Abstract
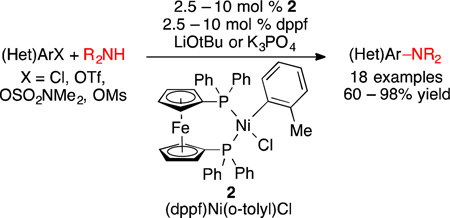
A new air-stable nickel precatalyst for C-N cross-coupling is reported. The developed catalyst system displays a greatly improved substrate scope for C-N bond formation to include both a wide range of aryl and heteroaryl electrophiles and aryl, heteroaryl, and alkylamines. The catalyst system is also compatible with weak base, allowing the amination of substrates containing base-sensitive functional groups.
The development of a general, robust, and operationally simple catalyst system for Ni-catalyzed C-N cross-coupling reactions has remained a significant challenge despite of 16 years of extensive catalyst development. After our first report,1 many groups have expanded Ni-catalyzed C-N cross-coupling to include new electrophiles such as aryl tosylates, carbamates, sulfamates, methyl ethers, phosphates, pivalates, and nitriles.2,3,4 However, many of these systems rely on air- and moisture-sensitive Ni(COD)2 as a catalyst precursor.1,3 While other catalyst systems have utilized air-stable Ni(II) sources, many of these systems required the use of an external reductant to generate the catalytically active Ni species.2c,5 Additionally, the overall substrate scope of all Ni-catalyst systems reported to date has remained relatively limited, with only a few successful examples of substrates containing base-sensitive functional groups.6,7,8,9 To address these challenges we sought to develop an air-stable, highly active Ni(II) pre-catalyst for C-N cross-coupling reactions.
Ni(II)-(σ-aryl) complexes, first reported by Shaw in 1960, were shown to be robust compounds, stable to moisture and air.10 In 2007, Yang reported the first use of these complexes in C-N cross-coupling by demonstrating that (Ph3P)2Ni(1-nap)Cl used in combination with an N-heterocyclic carbene ligand (IPr•HCl) generated an effective catalyst system for the amination of aryl chlorides.11,12,13 Based on these precedents14 and our previous success using dppf as a supporting ligand in Ni-based catalysis,1 we sought to investigate the use of a dppf-ligated Ni(II)-(σ-aryl) complexes of as a precatalyst in C-N bond-forming reactions.
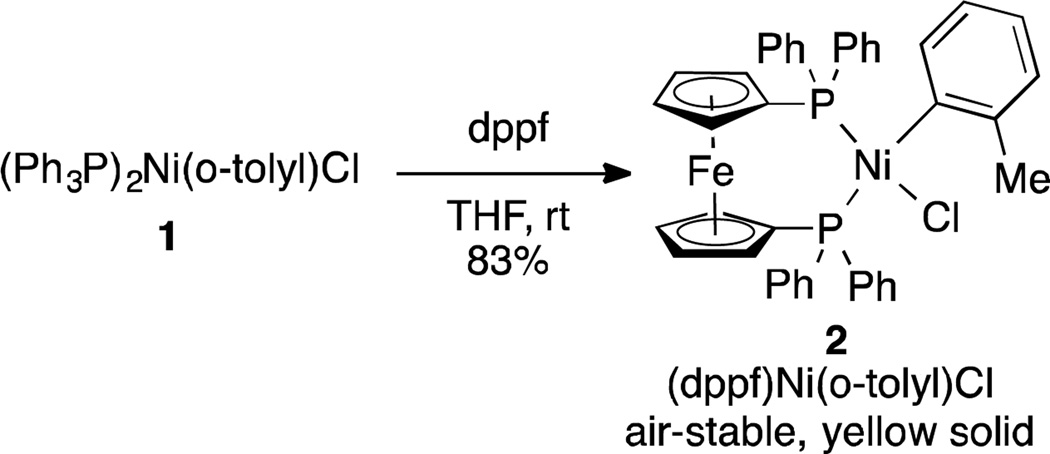
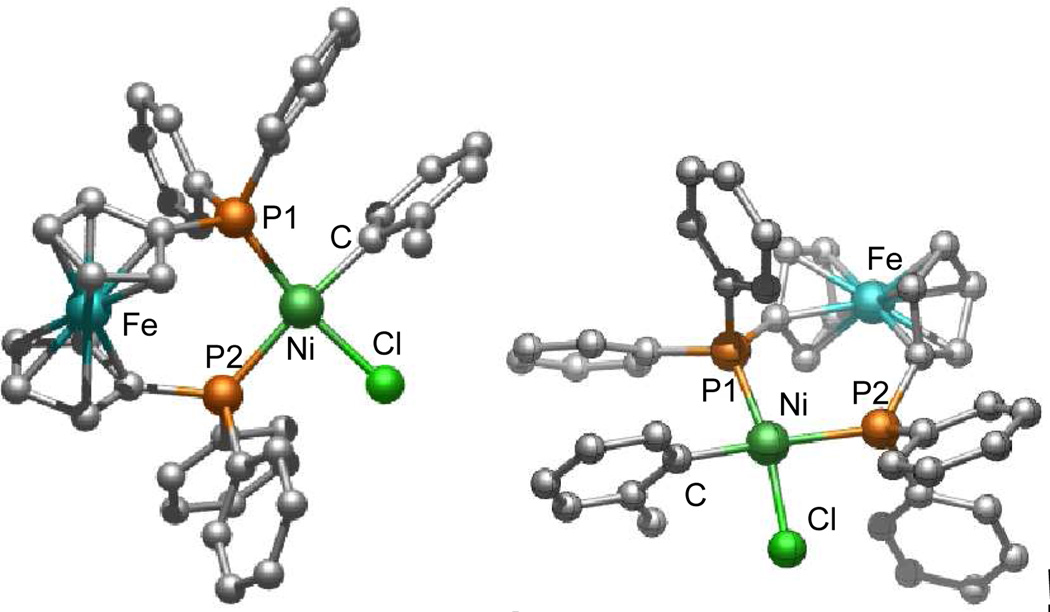
