Abstract
Endomorphin 1 (EM-1) and endomorphin 2 (EM-2) were tested for their capacity to alter immune function. Addition of either of these peptides to murine spleen cells in vitro inhibited antibody formation to sheep red blood cells in a bi-phasic dose dependent manner. Maximal inhibition was achieved at doses in the range of 10−13 to 10−15 M. Neither naloxone (general opioid receptor antagonist) nor CTAP (selective mu opioid receptor antagonist) blocked the immunosuppressive effect. To show that there was specificity to the immunosuppressive activity of the peptides, affinity purified rabbit antibodies were raised against each of the synthetic EM peptides haptenized to KLH and tested for capacity to inhibit immunosuppression. Antibody responses were monitored by a standard solid phase antibody capture ELISA assay, and antibodies were purified by immunochromatography using the synthetic peptides coupled to a Sepharose 6B resin. Verification of the specificity of affinity-purified antisera was performed by immunodot-blot and solid-phase RIA assays. The antisera specific for both EM-1 and EM-2 neutralized the immunosuppressive effects of their respective peptides in a dose-related manner. Control normal rabbit IgG had no blocking activity on either EM-1 or EM-2. These studies show that the endomorphins are immunomodulatory at ultra-low concentrations, but the data do not support a mechanism involving the mu opioid receptor.
Introduction
Endomorphin 1 (EM-1) and endomorphin 2 (EM-2) are two C-terminal amidated tetrapeptides, first isolated from bovine brain (Zadina et al., 1997) and then from human brain cortex (Hackler et al., 1997). Endomorphins (EMs) display the highest selectivity and affinity for the mu-opioid receptor (MOR) in the brain (Zadina et al., 1997) and produce a dose-dependent antinociception after i.c.v (Zadina et al., 1997) or i.t. injection in mice, which is blocked by pretreatment with CTAP, naloxone, and/or funaltrexamine (β-FNA) (Goldberg et al., 1998; Soignier et al., 2000; Huang et al., 2000; Przewlocka et al., 1999; Przewlocki et al., 1999; Stone et al., 1997; Ohsawa et al., 2001). Based on the extensive data showing the anatomical distribution of EM-like immunoreactivity, near the localization of MORs in several areas of the rat brain (Martin-Schild et al., 1997; Pierce et al., 1998; Schreff et al., 1998; Zadina, 2002), including primary afferents and their terminals in the spinal cord dorsal horn (Pierce et al., 1998; Schreff et al., 1998), both peptides have been implicated in the natural modulation of nociceptive transmission and pain (Zadina et al., 1997; Przewlocka et al., 1999; Przewlocki et al., 1999). At the cellular level, EMs have been found to activate G proteins (Alt et al., 1998; Sim et al., 1998; Harrison et al., 1998; Monory et al., 2000), regulate different types of adenylyl cyclase isoenzymes (Nevo et al., 2000), inhibit membrane-calcium currents (Mima et al., 1997; Higashida et al., 1998), activate inward K+ currents (Gong et al., 1998), and modulate the differential expression of MOR mRNA and MOR function in SHSY-5Y cells (Yu et al., 2003). Moreover, these peptides display many physiological activities normally attributed to opiate alkaloids, such as pain modulation (Przewlocka et al., 1999; Przewlocki et al., 1999; Ohsawa et al., 2001; Zadina, 2002), feeding responses (Asakawa et al., 1998), oxygen consumption (Asakawa et al., 2000), vasodepressor and cardiorespiratory regulation (Champion et al., 1997; Kwok and Dun, 1998; Czapala et al., 2000), neuroendocrine modulation (Coventry et al., 2001; Doi et al., 2001), learning and memory behavioral responses (Ukai et al., 2001), and immune regulation (Azuma and Ohura, 2002b)
EMs have been shown to be present in cells and tissues of the immune system (Jessop et al., 2000; Jessop et al., 2002; Mousa et al., 2002; Seale et al., 2004), and to alter a variety of immune parameters (Azuma et al., 2000; Azuma et al., 2002; Azuma and Ohura, 2002a; Azuma and Ohura, 2002b). We extend these studies by examining the effect of EM-1 and EM-2 on the capacity of mouse spleen cells to mount an in vitro antibody response and show that these opioid peptides are immunosuppressive at ultra-low doses in the femtomolar range. Further, their immunosuppressive activity is not blocked by naloxone or CTAP, indicating that the peptides are not acting via the mu opioid receptor.
Materials and Methods
Animals
New Zealand White male 2.5 kg rabbits were purchased from Harlan S.A., Mexico.
Six week-old, specific pathogen-free C3HeB/FeJ female mice were purchased from Jackson Laboratories (Bar Harbor, Maine).
Source of reagents
The Peptide Chemical Synthesis Program of the National Institute of Mental Health (Bethesda, MD) generously donated the synthetic EM-1 and EM-2 for immunization and antibody production. Peptide was synthesized on 2-chlorotrityl resin (AnaSpec, San Jose, CA) using standard Fmoc solid phase procedures (Hockfield et al., 1993). Purity was achieved with reverse-phase, high performance liquid chromatography (HPLC) and fast atom bombardment mass spectroscopy (FAB) was used to determine structural homogeneity and peptide purity. EM-1 and EM-2 used for in vitro assays of antibody production were obtained from Research Biochemicals International, Natick, MA. Naloxone was obtained from Endo Pharmaceuticals, Chadds Ford, PA. CTAP (D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2) was obtained from Multiple Peptide Systems, San Diego, CA. Normal rabbit serum was purchased from BD Biosciences, Franklin Lakes, NJ.
Production of rabbit polyclonal antibodies to EMs
For immunization, either EM-1 or EM-2 were coupled to the carrier protein, keyhole limpet hemocyanin (KLH, Sigma Chemical Co., St. Louis, MO) using a standard covalent coupling procedure with glutaraldehyde (Harlow et al., 1988). In brief, 5 mg of peptide was dissolved in 1 ml of phosphate-buffered saline (PBS), pH 7.2. The peptide fragment was covalently conjugated to 50 mg of the carrier protein using a final concentration of 0.2% glutaraldehyde. After 1 hr incubation at room temperature, the cross-linking reaction was terminated by adding to the conjugate solution a final concentration of 0.1 M glycine. The conjugate was dialyzed for 48 hr against PBS to remove the unreacted aldehydes, diluted with PBS to the equivalent of 1 mg/ml of conjugate, frozen and stored at −20° C until use. Antisera to the conjugated peptides were raised in New Zealand White male rabbits. Animals were initially immunized with a subcutaneous injection of 2 ml of a 1:1 emulsion of 1 mg/ml PBS solution of conjugate with Complete Freund’s Adjuvant (CFA; Sigma). Twelve sequential boosts were carried out at one-month intervals using 1 ml of a 1:1 emulsion of 0.5 mg/ml conjugate in PBS in IFA. Rabbits were usually bled 2 weeks after each boost. The collected blood was centrifuged at 3000 rpm at 4° C and sera stored at −20° C until use.
