Abstract
Background: Distant metastasis, generally to lung and bone, is rare in differentiated thyroid carcinoma (DTC) and the prognosis is still elusive. We investigated long-term outcomes of lung metastasis in DTC patients and its prognostic factors.
Methods: A retrospective review was performed of 4572 patients who underwent surgery for DTC from 1962 to 2009 at Seoul National University Hospital. Among them, 164 patients were identified with lung metastasis and 152 patients were enrolled in the final analysis. Poor prognosis was defined as progressive disease or death.
Results: Of these 152 patients, 10- and 20-year survival rates were 85.0% and 71.0%, respectively. No evidence of disease, stable disease, progressive disease, and death was identified in 22.4%, 28.3%, 35.5%, and 13.8%, respectively, after 11 years of median follow-up (range 2–41 years). Older age at diagnosis (≥45 years), primary tumor size ≥2 cm, follicular thyroid cancer, metastasis diagnosed after initial evaluation or 131I remnant ablation (late metastasis), multiple metastases other than lung, 131I nonavidity, and the presence of macronodules (≥1 cm) were more frequent in poor prognoses. Cox proportional hazard ratio for progression-free survival showed that 131I nonavidity was the only independent predictive factor for poor prognosis.
Conclusions: The prognosis of lung metastasis from DTC in Korea within this study was favorable. 131I nonavidity, observed more frequently in late metastasis, was the only independent factor predicting poor prognosis.
Introduction
Differentiated thyroid carcinoma (DTC), which includes papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC), is one of the most curable endocrine cancers. Distant metastasis occurs in 4–23% of DTC patients and the lung is the most frequent site of distant metastasis (1–7). The 10-year survival rate after a diagnosis of metastatic DTC ranges from 25% to 70%, and patients with metastatic DTC have markedly varying clinical outcomes from rapid progression and death to complete remission, suggesting a heterogeneity in those patients (2,6,8–14). Several retrospective studies have shown that the prognostic factors for the survival of metastatic DTC include age at diagnosis (9,14,15), histology of the tumor (14,16), site of metastasis (14,15), and 131I avidity (9,15,16).
Recently, the incidence of DTC has increased worldwide. The reason for these increasing incidence rates is not only explained by early detection from increased health screening with high-resolution ultrasonography (17–19), but may also be associated with environmental changes or radiation exposure (20–22). In Korea, the incidence of thyroid cancer is also rapidly increasing; the incidence rates per 100,000 persons adjusting for age increased from 2.1 (in 1999) to 15.4 (in 2009) in males and 10.4 (in 1999) to 79.6 (in 2009) in females (23)—rates that are higher than in any other country studied (24). Furthermore, Korean DTCs have several distinguishing characteristics: (i) family history of DTC is twofold higher than in other studies (25), (ii) PTC is more frequent (more than 90%) (26), and (iii) DTC patients had higher rates of BRAF V600E mutations (58–81%) (27–29) in Korea than in any other country (30). Environmentally, Korea is one of the iodine-sufficient areas in the world (31). Based on these distinct features, the long-term prognosis of metastatic DTC might be different from that in other countries.
The present study is a retrospective chart review of 152 DTC patients with lung metastasis, with a median follow-up period of 12 years. The aim of this study was to investigate the long-term prognosis of lung metastasis of DTC and the factors associated with long-term prognosis in Korea. We found that patients with lung metastasis of DTC in Korea had usually excellent long-term survival: the 20-year survival rate was ∼65.2% with aggressive treatment that included total thyroidectomy, lymph node (LN) dissection, and radioactive iodine ablation. 131I nonavidity in whole-body scan (WBS) was the most predictive factor for a poor prognosis.
Materials and Methods
Patients
A retrospective chart review of 4572 patients who were diagnosed with thyroid cancer (International Classification of Diseases code C73) and treated at Seoul National University Hospital from 1962 to 2009 was carried out. A total of 4393 patients had DTC and 164 (3.7%) patients were diagnosed with lung metastasis. Among them, 3 patients who had other coexisting cancers and 9 patients who did not receive any further regular follow-ups were excluded; therefore, 152 patients were finally enrolled in the analysis. This study was conducted according to the guidelines of the Declaration of Helsinki, and the research protocol was approved by the Institutional Review Board committee of Seoul National University Hospital (No. H-0912-009-302).