For our study, we selected (dppf)Ni(o-tolyl)Cl (2) as a precatalyst for C-N bond formation (Scheme 1). The synthesis of 2 can be accomplished by exchanging the triphenylphosphine ligands on (Ph3P)2Ni(o-tolyl)Cl (1) with dppf in THF at room temperature, resulting in an 83% yield (Scheme 1). (Ph3P)2Ni(o-tolyl)Cl (1) can be easily prepared directly from commercially available (Ph3P)2NiCl2 or in two steps from NiCl2•6H2O.15 Similar to other Ni(II)-(σ-aryl) complexes,2a–b,5j,10,12,13,14 2 was found to be air-stable.16 A crystal structure of 2 shows a cis-ligated nickel dppf complex with square planar geometry (Figure 1).17
Scheme 1.
Synthesis of (dppf)Ni(o-tolyl)Cl (2)
Figure 1.
X-ray crystal structure of (dppf)Ni(o-tolyl)Cl (2) (representation from two different angles). THF molecule and hydrogen atoms are omitted for clarity.
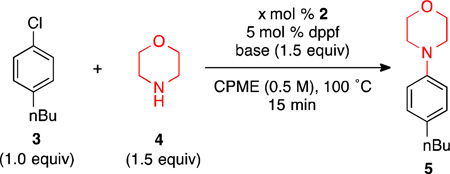
Having prepared and characterized 2, we set out to investigate its use as a precatalyst in the amination of 4-n-butylchlorobenzene (3) with morpholine (4) (Table 1). Performing the reaction using 5 mol % of 2 and 5 mol % dppf as the catalyst with NaOtBu as the base in CPME for 15 minutes at 100 °C resulted in 10% yield (entry 1, Table 1). KOtBu as a base provided a similar result (9% yield), while the use of LiOtBu led to a marked improvement in reactivity, giving the coupled product (5) in 49% yield (entries 2 and 3, Table 1), demonstrating that the choice of counterion is crucial to the success of the reaction when using tert-butoxide bases.18 Previously, Hartwig has shown that the addition of benzonitrile improved the reactivity in Ni-based systems–potentially stabilizing the catalytically active Ni-species.19 Similarly, we found that the addition of 1 equivalent of acetonitrile greatly improved the reactivity of 2, producing the product 5 in 86% yield (entry 4, Table 1). Extending the reaction time to 45 minutes gave full conversion of the aryl chloride, and product 5 was isolated in 85% yield (entry 5, Table 1). The use of the acetonitrile additive with either NaOtBu or KOtBu, however, still resulted in low yields (entry 6 and 7, Table 1). Conducting the reaction without an additional equivalent of the dppf ligand led to a diminshed yield of 5 (entry 8, Table 1), and lowering the catalyst loading had a similarly detrimental effect, with the reaction failing to reach full conversion even after extended reaction times (entries 9 and 10, Table 1).