Immunopurification of anti-EM polyclonal antibodies
Antigen affinity-purified antisera (A-APA) for EM-1 and EM-2 were prepared according to a standard chromatographic protocol described by Harlow and Lane (1988) using the peptides as antigens coupled to epoxy-activated Sepharose 6B resin (Amersham Pharmacia Biotech, NJ). Covalent coupling of peptides to resin was carried out according to a standard protocol described by the resin’s manufacturer. In brief, 5 mg of peptide was dissolved in 10 ml of a solution of 0.1 M sodium carbonate (pH 11), and incubated for 24 hr at 4° C with 5 ml of drained resin. After coupling, the peptide-resin conjugate was washed on a sintered funnel with 300 ml of 0.1 M Tris-HCl, pH 8. The remaining reactive epoxy-groups were blocked by a 24 hr incubation at room temperature of the peptide-resin fraction in a solution of 0.1 M triethanolamine, pH 8, and then washed 3X with PBS, pH 7.4. For affinity purification, fractions of 5 ml of whole, reactive antisera were diluted with 45 ml of 10 mM Tris-HCl, pH 7.5, and incubated for 24 hr at 4° C with 5 ml of drained peptide-resin solid phase. Specific antibodies were recovered from the column solid phase by elution with 5 gel volumes of a solution of 3 M potassium thiocyanate (KSCN) in 50 mM Tris-HCl, pH 8. The eluents were extensively dialysed against PBS/0.1% sodium azide, and stabilized with the addition of 4 mg/ml bovine serum albumin (BSA) and 50% glycerol. Aliquots of purified antibodies were stored at −20° C until use. The recovery of specific antibodies after immunopurification was verified by a standard antibody capture ELISA. C-14 antibody was raised using EM-1 as the antigen, while C-16 antibody was raised using EM-2 as antigen.
Specificity of A-APA for EM-1 and EM-2 by dot blots
Dot blot assays were initially used to screen A-APA C-14 and C-16 specificities to EM-1 and EM-2, respectively. Competitive peptide antigens such as Substance P, Met-enkephalin, Leu-enkephalin, and Tyr-W-MIF-1 were also tested as potential cross-reactive antigens. Tyr-W-MIF-1 is an opioid peptide extracted from bovine hypothalamus and human cortex (Hackler et al., 1993; Hackler et al., 1994). Synthetic peptides of EM-1 and EM-2 were used as homologous antigens to evaluate the cross-reactivity of both A-APA C-14 and C-16 antisera. The immunodot-bloting followed the procedures of Martin-Schild (Martin-Schild et al., 1997), as adapted from Loi (Loi et al., 1997). Briefly, 0.22 μ pore size PVDF Immunolon membranes (Millipore, Billerica, MA) were soaked in 100% methanol, dried out at room temperature, and washed in 10 mM PBS for 15 minutes. Peptides were initially diluted in 50% methanol (Mallinckrodt Baker, Phillipsburg, NJ) to obtain stock dilutions ranging from 2 × 10−2 to 2 × 10−7 M. 1.0 μl of these dilutions (2 × 10−9 to 2 × 10−14 M) were spotted on the membranes, which were then incubated 2 hours in a blocking solution of 3% teleostan gelatin (Sigma) in 10 mM PBS containing 0.05% Tween-20 (Sigma) (PBS-Tween). Membranes were then incubated overnight, with mild agitation, in solutions of C-14 (2.5 mg/ml) or C-16 (2.4 mg/ml) antibody in the blocking solution. Afterwards, membranes were washed 5X in PBS-Tween, 10 minutes per wash, in order to remove excessive non-bound primary antibody. Membranes were then incubated 2 hours in a 1:2000 dilution of Biotinylated goat anti-rabbit antibody (Jackson Immunoresearch, West Grove, PA) in PBS-Tween. Membranes were washed 5X in PBS-Tween, 10 minutes per wash, then incubated 1 hr in avidin-biotin complex solution (Vector Labs, Burlingame, CA) in PBS-Tween. After washing as done previously, membranes were developed by incubation in a solution of 0.03% DAB (Sigma) with 0.0003% H2O2.
Solid-phase radioimmunoassay (RIA) for EM1 and 2
A solid-phase radioimmunoassay (RIA) for EM-1 and EM-2, sensitive in the fmol range, was adapted from standard procedures previously described by Hockfield et al. (1993). Briefly, synthetic EM-1 or EM-2 were iodinated using the chloramine-T method and purified by HPLC using Hypersil wide-pore 5-μm, C-8/2 × 150 mm columns. The solid phase of the assay was prepared by adding a 100 of μl solution containing 500 ng of Protein-A (SIGMA)/0.1 M NaHCO3, pH 9, to Immunolon II-removable wells (VWR), followed by washing with RIA buffer (0.15 M K2HPO4, 0.2% Tween-20, 0.1% gelatin, pH 7.5). Treated wells were incubated for 2 h with 50-μl of a solution of RIA buffer containing a 1:30 dilution of C-14 A-APA, or a 1:20 dilution of C-16 A-APA. (These dilutions were determined previously to provide approximately 20–30 % binding). After removing the antibody solution and washing with RIA buffer, 50 μl of RIA buffer containing competitive peptides (0.1 fmol-0.5 nmol/well, in quadruplicate), plus radiolabeled EM-1 and EM-2 as peptide tracers, were incubated with adsorbed antibody for 2 h at room temperature. Typical standard displacement curves used approximately 5000 c.p.m. of [125I]-EM-1 or [125I]-EM-2 as tracer plus non-labeled EM-1, EM-2, or structurally related peptides to EMs in competitive RIAs. Treated wells were washed-out and counted for 4 min in a ten-channel gamma counter ISO DATA 500 (Hewlett-Packard).
Secondary in vitro antibody response
14 days prior to harvest of spleens, mice were primed with an intraperitoneal (ip) injection of 0.2 ml of a 10% (v/v) suspension of sheep red blood cells (SRBC) (Rockland, Gilbertsville, PA) in PBS. Immune function was assessed using an in vitro plaque-forming cell (PFC) assay, which measures the capacity of spleen cells to mount a secondary antibody response to sheep red blood cells (SRBCs) according to the method of Mishell and Dutton (Mishell and Dutton, 1967). Mice were sacrificed and their spleens aseptically removed. A single cell suspension of spleen cells was obtained by pushing the spleen through nylon mesh bags in RPMI supplemented with 5% fetal bovine serum (FBS). Red blood cells were lysed by hypotonic shock with sterile water. Cells were washed twice and resuspended in tissue culture medium consisting of RPMI supplemented with 10% FBS, 2mM L-glutamine, 50 μg/ml gentamicin, 1 mM nonessential amino acids, 1 mM sodium pyruvate, 10 μg/ml of adenosine, uridine, cytosine, and guanosine, and 0.05 mM of 2-mercaptoethanol. The cells were counted and resuspended to 1.0 × 107 cells/ml and dispensed into flat-bottom 24-well tissue culture plates (Corning Costar). Cultures were treated with dilutions of polyclonal anti-EM-1 or anti-EM-2 for 2 hours, after which EM-1 or EM-2 was added at concentrations ranging from 10−7 M to 10−21 M. Each condition was done in triplicate. For untreated spleen cells, at least nine replicate wells were used to establish the normal, baseline response level. In experiments using the inhibitors, naloxone or CTAP, they were added at 10−6 M 2 hr before the EM-1 or EM-2. Cultures were incubated for 5 days. On day 5, cells were harvested, washed in RPMI, and the number of direct PFCs (cells producing IgM antibodies against SRBCs) quantitated using the Cunningham modification of the Jerne hemolytic plaque assay (Cunningham and Szenberg, 1968). Results are expressed as a Suppression Index, where untreated spleen cells are given a value of 1.00 (100%), and responses of cultures receiving treatment with EMs are calculated as:
Statistics
The dependent variable, Suppression Index, was treated as a continuous variable for all analyses. The null hypothesis was that there would be no difference between group or dose. As the data were significantly non-normal using the Shapiro-Wilk test, a ‘normalized-rank’ transformation was applied to the suppression index in order to apply ANOVA methods. The rank-transformed data was analyzed using a two factor ANOVA followed by multiple comparisons to detect significant differences between means (groups and doses). Multiple pair-wise comparisons (groups and doses) were based on the least significant difference method) using type I error of 0.05 for statistical significance.
