi: 10.4161/auto.7.10.16626. [DOI] [PubMed] [Google Scholar]
- 87.Vives-Bauza C., Zhou C., Huang Y., Cui M., de Vries R.L., Kim J., May J., Tocilescu M.A., Liu W., Ko H.S., Magrane J., Moore D.J., Dawson V.L., Grailhe R., Dawson T.M., Li C., Tieu K., Przedborski S. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl. Acad. Sci. USA. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang W., Fang H., Groom L., Cheng A., Zhang W., Liu J., Wang X., Li K., Han P., Zheng M., Yin J., Wang W., Mattson M.P., Kao J.P., Lakatta E.G., Sheu S.S., Ouyang K., Chen J., Dirksen R.T., Cheng H. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wei L., Dirksen R.T. Perspectives on: SGP symposium on mitochondrial physiology and medicine: mitochondrial superoxide flashes: from discovery to new controversies. J. Gen. Physiol. 2012;139:425–434. doi: 10.1085/jgp.201210790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schwarzlander M., Murphy M.P., Duchen M.R., Logan D.C., Fricker M.D., Halestrap A.P., Muller F.L., Rizzuto R., Dick T.P., Meyer A.J., Sweetlove L.J. Mitochondrial ‘flashes’: a radical concept repHined. Trends Cell Biol. 2012;22:503–508. doi: 10.1016/j.tcb.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 91.Frokjaer-Jensen C., Davis M.W., Hopkins C.E., Newman B.J., Thummel J.M., Olesen S.P., Grunnet M., Jorgensen E.M. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat. Genet. 2008;40:1375–1383. doi: 10.1038/ng.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ormo M., Cubitt A.B., Kallio K., Gross L.A., Tsien R.Y., Remington S.J. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 1996;273:1392–1395. doi: 10.1126/science.273.5280.1392. [DOI] [PubMed] [Google Scholar]
- 93.Christie J.M., Hitomi K., Arvai A.S., Hartfield K.A., Mettlen M., Pratt A.J., Tainer J.A., Getzoff E.D. Structural tuning of the fluorescent protein iLOV for improved photostability. J. Biol. Chem. 2012;287:22295–22304. doi: 10.1074/jbc.M111.318881. [DOI] [PMC free article] [PubMed] [Google Scholar]