Table 1.
Optimization of Reaction Conditionsa
 | |||||
|---|---|---|---|---|---|
| entry | mol % 2 | additive | base | conversionb | yieldb |
| 1 | 5 | None | NaOtBu | 21% | 10% |
| 2 | 5 | None | KOtBu | 23% | 9% |
| 3 | 5 | None | LiOtBu | 54% | 49% |
| 4 | 5 | MeCN | LiOtBu | 90% | 86% |
| 5c | 5 | MeCN | LiOtBu | 100% | 91% |
| 6 | 5 | MeCN | NaOtBu | 14% | 5% |
| 7 | 5 | MeCN | KOtBu | 4% | <4% |
| 8d | 5 | MeCN | LiOtBu | 79% | 68% |
| 9 | 2.5 | MeCN | LiOtBu | 58% | 50% |
| 10e | 2.5 | MeCN | LiOtBu | 68% | 60% |
Reaction conditions: 3 (0.25 mmol), 4 (0.375 mmol), base (0.375 mmol) 2 (2.5 – 5 mol %), dppf (2.5 – 5 mol %), additive (0.25 mmol), CPME (0.5 mL), 100 °C, 15 min.
Determined by GC using dodecane as the internal standard.
Reaction time was 45 min. Isolated yield was 85%, 1 mmol scale, average of two runs.
No additional dppf was added.
Reaction time was 1 h.
CPME = cyclopentyl methyl ether.
In addition to the desired arylamine product 5, a small amount of the corresponding aminated o-tolyl product (2%) was also observed. This byproduct is likely the result of catalyst activation, and similar byproducts have been observed in other systems using Ni(II)-(σ-aryl) complexes as precatalysts.20,21 We note that the corresponding aminated o-tolyl byproducts from 2 were not observed in many of the reactions using 2. In the instances when they were formed, the small amounts (0.5 – 5%) were easily separated during purification.22
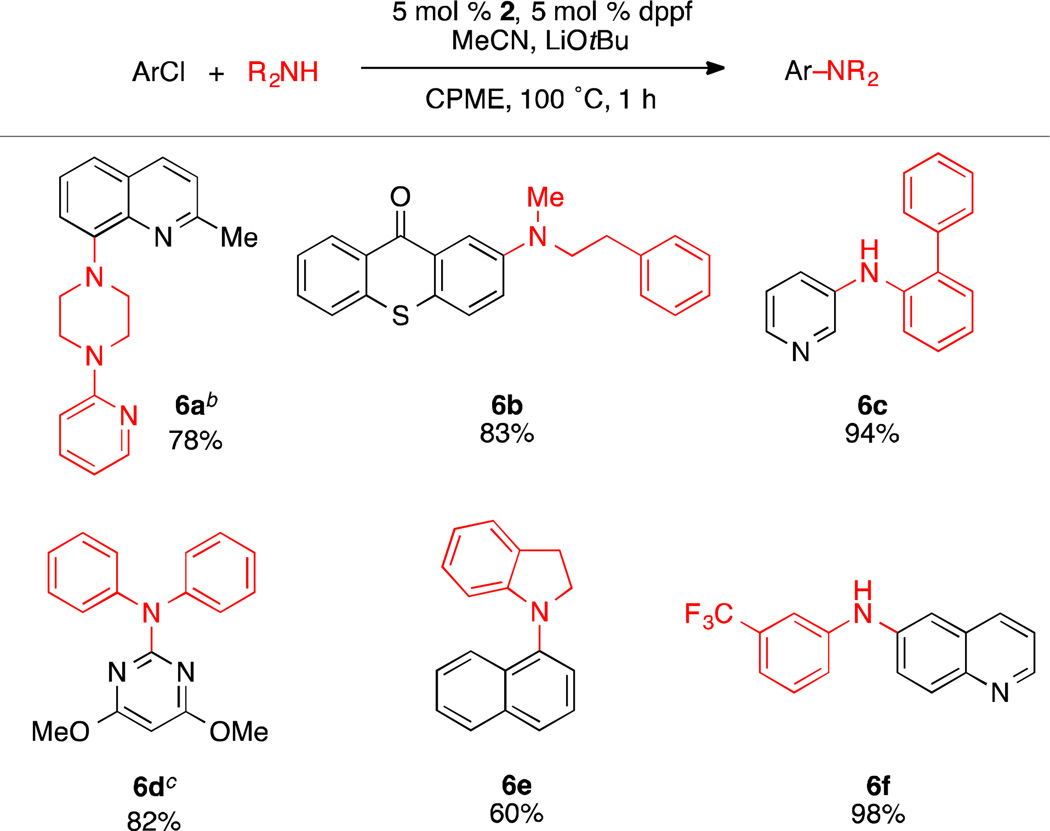
With the conditions for amination identified, we investigated the scope of this reaction with aryl and heteroaryl chlorides. The catalyst system was found to tolerate both cyclic and acyclic secondary alkylamines, which were coupled in high yield (6a and 6b, Scheme 2). Primary anilines, including the