Results
Effect of EM-1 and EM-2 on secondary PFC responses
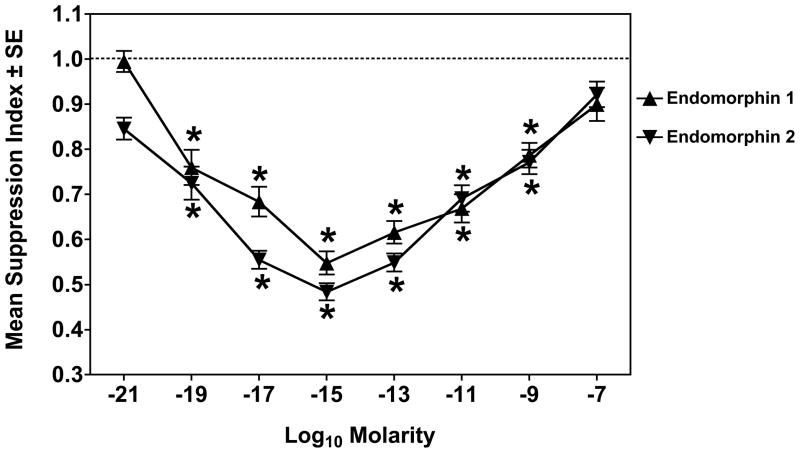
Figure 1 shows the effect of EM-1 and EM-2 on the secondary PFC response of mouse spleen cells. Each EM was titrated over a wide range of doses. As compared to the PFC response of untreated cells, there is a biphasic response to both EMs, with maximal immunosuppression of approximately 50% of control seen at a concentration range of 10−13 to 10−15 M.
Figure 1.
EM-1 and EM-2 are immunosuppressive. Dose response curves of EM-1 (▲) and EM-2 (▼) in a secondary PFC assay. Data are the pooled results from 12 experiments. *Significantly different from control (1.0) by 95% confidence limit.
Mu opioid receptor antagonists do not block immunosuppression by EM-1 or EM-2
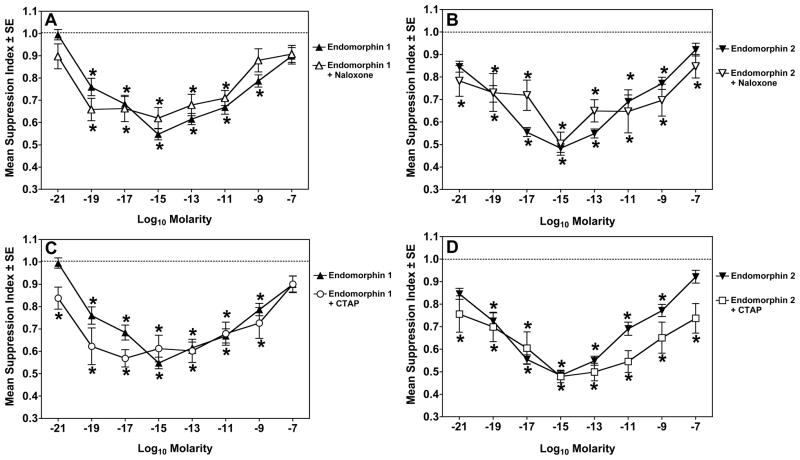
To determine if the activity of the endomorphin peptides on mouse spleen cells was via the mu opioid receptor, experiments were repeated with pretreatment of the cells for 2 hrs with 10−6 M naloxone or 10−6 M CTAP before addition of EM-1 or EM-2. Naloxone, at this concentration, should block mu, kappa, and delta opioid receptors. CTAP is a selective antagonist at the mu receptor. As shown in Fig. 2, neither opioid receptor antagonist blocked the immunosuppressive activity of either endomorphin.
Figure 2.
Naloxone does not block the immunosuppressive effects of endomorphins. Panel A: Dose response curves of endomorphin 1 alone (▲) or with 2 hr pretreatment with 10−6 M naloxone (△). Panel B: Dose response curves of endomorphin 2 alone (▼) or with 2 hr pretreatment with 10−6 M naloxone (▽). Panel C: Dose response curves of endomorphin 1 alone (▲) or with 2 hr pretreatment with 10−6 M CTAP (○). Panel D: Dose response curves of endomorphin 2 alone (▼) or with 2 hr pretreatment with 10−6 M CTAP (□). *Significantly different from control (1.0) by 95% confidence limit. Data are from 3 experiments.
Neutralization of immunosuppression by EM-specific polyclonal antibodies
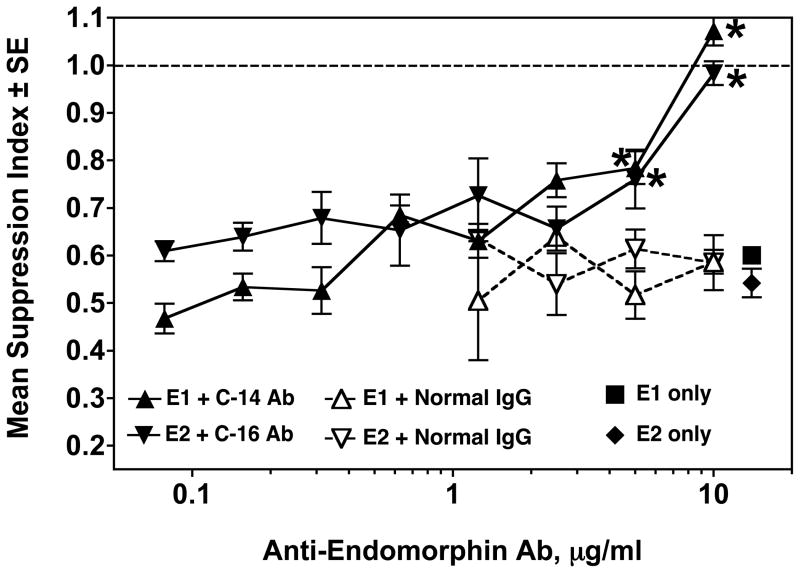
To prove specificity of the agonist effects of the EMs, the blocking capacity of anti-EM antibodies was tested. Spleen cells were pretreated with either C-14 or C-16 antibody, ranging in concentration from 10 to 0.078 μg/ml, for 2 hours prior to addition of the corresponding EM. As a control, normal rabbit IgG was added to separate wells at concentrations from 10 to 1.25 μg/ml, for 2 hours, before addition of either EM. EM-1 or EM-2 was then added to the respective wells at a final concentration of 10−15 M. As a positive control, wells were treated with 10−15 M EM-1 or EM-2 only. Figure 3 shows that both anti-EM antibodies completely blocked the immunosuppressive effect of their respective EMs at a concentration of 10 μg/ml, and partially blocked at 5 μg/ml. Upon titration, both antibodies exhibited a loss of neutralization when concentrations decreased to 2.5 μg/ml. Normal rabbit serum showed no effect on the immunosuppressive capacity of either EM.
Figure 3.