Treatment protocols
All 152 patients underwent thyroidectomy, and neck dissection was performed if there was any enlarged lymph node suspicious for malignancy present in the preoperative radiologic examinations. Patients (n=151) who had extrathyroidal invasion, LN metastasis, a larger tumor size (≥4 cm), or any evidence of distant metastasis received postoperative 131I administration (from 30 to 200 mCi) to ablate the normal thyroid and residual disease or to treat recurrent and metastatic lesions. One patient who was diagnosed with thyroid cancer by an initial lung mass did not receive 131I therapy due to a rapid disease progression and poor performance status. To exclude the effects of previous administrations of intravenous contrast on 131I uptakes, all 131I therapies were performed at least more than 2 months later from any previous use of intravenous contrast.
A higher dose (≥100 mCi) was given when lung metastases were detected by a prior WBS. Serum thyrotropin (TSH), thyroglobulin (Tg) and anti-Tg antibody (Tg-Ab) levels were measured in all patients immediately before 131I administration under TSH stimulation (≥30 μIU/mL) with the withdrawal of thyroxine (T4). WBS was performed 3–5 days after 131I administration. All patients were treated with thyroxine to suppress TSH. Regular follow-up evaluations were performed every 6 months after surgery with clinical assessment and measurement of serum TSH, Tg, and Tg-Ab.
The WBS images were interpreted by two experienced nuclear medicine physicians. If any abnormal 131I uptake suggesting recurrence or metastasis was observed, further radiological studies, such as chest X-ray (82%), chest computed tomography (CT; 86%) and/or fluoro-18 fluorodeoxy glucose positron emission tomography (18F-FDG-PET, 60%), were performed. False-positive radioactive iodine uptake was confirmed when concordant benign lung lesions (e.g., infections, bronchiectasis, and atelectasis) were observed in the chest CT. Neck lesions were further evaluated by fine-needle aspiration cytology. To improve the efficacy of 131I therapy, all patients who had measureable metastatic lesions were considered for surgical resection whenever possible, whether those were locoregional or distant. Negative WBSs with clinically suspected lung metastasis such as elevated Tg levels with lung lesions in other radiological studies (chest CT or chest X-ray) were defined as “nonavid” lesions. Collectively, diagnosis of lung metastases could be categorized into three different groups according to the timing of metastasis as follows: (i) diagnosed by radiologic images (chest X-ray or CT) during initial preoperative work-up (“pre-op metastasis”), (ii) detected by WBS during initial 131I remnant ablation (“immediate metastasis”), or (iii) diagnosed by WBS or radiologic examination performed due to increases in T4-suppressed Tg or stimulated (off T4) Tg after initial evaluation or 131I remnant ablation (“late metastasis”).
Diagnosis of lung metastasis
Among the 152 patients, 132 patients were clinically diagnosed with lung metastasis by radiologic examinations, including 131I (WBS), chest CT, and/or chest X-ray, and 20 patients underwent lung biopsy and had a pathologic diagnosis.
Eleven patients were diagnosed by preoperative radiologic examinations (chest CT/X-ray). Among the 79 patients who were diagnosed with lung metastasis immediately after the operation, 56 (71%) patients were diagnosed by 131I WBS, and 23 (29%) were diagnosed by chest CT/X-ray. Fifty-two patients who were not diagnosed with lung metastasis during the initial remnant ablation periods underwent further studies including neck ultrasonography, chest CT/X-ray, or 131I WBS due to increased serum Tg or Tg-Ab levels, and were diagnosed with lung metastasis by 131I WBS (n=15) or chest CT/X-ray (n=37). Ten patients were diagnosed with lung metastasis incidentally by chest CT.
Except for one patient who had no opportunity to receive 131I therapy due to a rapid disease progression, all 151 patients underwent 131I therapy: 40 were 131I nonavid, and 111 had 131I-avid lesions in the post-therapy WBS. Among the 40 131I nonavid patients who had lung lesions in the chest CT with elevated Tg or Tg-Ab levels, 32 patients had lung lesions in the 18F-FDG-PET/CT. Lung biopsy was performed in 20 of the 40 nonavid patients. The other 20 patients who did not have lung biopsies performed were clinically diagnosed based on their compatible CT images of lung lesions (miliary or multiple lung lesions in both lobes) and elevated T4-off/on Tg levels. Lung metastasis was defined as lung parenchymal metastases and did not include mediastinal or pleural metastases.