bulky, ortho-substituted 2-aminobiphenyl, were readily arylated in excellent yield (6c and 6f, Scheme 2). Diphenylamine also underwent facile cross-coupling with 2-chloro-4,6-dimethoxypyrimidine in 82% yield, although the reaction required increased catalyst loading and higher temperatures to achieve full conversion (6d, Scheme 2). The arylation of indoline with 1-chloronaphthalene suffered from significant reduction of the aryl halide presumably due to competing β-hydride elimination, but the desired product was still isolated in 60% yield (6e, Scheme 2).
Scheme 2.
Amination of Aryl Chlorides using LiOtBua
aReaction conditions: aryl chloride (1.0 mmol), amine (1.5 mmol), LiOtBu (1.5 mmol), 2 (5 mol %), dppf (5 mol %), MeCN (1.0 mmol), CPME (2 mL), 100 °C, 1 h. Yields are of the isolated product, average of two runs. bReaction time 16 h. c2 (10 mol %) and dppf (10 mol %), 130 °C, 16 h.
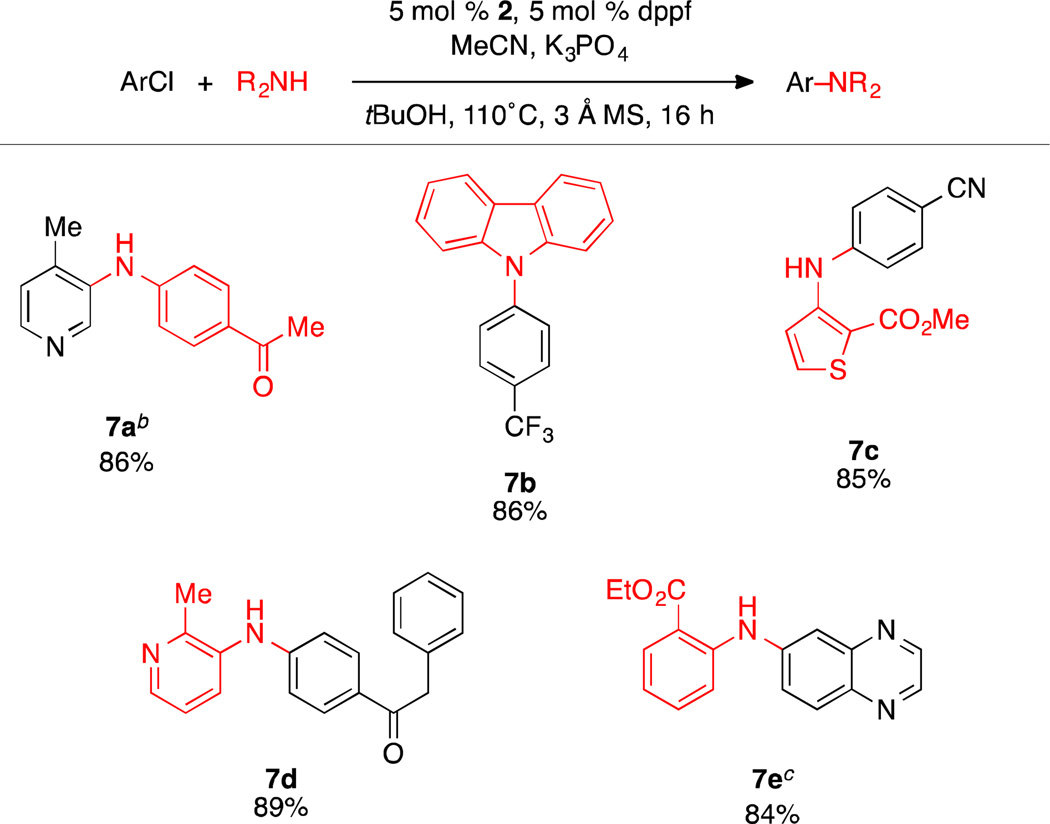
Although our catalyst system proved to be highly effective for amination of aryl and heteroaryl chlorides using LiOtBu, we were interested in expanding the scope of the reaction to include substrates bearing base sensitive functional groups. Due to their lower pKa values relative to alkylamines, we felt that anilines would be readily deprotonated during the transmetallation step (amine binding and deprotonation) under weakly basic conditions.23 Thus, by changing the solvent to tBuOH and employing K3PO4 as the base,24 we found that a wide variety of primary and secondary anilines could be efficiently arylated (Scheme 3). These conditions were found to tolerate base-sensitive functional groups as well as ortho-substitution on the aryl halide and the amine nucleophiles, including anilines containing an ester substituent in the ortho position (Scheme 3).