Secondary plaque-forming cell (PFC) assay showing immunosuppression produced by 10−15 M EM-1 (■) or EM-2 (◆), and the neutralization of immunosuppression by pretreatment with C-14 (anti-EM-1) (▲) or C-16 (anti-EM-2) (▼) affinity-purified antibodies. Pretreatment with normal rabbit IgG (△ .▽) had no effect on immunosuppression of either EM. Data are pooled from 2 experiments. * p< 0.05 for C-14 or C-16 antibody treatment vs. normal rabbit IgG.
Characterization of specificity of EM antibodies
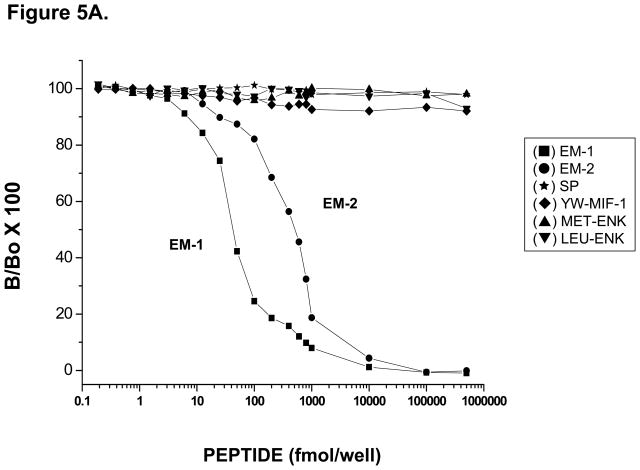
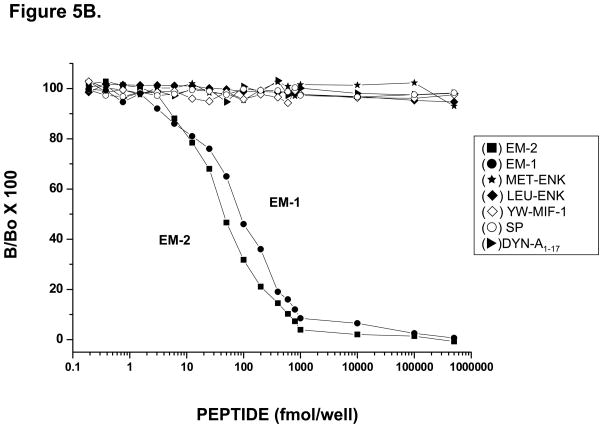
Immunodot-blot assays were initially carried out in order to determine potential cross-reactivity of both C-14 and C-16 A-APA antisera to other structurally related endogenous peptides. Figure 4 shows blots of EM-1, EM-2, Substance P, Met-enkephalin, Leu-enkephalin, and Tyr-W-MIF-1, each run at concentrations from 2 × 10−9 M to 2 × 10−14 M, blotted against C-14 antibody (Panel A) at 2.5 μg/ml, or C-16 antibody (Panel B) at 2.4 μg/ml. No cross-reactivity was observed by either antiserum to any of the peptides tested in the assay except EM-1 and EM-2. Interestingly, both antibodies reacted with EM-1 and EM-2, a result that is compatible with the very high (approx. 75%) structural homology shared by both peptides. To further verify and assess the specificity of both C-14 and C-16 A-APA antisera, sensitive and specific solid-phase RIAs to EM-1 and EM-2 were developed using as labeled tracers either [125I]-EM-1 or [125I]-EM-2. A representative RIA showing typical displacement curves by synthetic [125I]-EM-1 using the C-14-A-APA is shown in Fig. 5A. This purified EM-1 antiserum was capable of detecting EM-1 in a concentration range of 42 ± 5 fmol/well at the IC50 value. Moreover, the smallest measurable displacement detected of synthetic EM-1 at IC20 was as low as 17 ± 5 fmol/well in the EM-1 assay, and 170 ± 10 fmol at IC80. The cross-reactivity for EM-2 at the IC50 was 10 ± 3 % E-2. In addition, no significant cross-reactivity against Substance P, Tyr-W-MIF-1, Met-enkephalin, or Leu-enkephalin was observed using 0.1–500 fmol of competitive peptides. Fig. 5B shows a typical displacement curve of synthetic [125I]-EM-2 using the C-16 A-APA. This purified EM-2 antisera was capable of detecting EM-2 in a concentration range of 44.8 ± 1.2 fmol/well at the IC50 value and the smallest measurable displacement detected of synthetic EM-2 at IC20 was as low as 10.4 ± 1.5 fmol/well and 220 ± 10 fmol at the IC80 in this assay. The cross-reactivity for EM-1 at the IC50 was >90 %. This assay showed no significant cross-reactivity for Dynorphin A 1–17, Tyr-W-MIF-1, Met-enkephalin, Leu-enkephalin and Substance P within a range of 0.1 fmol-0.5 nmol of competitive peptides.
Figure 4.
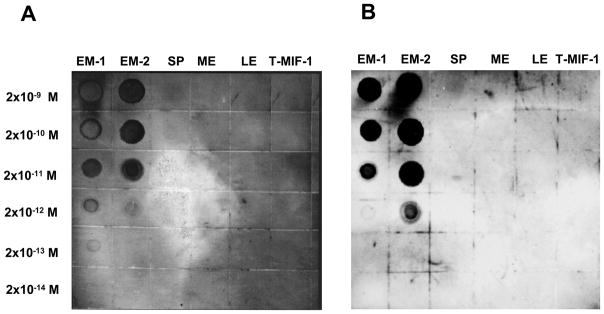
Immunodot-blot assay for specificity of anti-EM antibodies. Panel A: Dot-blots obtained using antiserum C-14 (anti-E1) at 2.5 mg/ml. Panel B: Dot-blots obtained using antiserum C-16 (anti-E2) at 2.4 mg/ml. Antibodies used in both panels were blotted onto indicated concentrations of EM-1, EM-2, substance P, met-enkephalin, leu-enkephalin, and Tyr-W-MIF-1 (T-MIF-1), a mammalian opiate peptide.
Figure 5.
Representative displacement curves for EM-1 and EM-2 in a solid-phase RIA using the affinity-purified antiserum C-14 for EM-1 (A), and C-16 for EM-2 (B). In panel A, the mean ± SEM for IC20, IC50 and IC80 values for the assay were 17 ± 3 fmol, 42 ± 5 fmol and 170 ± 10 fmol for E-1. The cross-reactivity at the IC50 was 10 ± 3 % for E-2. In panel B, the mean ± SEM for IC20, IC50 and IC80 values for the assay were 10.4 ± 1.5 fmol, 44.8 ± 1.2 fmol and 220 ± 10 fmol for EM-2. The cross-reactivity at the IC50 was >90% for EM-1. No significant cross-reactivity for substance P, Tyr-W-MIF-1, Met-enkephalin and Leu-enkephalin was observed using a range of 0.1–1000 fmol of competitive peptides in either assay.