Classification of disease status
Based on the radiological images, including WBS and serum Tg levels, the disease status was classified as follows: no evidence of disease (NED), stable disease (SD), progressive disease (PD), and death. NED was defined as undetectable T4-off Tg levels (<1.0 ng/mL) with NED in the WBS or other imaging studies (neck ultrasonography, chest CT, or 18F-FDG-PET/CT scans). The sensitivity of our Tg measurements changed during the study period: lower detection limits changed from 1.0 to 0.1 ng/mL. After this sensitivity change, six patients showed Tg levels ranging from 0.1 to 1.0 ng/mL. They showed NED in the neck ultrasonography, chest CT, bone scan, and/or PET CT and were classified as NED. SD was defined as positive Tg levels (suppressed or stimulated) with or without persistent cervical uptake and/or metastasis in WBS, but without progressive elevation of Tg levels and no new metastatic foci. PD was defined as increasing suppressed or stimulated Tg levels despite 131I therapy and/or new metastatic foci detected by imaging modalities. Data on thyroid-cancer-related death, which was not confirmed by the medical records, were assessed by the Korean Statistical Information Service. Patients with NED or SD were defined as “good prognosis,” and PD or thyroid-cancer-related death was defined as “poor prognosis.”
Statistical methods
Chi-square or Fisher's exact test was used to estimate the differences between groups (p<0.05 was considered significant). The Cox proportional hazard model was used to examine the association of related prognostic factors with the progression-free survival (PFS). The hazard ratio (HR), 95% confidence interval (CI), and p-value were reported. Cumulative survival plots were constructed using the Kaplan–Meier method. Log rank test was performed to evaluate the difference in the progression-free survival time between groups. All reported p-values are two-sided. All statistical analyses were performed using SPSS statistical software.
Results
Clinical characteristics of the patients with lung metastasis and their long-term prognosis
Among the 152 patient, 119 were women and 33 were men (F:M=3.6:1). One hundred twenty-five (82.2%) had PTC and 27 (17.8%) had FTC. The median age at diagnosis for the primary cancer was 48 years (range 10–75 years), and the median follow-up was 11 years (range 2–41 years). One hundred forty-one (92.8%) of the 152 patients underwent total thyroidectomy; 26 (17.1%) patients underwent a second operation consisting of a neck dissection for lymph node recurrence; 11 (7.2%) patients underwent a lobectomy or wedge resection of the lung due to macronodular lung metastasis. Of the 152 patients who were diagnosed with lung metastasis, 125 (82.2%) patients had lung metastasis only and 21 (13.8%) patients had both lung and bone metastases.
At last follow-up, 131 (86.2%) patients were alive and 21 (13.8%) were dead. Among the deceased patients, 18 (85.7%) were found to have disease-specific mortality and 3 (14.3%) succumbed to hematologic malignancies after receiving a high cumulative dose of radioactive iodine therapy (>1000 mCi). Among the patients who are alive, 97 (74.0%) still have evidence of disease and 34 (26.0%) have NED. Collectively, 77 (50.7%) patients showed a good prognosis (NED to SD) and 75 (49.3%) showed a poor prognosis (PD to death) at last follow-up.
The overall survival rate was 86% and the 10- and 20-year survival rates were 85% and 71%, respectively. The 10-year survival rate in the lung-metastasis-only group (n=125) was 89%, whereas the 5- and 10-year survival rates in the multiple metastasis group (n=21) were 72% and 51%, respectively.
Because the survival rates of the present study were very high, the patients were divided into two groups according to their prognosis: the good prognosis (NED to SD) group and poor-prognosis (PD to death) group. The predictable factors for a poor prognosis were analyzed. Table 1 shows a comparison of the clinical characteristics according to the prognostic groups for lung metastasis. The age at diagnosis for the primary cancer (52.3±13.0 vs. 39.1±17.3 cm, p<0.001) was higher; the initial size of the primary thyroid tumor was larger (2.4±1.7 vs. 3.3±2.0 cm, p=0.015), and FTC (10.3% vs. 25.7%, p=0.012) was more frequent in the poor-prognosis group rather than in the good-prognosis group. However, other pathologic findings such as multiplicity, the presence of extrathyroidal invasion, or cervical LN metastasis showed no difference between the groups.
Table 1.