Scheme 3.
Amination of Aryl Chlorides using K3PO4a
aReaction conditions: aryl chloride (1.0 mmol), amine (1.5 mmol), K3PO4 (3 mmol), 2 (5 mol %), dppf (5 mol %), MeCN (1.0 mmol), tBuOH (2 mL), 3 Å MS (300 mg), 110 °C, 16 h. Yields are of the isolated product, average of two runs. bIn cases where using the standard conditions do not give full conversion, omission of the 3 Å MS or using 6 mmol of K3PO4 were found to allow the reaction to reach completion. cK3PO4 (6 mmol), Dioxane (4 mL).
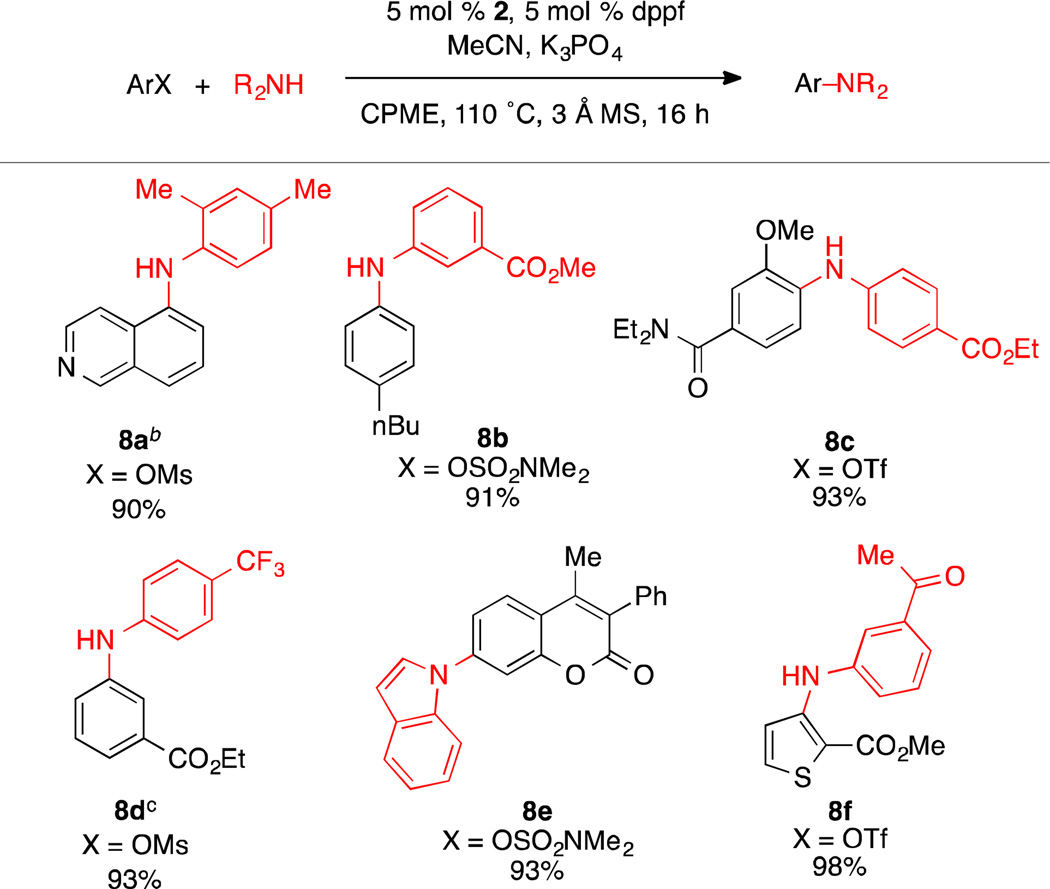
Having established the conditions for the arylation of anilines using K3PO4, we sought to expand the reaction scope further to include phenol-derived aryl electrophiles. While the amination of aryl sulfamate electrophiles is well-established,2c,3d,g the amination of aryl mesylates with Ni-catalyst systems remains unknown.25,26 Furthermore, only one successful example of Ni-catalyzed amination of an aryl triflate has been reported.27 As aryl mesylate and some aryl triflate electrophiles are known to be sensitive to strong bases,24a,28 we felt that application of the developed weak base conditions would enable the successful amination of these electrophiles. However, the use of tBuOH proved to be detrimental to the reaction, resulting in modest yields of the desired product. By using CPME as the solvent and performing the reaction at a slightly lower concentration a dramatic improvement of the product yields was observed. As with the aryl chloride substrates, these conditions were able to tolerate base sensitive functional groups as well as orthosubstituents on either the electrophile or the nucleophile (Scheme 4).
Scheme 4.
Amination of Aryl Mesylates, Triflates, and Sulfamatesa
aReaction conditions: aryl chloride (1.0 mmol), amine (1.5 mmol), K3PO4 (3 mmol), 2 (5 mol %), dppf (5 mol %), MeCN (1.0 mmol), CPME (4 mL), 3 Å MS (300 mg), 110 °C, 16 h. Yields are of the isolated product, average of two runs. bIn cases where using the standard conditions do not give full conversion, using 6 mmol of K3PO4 were found to allow the reaction to reach completion. c2 (2.5 mol %), dppf (2.5 mol %), 4 h.
In summary, we have developed a highly active, dppf-ligated nickel precatalyst (2) for use in C-N cross-coupling reactions. This robust precatalyst can be easily prepared from readily available Ni(II) sources and is air-stable. Furthermore, this catalyst system has been demonstrated to cross-couple a wide array of amine nucleophiles efficiently with aryl and heteroaryl electrophiles, including substrates containing base sensitive functional groups.