Cross-neutralizing capacity of anti-EM antibodies
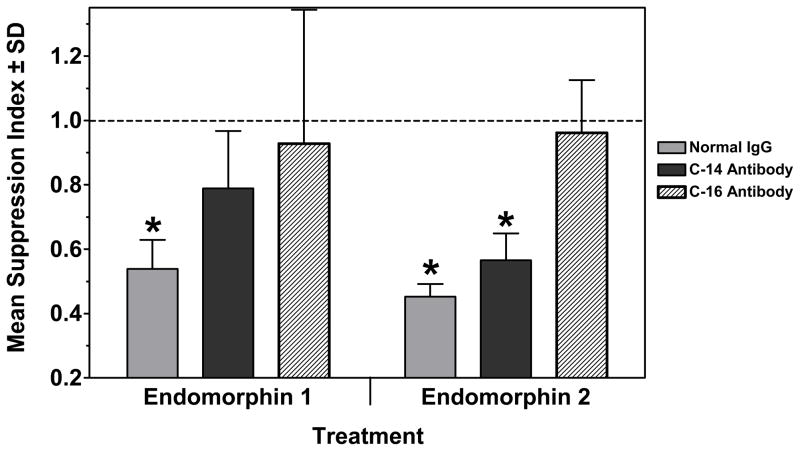
To determine if there was any cross-neutralizing ability between the two antibodies in regard to the EMs, an experiment was carried out in which 10 μg/ml of C-14, C-16 or normal IgG, were added to cultures 2 hrs before treatment using EM-1 or EM-2 (10−15 M) (Fig. 6). While normal IgG had no effect on immunosuppresion by either EM, C-14 antibody blocked only the EM-1 immunosuppression, not the EM-2 immunosuppression. However, the C-16 antibody blocked the immunosuppressive effects of both EMs.
Figure 6.
Cross-reactivity of anti-EM antibodies. Normal rabbit IgG, C-14 (anti-EM-1) and C-16 (anti-EM-2) Abs (10 μg/ml) were used to neutralize EM-1 or EM-2 at 10−15 M. Data are from a single secondary PFC assay. *Significantly different from control (1.0) by 95% confidence limit.
Discussion
These results show that the related tetrapeptides, EM-1 and EM-2, inhibit spleen cell antibody formation in a biphasic dose dependent manner, when added to in vitro culture systems at concentrations in the femtomolar range. The immunosuppressive activity was not inhibited by naloxone or CTAP, opioid receptor antagonists. The specificity of the activities of these peptides was demonstrated by their inhibition using specific affinity-purified rabbit antisera raised against either EM-1 or EM-2 peptides haptenized to KLH as immunogenic carrier protein. These studies also validate the specificity of the antisera.
Immunoreactive material to both EM-1 and EM-2 has been demonstrated to be present in normal rat and human spleens (Jessop et al., 2000), in rat popliteal lymph nodes (Mousa et al., 2002), and in human peripheral blood leukocytes (Jessop et al., 2002) as assessed by radioimmunoassay (RIA) or immunohistochemical analysis. There is evidence that inflammation increases immunoreactive-like material to EM-1 and EM-2 in synovial tissue from rats with adjuvant arthritis (Jessop et al., 2002) and in draining popliteal lymph nodes of animals given Freund’s complete adjuvant. Immunoreactivity has been localized to macrophages and B cells in rat spleen (Seale et al., 2004), both of which are cells involved in mounting an ex vivo response to sheep red blood cells in the assay used in the present studies. There are a limited number of other reports in the literature documenting functional effects of EM-1 and EM-2 on the immune system. Ohura’s group has shown that both tetrapeptides suppress LPS induced cytokine production (IL-12 and IL-10) in a human macrophage cell line (THP-1) (Azuma and Ohura, 2002b) and in rat primary peritoneal macrophages (Azuma and Ohura, 2002a). Further, they both also decreased phagocytosis, chemotaxis, and hydrogen peroxide production by THP-1 cells, and EM-2 had similar effects in the rat peritoneal macrophages. In rat cells, EM-2 also inhibited TNF-α, but potentiated IL-β secretion and Mac-1 expression (Azuma and Ohura, 2002a). Rat neutrophils were also shown to respond to EM-1 and EM-2 with reduced superoxide anion production following PMA stimulation, but with increased production when added to unstimulated cells (Azuma et al., 2000). Their adhesion to fibronectin was inhibited by EMs (Azuma et al., 2002). In contrast, to the immunosuppressive activities, they did not affect phagocytosis by neutrophils, and potentiated chemotaxis (Azuma et al., 2002). Another group has shown that EM-1 inhibits IL-8 production by the Caco-2 intestinal cell line stimulated with IL-1B(Neudeck and Loeb, 2002). Peterson et al (Peterson et al., 1999) reported that EM-1, but not EM-2, increased HIV replication in human microglia in vitro. Interestingly, Carrigan et al found that EM-1 was analgesic in rats, but had no immunmodulatory activity (Lynn and Herkenham, 1994). The paradigm used in these studies is different from the one used in the data presented here, as the peptides were injected ICV, rather than being added to cells of the immune system in vitro. In contrast, based on the studies using adjuvant arthritis, where EMs were up-regulated in the peripheral immune system, Jessop (Jessop, 2006) has proposed that the EMs could be used as anti-inflammatory agents in vivo.
One aspect of the data in the present study is unusual, namely that the activity of both EMs was observed at ultra-low concentrations in the femtomolar range. Azuma et al (Azuma et al., 2000) reported that EM-1 and EM-2 depressed superoxide anion production in rat neutrophils at doses ranging from 10−6 to 10−18 M, with maximal activity at 10−8 M. Jessop cited unpublished data supporting efficacy of doses of EM-1 as low as 100 fmol injected in vivo into the ankle joint, in a rat model of adjuvant arthritis (Jessop, 2006). There are several reports in the literature on activity of ultra-low doses of opioids, including EMs, on microglia. Peterson et al (Peterson et al., 1999) found that EM-1 potentiated HIV replication in human microglial cells in culture at doses ranging from 10−10 to 10−16 M. The dose response curve was biphasic, and similar in shape to that observed in the present studies, although the maximal effect was at 10−10 M. Similar effects were observed in a mixed glial/neuronal cell cultures infected with HIV, although the active doses of EM-1 were not as extensive, 10−10 to 10−14 M (Peterson et al., 1999). Other work from this group showed that morphine inhibited phagocytosis of Cryptococcus neoformans, a yeast, by swine microglia, at doses as low as 10−18 M. The dose response was not biphasic in these experiments. Additionally, Peterson’s group showed that U50, 488, a kappa agonist, inhibited HIV replication in human microglia in culture at 10−12 M (Chao et al., 1996), and morphine, at the same concentration, augmented HIV replication (Peterson et al., 1994). A recent report by Qian et al (Qian et al., 2007), demonstrated that morphine inhibited neuron-glial culture production of LPS-stimulated toxicity and of drug-induced dopaminerigic neurotoxicity at doses as low as 10−14 M. Observations of activity at femtomolar concentrations of other opioid peptides, including dynorphin, on microglia, have also been made by Hong’s group (Liu et al., 2001).
The question arises as to the mechanism of action of the EMs at these ultra-low concentrations. In most published studies of EM activity, mu opioid receptor antagonists blocked their effects. For example, Azuma et al found that β-funaltrexamine (β-FNA) was able to block the effects of EM-1 and EM-2 on rat neutrophil respiratory burst (Azuma et al., 2000), and on superoxide production (Azuma et al., 2002). Neudeck and Loeb also were able to block the effect of EM-1 on Caco-1 cells secretion of IL-8 with β-FNA (Neudeck and Loeb, 2002). Yet, there are a number of reports where questions have been raised as whether the activities of opioids at very low concentrations are mediated by classical opioid receptors. Peterson’s group found that the potentiating effect of EM-1 on HIV replication in glial/neuronal cell cultures, exhibiting a bell-shaped dose response curve, could be blocked by β-FNA. However, other opioids, including morphine and DAMGO, over a wide range of doses, had no effect on viral replication (Peterson et al., 1999). These, and other observations, led these investigators to conclude that the EMs might be acting by an ‘atypical μ-opioid receptor”. Hong’s group has presented extensive evidence for a “conventional opioid receptor-unrelated mechanism” by which dynorphin and morphine, at concentrations at 10−14 to 10−15 M, inhibit glia-mediated neurotoxicity (Liu et al., 2001; Qian et al., 2007). They conclude that inhibition of the enzyme, NADPH oxidase, is the mechanism of action (Qian et al., 2007). We tested the capacity of naloxone and of CTAP, at 10−6 M, to inhibit the immunosuppressive activity of EM-1 and of EM-2 across the full range of their dose response curves, and found no effect by either antagonist. Each experiment was repeated three times, validating the lack of effect. The capacity of the antagonists to block immunosuppression was tested by adding them to the cultures for two hours before adding the endomorphins. A two hour pretreatment with naloxone at 10−6 M has been shown by us previously to effectively block in vitro immunosuppression mediated by morphine, and a two hr pretreatment with nor-BNI at 10−6 M blocked immunosuppression mediated by the kappa agonist, U50, 488H (Eisenstein et al., 1995). The current results, where naloxone and CTAP failed to block suppression by the endomorphins, support a nonopioid receptor dependent mechanism for the immunosuppressive activity of EM-1 and EM-2. The inhibition of the immunosuppression by the antibodies used in the present studies, does indicate that the effects of the endomorphins on immune function is real, as it can be blocked by specific antibodies, and not by normal rabbit immunoglobulin. These studies also validate the efficacy of the antibodies, showing that they have neutralizing capacity against these neuropeptides. The mechanism by which the endomorphins are acting in the in vitro assay of antibody formation used in the present studies is not known. As mentioned above, Hong’s group provides evidence that the effect of opioids at ultra-low concentrations on microglia may be by inhibition of the enzyme, NADPH oxidase (Qian et al., 2007). There is actually considerable evidence in the literature for the existence of a nonclassical opioid receptor on cells of the immune system that binds β-endorphin. The first report of such a site on cultured human lymphocytes was in 1979 (Hazum et al., 1979). This publication and reports by others, using a human glioblastoma cell line and murine bone marrow macrophages, in addition to human lymphocytes, suggests that this site binds the C-terminal, rather than the N-terminal, end of the peptide (Westphal and Li, 1984; Borboni et al., 1989; Gelfand et al., 1995). Sharp’s group identified a naloxone-resistant binding site for β-endorphin on the human macrophage cell line, U937, on murine peritoneal macrophages, and most importantly, on murine splenocytes (Shahabi et al., 1990b; Woods et al., 1997; Shahabi et al., 1990a). Roy has also postulated a nonopioid morphine binding site on immune cells based on modulation of certain immune functions by this opioid that were still observed using cells from mu opioid receptor knock-out mice (Roy et al., 1998). Whether the endomorphins can bind to the β-endorphin site, or whether there are other receptors for opioid peptides on leukocytes, is not known at this time, but would certainly be of interest.
The present report adds another functional activity of endomorphins on the immune system, namely inhibition of in vitro antibody formation by mouse spleen cells. Since murine splenocyte cultures contain B-cells, T-cells, and macrophages, all of which are needed to make a plaque-forming cell response, identification of the cell type whose function is down-regulated by the endomorphins remains to be identified. These studies add to the growing literature showing a robust neuroimmune connection mediated by cytokines and neuropeptides.
Acknowledgments
This work was supported by NIDA grants DA06650 and DA13429, and by SEP-CONACyT-2004-CO1-47804, UNAM MacroProject MP6-18, INPRFM-Subcuenta-100.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alt A, Mansour A, Akil H, Medzihradsky F, Traynor JR, Woods JH. Stimulation of guanosine-5′-O-(3-[35S]thio)triphosphate binding by endogenous opioids acting at a cloned mu receptor. J Pharmacol Exp Ther. 1998;286:282–288. [PubMed] [Google Scholar]
- Asakawa A, Inui A, Momose K, Ueno N, Fujino MA, Kasuga M. Endomorphins have orexigenic and anxiolytic activities in mice. NeuroRep. 1998;9:2265–2267. doi: 10.1097/00001756-199807130-00022. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Ueno N, Fujino MA, Kasuga M. Endomorphin-1, an endogenous μ-opioid receptor selective agonist, stimulates oxygen consumption in mice. Horm Metab Res. 2000;32:51–52. doi: 10.1055/s-2007-978587. [DOI] [PubMed] [Google Scholar]
- Azuma Y, Ohura K. Endomorphin-2 modulates productions of TNF-α, IL-1β, IL-10, and IL-12, and alters functions related to innate immune of macrophages. Inflammation. 2002a;26:223–232. doi: 10.1023/a:1019766602138. [DOI] [PubMed] [Google Scholar]
- Azuma Y, Ohura K. Endomorphins 1 and 2 inhibit IL-10 and IL-12 production and innate immune functions, and potentiate NF-κB DNA binding in THP-1 differentiated to macrophage-like cells. Scand J Immunol. 2002b;56:260–209. doi: 10.1046/j.1365-3083.2002.01128.x. [DOI] [PubMed] [Google Scholar]
- Azuma Y, Ohura K, Wang PL, Shinohara M. Endomorphins delay constitutive apoptosis and alter the innate host defense functions of neutrophils. Immunol Lett. 2002;81:31–40. doi: 10.1016/s0165-2478(01)00335-2. [DOI] [PubMed] [Google Scholar]