Supplementary Material
Acknowledgment
Research reported in this publication was supported by the National Institutes of Health under award number GM58160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. N.H.P acknowledges a National Science Foundation Graduate Research Fellowship. We thank Dr. Aaron C. Sather (MIT), Dr. Sean M. Smith (MIT), and Ekaterina V. Vinogradova (MIT) for aid in the preparation of this manuscript.
Footnotes
Supporting Information Available Experimental procedures along with experimental and spectroscopic data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Wolfe JP, Buchwald SL. J. Am. Chem. Soc. 1997;119:6054. [Google Scholar]
- 2.(a) Gao C-Y, Yang L-M. J. Org. Chem. 2008;73:1624. doi: 10.1021/jo7022558. [DOI] [PubMed] [Google Scholar]; (b) Huang J-H, Yang L-M. Org. Lett. 2011;13:3750. doi: 10.1021/ol201437g. [DOI] [PubMed] [Google Scholar]; (c) Hie L, Ramgren SD, Mesganaw T, Garg NK. Org. Lett. 2012;14:4182. doi: 10.1021/ol301847m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Bolm C, Hildebrand JP, Rudolph J. Synthesis. 2000;7:911. [Google Scholar]; (b) Tobisu M, Shimasaki T, Chatani N. Chem. Lett. 2009;38:710. [Google Scholar]; (c) Shimasaki T, Tobisu M, Chatani N. Angew. Chem. Int. Ed. 2010;49:2929. doi: 10.1002/anie.200907287. [DOI] [PubMed] [Google Scholar]; (d) Ackermann L, Sandmann R, Song W. Org. Lett. 2011;13:1784. doi: 10.1021/ol200267b. [DOI] [PubMed] [Google Scholar]; (e) Ackermann L, Song W, Sandmann R. J. Organomet. Chem. 2011;696:195. [Google Scholar]; (f) Mesganaw T, Silberstein AL, Ramgren SD, Nathel NFF, Hong X, Liu P, Garg NK. Chem. Sci. 2011;2:1766. doi: 10.1039/c1sc00230a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Ramgren SD, Silberstein AL, Yang Y, Garg NK. Angew. Chem. Int. Ed. 2011;50:2171. doi: 10.1002/anie.201007325. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Tobisu M, Yasutome A, Yamakawa K, Shimasaki T, Chatani N. Tetrahedron. 2012;68:5157. [Google Scholar]
- 4.Miller J, Dankwardt J, Penney J. Synthesis. 2003:1643. [Google Scholar]
- 5.(a) Brenner E, Fort Y. Tetrahedron Lett. 1998;39:5359. [Google Scholar]; (b) Brenner E, Schneider R, Fort Y. Tetrahedron. 1999;55:12829. [Google Scholar]; (c) Brenner E, Schneider R, Fort Y. Tetrahedron Lett. 2000;41:2881. [Google Scholar]; (d) Lipshutz BH, Ueda H. Angew. Chem. Int. Ed. 2000;39:4492. [PubMed] [Google Scholar]; (e) Gradel B, Brenner E, Schneider R, Fort Y. Tetrahedron Lett. 2001;42:5689. [Google Scholar]; (f) Desmarets C, Schneider Rl, Fort Y. J. Org. Chem. 2002;67:3029. doi: 10.1021/jo016352l. [DOI] [PubMed] [Google Scholar]; (g) Omar-Amrani R, Thomas A, Brenner E, Schneider R, Fort Y. Org. Lett. 2003;5:2311. doi: 10.1021/ol034659w. [DOI] [PubMed] [Google Scholar]; (h) Chen C, Yang L-M. Org. Lett. 2005;7:2209. doi: 10.1021/ol050608i. [DOI] [PubMed] [Google Scholar]; (i) Manolikakes G, Gavryushin A, Knochel P. J. Org. Chem. 2008;73:1429. doi: 10.1021/jo702219f. [DOI] [PubMed] [Google Scholar]; (j) Gao C-Y, Cao X, Yang L-M. Org. Biomol. Chem. 2009;7:3922. doi: 10.1039/b911286c. [DOI] [PubMed] [Google Scholar]
- 6.Bolm has reported the use of Cs2CO3 with Ni(COD)2 and BINAP in the amination of aryl tosylates with sulfoximines. See reference 3a.
- 7.Yang has reported a successful example containing an ethyl ester. See reference 2b.
- 8.Singh has reported the arylation of amines using boronic acids and a nickel catalyst using DBU as the base. See: Raghuvanshi DS, Gupta AK, Singh KN. Org. Lett. 2012;14:4326. doi: 10.1021/ol3021836.
- 9.Johnson has reported Ni-catalyzed electrophilic amination of an an arylzinc reagent containing an ethyl ester. See: Johnson JS, Berman AM. Synlett. 2005:1799.
- 10.Chatt J, Shaw BL. J. Chem. Soc. 1960:1718. [Google Scholar]
- 11.Concurrent with the advent of Ni(II)-(σ-aryl) complexes as pre-catalysts, the groups of Nolan, Matsubara, and Nicasio have made excellent use of pre-ligated, air-stable Ni-NHC complexes for use in C-N cross-coupling and other Ni-catalyzed cross-coupling reactions. See the following references: Kelly RA, Scott NM, Díez-González S, Stevens ED, Nolan SP. Organometallics. 2005;24:3442. Matsubara K, Ueno K, Koga Y, Hara K. J. Org. Chem. 2007;72:5069. doi: 10.1021/jo070313d. Iglesias MJ, Prieto A, Nicasio MC. Adv. Synth. Catal. 2010;352:1949. Iglesias MJ, Blandez JF, Fructos MR, Prieto A, Álvarez E, Belderrain TR, Nicasio MC. Organometallics. 2012;31:6312. Martin AR, Makida Y, Meiries S, Slawin AMZ, Nolan SP. Organometallics. 2013;32:6265.
- 12.Chen C, Yang L-M. J. Org. Chem. 2007;72:6324. doi: 10.1021/jo0709448. [DOI] [PubMed] [Google Scholar]
- 13.This system was also further applied to many C-N cross-coupling reactions see references: 2a, 2b, 5j, 12 and the following: Fan X-H, Li G, Yang L-M. J. Organomet. Chem. 2011;696:2482.