- Azuma Y, Wang PL, Shinohara M, Ohura K. Immunomodulation of the neutrophil respiratory burst by endomorphins 1 and 2. Immunol Lett. 2000;75:55–59. doi: 10.1016/s0165-2478(00)00274-1. [DOI] [PubMed] [Google Scholar]
- Borboni P, Di Cola G, Sesti G, Marini MA, Del Porto P, Gilardini Montani MS, Lauro R, De Pirro R. Beta-endorphin receptors on cultured and freshly isolated lymphocytes from normal subjects. Biochem Biophys Res Comm. 1989;163:642–648. doi: 10.1016/0006-291x(89)92185-2. [DOI] [PubMed] [Google Scholar]
- Champion HC, Zadina JE, Kastin AJ, Hackler L, Ge LJ, Kadowitz PJ. Endomorphin 1 and 2, endogenous ligands for the μ-opioid receptor, decrease cardiac output, and total peripheral resistance in the rat. Peptides. 1997;18:1393–1397. doi: 10.1016/s0196-9781(97)00210-6. [DOI] [PubMed] [Google Scholar]
- Chao CC, Gekker G, Hu S, Sheng WS, Shark KB, Bu DF, Archer S, Bidlack JM, Peterson PK. κ opioid receptors in human microglia downregulate human immunodeficiency virus-1 expression. Proc Natl Acad Sci USA. 1996;93:8051–8056. doi: 10.1073/pnas.93.15.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coventry TL, Jessop DS, Finn DP, Crabb MD, Kinoshita H, Harbuz MS. Endomorphins and activation of the hypothalamo-pituitary-adrenal axis. J Endocrinol. 2001;169:185–193. doi: 10.1677/joe.0.1690185. [DOI] [PubMed] [Google Scholar]
- Cunningham A, Szenberg A. Further improvements in the plaque technique for detecting single antibody producing cells. Immunology. 1968;14:599–601. [PMC free article] [PubMed] [Google Scholar]
- Czapala MA, Gozal D, Alea OA, Beckerman RC, Zadina JE. Differential cardiorespiratory effects of endomorphin 1, endomorphin 2, DAMGO, and morphine. Amer J Resp Crit Care Med. 2000;162:994–999. doi: 10.1164/ajrccm.162.3.9911102. [DOI] [PubMed] [Google Scholar]
- Doi N, Brown CH, Cohen HD, Leng G, Russell JA. Effects of the endogenous opioid peptide, endomorphin 1, on supraoptic nucleus oxytocin and vasopressin neurones in vivo and in vitro. Brit J Pharmacol. 2001;132:1136–1144. doi: 10.1038/sj.bjp.0703911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein TK, Meissler JJ, Jr, Rogers TJ, Geller EB, Adler MW. Mouse strain differences in immunosuppression by opioids in vitro. J Pharmacol Exp Ther. 1995;275:1484–1489. [PubMed] [Google Scholar]
- Gelfand RA, Wepsic HT, Parker LN, Jadus MR. Prosaglandin E2 induces up-regulation of murine macrophage β-endorphin receptors. Immunol Lett. 1995;45:143–148. doi: 10.1016/0165-2478(94)00245-m. [DOI] [PubMed] [Google Scholar]
- Goldberg IE, Rosi GC, Letchworth SR, Mathis JP, Ryan-Moro J, Leventhal L, Su W, Emmel D, Bolan EA, Pasternak GW. Pharmacological characterization of endomorphin-1 and endomorphin-2 in mouse brain. J Pharmacol Exp Ther. 1998;286:1007–1013. [PubMed] [Google Scholar]
- Gong J, Strong JA, Zhang S, Yue X, DeHaven RN, Daubert JD, Cassel JA, Yu G, Mansson E, Yu L. Endomorphins fully activate a cloned human mu opioid receptor. FEBS Lett. 1998;439:152–156. doi: 10.1016/s0014-5793(98)01362-3. [DOI] [PubMed] [Google Scholar]
- Hackler L, Kastin AJ, Erchegyi J, Zadina JE. Isolation of Tyr-W-MIF-1 from bovine hypothalami. Neuropeptides. 1993;24:159–164. doi: 10.1016/0143-4179(93)90080-t. [DOI] [PubMed] [Google Scholar]
- Hackler L, Kastin AJ, Zadina JE. Isolation of a novel peptide with a unique binding profile from human brain cortex: Tyr-K-MIF-1 (Tyr-Pro-Lys-Gly-NH2) Peptides. 1994;15:945–950. doi: 10.1016/0196-9781(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Hackler L, Zadina JE, Ge LJ, Kastin AJ. Isolation of relatively large amounts of endomorphin-1 and endomorphin-2 from human brain cortex. Peptides. 1997;18:1635–1639. doi: 10.1016/s0196-9781(97)00259-3. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies, A Laboratory Manual. Cold Spring Harbor Press; Cold Spring Harbor, NY: 1988. [Google Scholar]
- Harrison LM, Kastin AJ, Zadina JE. Differential effects of endomorphin-1, endomorphin-2, and Tyr-W-MIF-1 on activation of G-proteins in SH-SY5Y human neuroblastoma membranes. Peptides. 1998;19:749–753. doi: 10.1016/s0196-9781(98)00022-9. [DOI] [PubMed] [Google Scholar]
- Hazum E, Chang KJ, Cuatrecasas P. Specific nonopiate receptors for beta-endorphin. Science. 1979;205:1033–1035. doi: 10.1126/science.224457. [DOI] [PubMed] [Google Scholar]
- Higashida H, Hoshi N, Knijnik R, Zadina JE, Kastin AJ. Endomorphins inhibit high-threshold Ca2+ channel currents in rodent NG108-15 cells overexpressing μ-opioid receptors. J Physiol. 1998;507:71–75. doi: 10.1111/j.1469-7793.1998.071bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockfield S, Carlson S, Evans C, Levitt P, Pintar J, Silberstein L. Selected methods for antibody and nucleic acid probes. Cold Spring Harbor Press; Cold Spring Harbor, NY: 1993. Molecular Probes of the Nervous System I. [Google Scholar]
- Huang C, Wang Y, Chang JK, Han JS. Endomorphin and μ-opioid receptors in mouse brain mediate the analgesic effect induced by 2 Hz but not 100 Hz electroacupuncture stimulation. Neurosci Lett. 2000;294:159–162. doi: 10.1016/s0304-3940(00)01572-x. [DOI] [PubMed] [Google Scholar]
- Jessop DS. Endomorphins as agents for the treatment of chronic inflammatory disease. Biodrugs. 2006;20:161–166. doi: 10.2165/00063030-200620030-00003. [DOI] [PubMed] [Google Scholar]
- Jessop DS, Major GN, Coventry TL, Kaye SJ, Fulford AJ, Harbuz MS, De Bree FM. Novel opioid peptides endomorphin-1 and endomorphin-2 are present in mammalian immune tissues. J Neuroimmunol. 2000;106:53–59. doi: 10.1016/s0165-5728(99)00216-7. [DOI] [PubMed] [Google Scholar]
- Jessop DS, Richards LJ, Harbuz MS. Opioid peptides endomorphin-1 and endomorphin-2 in the immune system in humans and in a rodent model of inflammation. Ann N Y Acad Sci. 2002;966:456–463. doi: 10.1111/j.1749-6632.2002.tb04247.x. [DOI] [PubMed] [Google Scholar]
- Kwok EH, Dun NJ. Endomorphins decrease heart rate and blood pressure possibly by activating vagal afferents in anesthetized rats. Brain Res. 1998;803:204–207. doi: 10.1016/s0006-8993(98)00623-4. [DOI] [PubMed] [Google Scholar]
- Liu B, Qin L, Yang SN, Wilson BC, Liu Y, Hong JS. Femtomolar concentrations of dynorphins protect rat mesencephalic dopaminergic neurons against inflammatory damage. J Pharmacol Exp Ther. 2001;298:1133–1141. [PubMed] [Google Scholar]
- Loi PK, McGraw HF, Tublitz NJ. Peptide detection in single cells using dot immunoblot assay. Peptides. 1997;18:749–753. doi: 10.1016/s0196-9781(97)00006-5. [DOI] [PubMed] [Google Scholar]
- Lynn AB, Herkenham M. Localization of cannabinioid receptors and non-saturable high-density cannabinoid binding sites in peripheral tissues of the rat: implications for receptor-mediated immune modulation by cannabinoids. J Pharmacol Exp Ther. 1994;268:1612–1623. [PubMed] [Google Scholar]