- 14.Ni(II)-(σ-aryl) complexes have also been well-studied in the context of C-C bond formation: Xing C-H, Lee J-R, Tang Z-Y, Zheng JR, Hu Q-S. Adv. Synth. Catal. 2011;353:2051. Fan X-H, Yang L-M. Eur. J. Org. Chem. 2011:1467. Leowanawat P, Zhang N, Safi M, Hoffman DJ, Fryberger MC, George A, Percec V. J. Org. Chem. 2012;77:2885. doi: 10.1021/jo3001194. Standley EA, Jamison TF. J. Am. Chem. Soc. 2013;135:1585. doi: 10.1021/ja3116718.
- 15.See Supporting Information.
- 16.No loss of catalyst activity was observed after seven months.
- 17.The bond angles for P1-Ni-Cl (167.1°) and P2-Ni-C (163.7°) show that the complex is distorted from the expected 180° for square planar geometry.
- 18.A similar observation on the differing effects of the tert-butoxide bases (LiOtBu, NaOtBu and KOtBu) on product yield was made by Lipschutz. See reference 5d.
- 19.Ge S, Hartwig JF. J. Am. Chem. Soc. 2011;133:16330. doi: 10.1021/ja2082087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.For examples where similar byproducts are formed from Ni(II)-(σ-aryl) precatalysts, see references 2a, 2b, 12, and 13.
- 21.Yang has reported that the cross-coupling of diphenylamine with aryl halides using Ni(II)-(σ-aryl) precatalysts did not produce the corresponding arylated diphenylamine byproduct from catalyst activation. See reference 5j.
- 22.The corresponding aminated o-tolyl products were observed in the crude reaction mixtures of the following substrates: 6c, 6d, 6f (Table 1); 7a, 7e (Table 2); and 8a, 8c, 8d, 8e (Table 3).
- 23.Meyers C, Maes BUW, Loones KTJ, Bal G, Lemière GLF, Dommisse RA. J. Org. Chem. 2004;69:6010. doi: 10.1021/jo049774e. [DOI] [PubMed] [Google Scholar]
- 24.The use of 3 Å molecular sieves in conjunction with K3PO4 was found to eliminate byproducts resulting from the hydrolysis of esters. However, in certain cases (7a, Scheme 3), their use lead to incomplete conversion of the aryl electrophile, suggesting a benefical role of small amounts of water. This phenomenon has been observed before with inorganic bases, see: Klapars A, Huang X, Buchwald SL. J. Am. Chem. Soc. 2002;124:7421. doi: 10.1021/ja0260465.
- 25.For examples of Pd-catalyzed aminaton of aryl mesylates, see: So CM, Zhou Z, Lau CP, Kwong FY. Angew. Chem. Int. Ed. 2008;47:6402. doi: 10.1002/anie.200802157. Fors BP, Watson DA, Biscoe MR, Buchwald SL. J. Am. Chem. Soc. 2008;130:13552. doi: 10.1021/ja8055358. Dooleweerdt K, Fors BP, Buchwald SL. Org. Lett. 2010;12:2350. doi: 10.1021/ol100720x. Alsabeh PG, Stradiotto M. Angew. Chem. Int. Ed. 2013;52:7242. doi: 10.1002/anie.201303305.
- 26.Ni-catalzyed C-C bond formation with aryl mesylates is well established. For selected recent examples, see: Kuroda J-I, Inamoto K, Hiroya K, Doi T. Eur. J. Org. Chem. 2009:2251. Molander GA, Beaumard F. Org. Lett. 2010;12:4022. doi: 10.1021/ol101592r. Muto K, Yamaguchi J, Itami K. J. Am. Chem. Soc. 2012;134:169. doi: 10.1021/ja210249h.
- 27.See reference 2c
- 28.Electron-deficient aryl triflates are known to undergo O-S bond cleavage in strongly basic conditions, see the following: Wolfe JP, Buchwald SL. J. Org. Chem. 1997;62:1264. Louie J, Driver MS, Hamann BC, Hartwig JF. J. Org. Chem. 1997;62:1268. Åhman J, Buchwald SL. Tetrahedron Lett. 1997;38:6363.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.