- Martin-Schild S, Zadina JE, Gerall AA, Vigh S, Kastin AJ. Localization of endomorphin-2-like immunoreactivity in the rat medulla and spinal cord. Peptides. 1997;18:1641–1649. doi: 10.1016/s0196-9781(97)00320-3. [DOI] [PubMed] [Google Scholar]
- Mima H, Morikawa H, Fukuda K, Kato S, Shoda T, Mori K. Ca2+ channel inhibition inhibition by endomorphins via the cloned μ-opioid receptor expressed in NG108-15 cells. Eur J Pharmacol. 1997;340:R1–R2. [PubMed] [Google Scholar]
- Mishell RI, Dutton RW. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967;126:423–424. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monory K, Bourin MC, Spetea M, Tömböly C, Tóth G, Matthes HW, Kieffer BL, Hanoune J, Borsodi A. Specific activation of the mu opioid receptor (MOR) by endomorphin 1 and endomorphin 2. Eur J Neruosci. 2000;12:577–584. doi: 10.1046/j.1460-9568.2000.00936.x. [DOI] [PubMed] [Google Scholar]
- Mousa SA, Machelska H, Schäfer M, Stein C. Immunohistochemical localization of endomorphin-1 and endomorphin-2 in immune cells and spinal cord in a model of inflammatory pain. J Neuroimmunol. 2002;126:5–15. doi: 10.1016/s0165-5728(02)00049-8. [DOI] [PubMed] [Google Scholar]
- Neudeck BL, Loeb JM. Endomorphin-1 alters interleukin-8 secretion in Caco-2 cells via a receptor mediated process. Immunol Lett. 2002;84:217–221. doi: 10.1016/s0165-2478(02)00198-0. [DOI] [PubMed] [Google Scholar]
- Nevo I, Avidor-Reiss T, Levy R, Bayewitch M, Vogel Z. Acute and chronic activation of the mu-opioid receptor with the endogenous ligand endomorphin differentially regulates adenylyl cyclase isozymes. Neuropharmacol. 2000;39:364–371. doi: 10.1016/s0028-3908(99)00155-0. [DOI] [PubMed] [Google Scholar]
- Ohsawa M, Mozoguchi H, Narita M, Nagase H, Kampine JP, Tseng LF. Differential antinociception induced by spinally administered endomorphin-1 and endomorphin-2 in the mouse. J Pharmacol Exp Ther. 2001;298:592–597. [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Anderson WR, Kravitz F, Portoghese PS, Balfour HH, Jr, Chao CC. Morphine amplifies HIV-1 expression in chronically infected promonocytes cocultured with human brain cells. J Neuroimmunol. 1994;50:167–175. doi: 10.1016/0165-5728(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Lokensgard J, Portoghese PS, Chao CC. Endomorphin-1 potentiates HIV-1 expression in human brain cell cultures: implications of an atypical μ-opioid receptor. Neuropharmacology. 1999;38:273–278. doi: 10.1016/s0028-3908(98)00167-1. [DOI] [PubMed] [Google Scholar]
- Pierce TL, Grahek MD, Wessendorf MW. Immunoreactivity for endomorphin-2 occurs in primary afferents in rats and monkey. NeuroRep. 1998;9:385–389. doi: 10.1097/00001756-199802160-00005. [DOI] [PubMed] [Google Scholar]
- Przewlocka B, Mika J, Labuz D, Tóth G, Przewlocki R. Spinal analgesic action of endomorphins in acute, inflammatory and neuropathic pain in rats. Eur J Pharmacol. 1999;367:189–196. doi: 10.1016/s0014-2999(98)00956-x. [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Labuz D, Mika J, Przewlocka B, Tömböly C, Tóth G. Pain inhibition by endomorphins. Ann NY Acad Sci. 1999;897:154–164. doi: 10.1111/j.1749-6632.1999.tb07887.x. [DOI] [PubMed] [Google Scholar]
- Qian L, Tan KS, Wei SJ, Wu HM, Xu Z, Wilson B, Lu RB, Hong JS, Flood PM. Microglia-mediated neurotoxicity is inhibited by morphine through an opioid receptor-independent reduction of NADPH oxidase activity. J Immunol. 2007;179:1198–1209. doi: 10.4049/jimmunol.179.2.1198. [DOI] [PubMed] [Google Scholar]
- Roy S, Barke RA, Loh HH. Mu-opioid receptor-knockout mice: role of μ-opioid receptor in morphine mediated immune functions. Mol Brain Res. 1998;61:190–194. doi: 10.1016/s0169-328x(98)00212-5. [DOI] [PubMed] [Google Scholar]
- Schreff M, Schulz S, Wiborny D, Hollt V. Immunofluorescent identification of endomorphin-2-containing nerve fibers and terminals in the rat brain and spinal cord. NeuroRep. 1998;9:1031–1034. doi: 10.1097/00001756-199804200-00014. [DOI] [PubMed] [Google Scholar]
- Seale JV, Jessop DS, Harbuz MS. Immunohistochemical staining of endomorphin 1 and 2 in the immune cells of the spleen. Peptides. 2004;25:91–94. doi: 10.1016/j.peptides.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Shahabi NA, Linner KM, Sharp BM. Murine splenocytes express a naloxone-insensitive binding site for β-endorphin. Endocrinology. 1990a;126:1442–1448. doi: 10.1210/endo-126-3-1442. [DOI] [PubMed] [Google Scholar]
- Shahabi NA, Peterson PK, Sharp B. Beta-endorphin binding to naloxone-insensitive sites on a human mononuclear cell line (U937): effects of cations and guanosine triphosphate. Endocrinology. 1990b;126:3006–3015. doi: 10.1210/endo-126-6-3006. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Liu Q, Childers SR, Selley DE. Endomorphin stimulated [35S]GTPχS binding in the rat brain: evidence for partial agonist activity at μ-opioid receptors. J Neurochem. 1998;70:1567–1576. doi: 10.1046/j.1471-4159.1998.70041567.x. [DOI] [PubMed] [Google Scholar]
- Soignier RD, Vaccarino AL, Brennan AM, Kastin AJ, Zadina JE. Analgesic effects of endomorphin-1 and endomorphin-2 in the formalin test in mice. Life Sci. 2000;67:907–912. doi: 10.1016/s0024-3205(00)00689-5. [DOI] [PubMed] [Google Scholar]
- Stone LS, Fairbanks CA, Laughlin TM, Nguyen HO, Bushy TM, Wessendorf MW, Wilcox GL. Spinal analgesic actions of the new endogenous opioid peptides endomorphin-1 and endomorphin-2. NeuroRep. 1997;8:3131–3135. doi: 10.1097/00001756-199709290-00025. [DOI] [PubMed] [Google Scholar]
- Ukai M, Watanabe Y, Kameyama T. Endomorphins 1 and 2, endogenous mu-opioid receptor agonists, impair passive avoidance learning in mice. Eur J Pharmacol. 2001;421:115–119. doi: 10.1016/s0014-2999(01)01009-3. [DOI] [PubMed] [Google Scholar]
- Westphal M, Li CH. β-endorphin: characterization of binding sites specific for the human hormone in human glioblastoma SF126 cells. Proc Natl Acad Sci USA. 1984;81:2921–2923. doi: 10.1073/pnas.81.9.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JA, Shahabi NA, Sharp BM. Characterization of a naloxone-insensitive β-endorphin receptor on murine peritoneal macrophages. Life Sci. 1997;60:573–586. doi: 10.1016/s0024-3205(96)00640-6. [DOI] [PubMed] [Google Scholar]
- Yu X, Mao X, Blake AD, Li WX, Chang SL. Morphine and endomorphins differentially regulate μ-opioid receptor mRNA in SHSY-5Y human neuroblastoma cells. J Pharmacol Exp Ther. 2003;306:447–454. doi: 10.1124/jpet.103.048694. [DOI] [PubMed] [Google Scholar]
- Zadina JE. Isolation and distribution of endomorphins in the central nervous system. Japan J Pharmacol. 2002;89:203–208. doi: 10.1254/jjp.89.203. [DOI] [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the μ-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]