Clinical Characteristics According to Prognosis
| Good prognosisa(n=78) | Poor prognosisb(n=74) | p | |
|---|---|---|---|
| Mean age at diagnosis (years) | 39.1±17.3 | 52.3±13.0 | <0.001 |
| Male:female (male %) | 13:65 (16.7) | 20:54 (27.0) | 0.121 |
| Pathology of primary tumor | |||
| Size (cm) | 2.4±1.7 | 3.3±2.0 | 0.015 |
| PTC:FTC (FTC %) | 70:8 (10.3) | 55:19 (25.7) | 0.012 |
| Multiplicity, present % | 40.0 | 48.6 | 0.443 |
| Extrathyroidal invasion, gross, present % | 27.8 | 30.8 | 0.783 |
| LN metastasis, present % | 42.6 | 58.5 | 0.123 |
| Timing of metastasisc | <0.001 | ||
| Pre-op, n (%) | 3 (27.3) | 8 (72.7) | |
| Immediate, n (%) | 53 (67.1) | 26 (32.9) | |
| Late, n (%) | 22 (35.5) | 40 (64.5) | |
| Sites of metastasis | |||
| Lung only:multiple (multiple %) | 73:3 (3.9) | 52:18 (25.7) | <0.001 |
| Pattern of lung uptake in WBS | <0.001 | ||
| Bilateral diffuse, n (%) | 35 (89.7) | 4 (10.3) | |
| Focal, n (%) | 27 (57.4) | 20 (42.6) | |
| Nonavid, n (%) | 6 (15.0) | 34 (85.0) | |
| Size of lung lesions from radiologic examd | |||
| Micro:macro (macro %) | 13:12 (48.0) | 8:50 (86.2) | <0.001 |
Good prognosis: no evidence of disease or stable disease.
Poor prognosis: progression of disease or death.
Pre-op: lung metastasis diagnosed during initial preoperative work-up; immediate: metastasis diagnosed by WBS during initial 131I remnant ablation; late: diagnosed by WBS or radiologic examination performed due to the increases of T4-suppressed Tg or stimulated (off T4) Tg after initial evaluation or 131I remnant ablation.
Micro: micronodule, where the largest size of the nodule is <1 cm; macro: macronodule, where the largest size of the nodule is ≥1 cm.
FTC, follicular thyroid carcinoma; LN, lymph node; PTC, papillary thyroid carcinoma; T4, thyroxine; WBS, whole-body scan.
Several metastasis-related factors, including the timing of metastasis, site of metastasis (lung only vs. multiple), pattern of lung uptake in WBS, and size of the lung lesions, were significantly different between the prognostic groups. According to the timing of metastasis, the pre-op group clearly had higher rates of poor prognosis (72.7%). Moreover, among patients who were diagnosed after thyroidectomy, the late metastasis group frequently had more poor prognoses than that of the immediate metastasis group (64.5% vs. 32.9%, p<0.001). Multiple metastatic sites rather than lung-only had higher rates of poor prognosis. Among the different patterns of lung uptake in WBS, focal uptake (42.6% vs. 10.3%, p<0.001) or 131I nonavidity (85.0% vs. 10.3%, p<0.001) was more frequent in the poor-prognosis group than that of bilateral diffuse uptake. Interestingly, radiologic images such as chest X-ray or CT revealed that all the metastatic lesions were multiple with variable sizes. There was no solitary lesion of lung metastasis. These lung lesions were further classified into two groups according to their size of largest nodule: micronodules (largest nodule <1 cm) and macronodules (largest nodule ≥1 cm). As expected, 86.2% of the macronodular lesions showed a poor prognosis, whereas 52.0% of the micronodular lesions showed a good prognosis. The number of or the largest size of macronodules within lesions showed no difference between the various prognoses groups (good/poor) (data not shown).
Clinical characteristics and prognosis of patients according to timing of metastasis
The clinical characteristics according to the timing of the metastasis are summarized in Table 2. The pre-op metastasis group had the highest rate of poor prognosis, with 75.0% having macronodular lung lesions. Because the initial pathologic findings (pathology, size, and LN status) or characteristics of the metastatic lesions (site of metastasis and the pattern of WBS) showed no distinctive features compared to the other groups (immediate or late metastasis), pre-op diagnosis of lung metastasis might be a valuable predictive factor for a poor prognosis.
Table 2.
Comparison of the Clinical Characteristics Between Different Timing of Metastasis
| Pre-op(n=11) | Immediate(n=79) | Late(n=62) | p* | p# | |
|---|---|---|---|---|---|
| Mean age at diagnosis (years) | 49.6±22.1 | 45.2±17.7 | 45.3±14.2 | 0.713 | 0.519 |
| Male:female (male %) | 1:10 (9.1) | 16:63 (20.3) | 16:46 (25.8) | 0.380 | 0.436 |
| Pathology of primary tumor | |||||
| Size (cm) | 3.4±1.9 | 2.7±1.7 | 2.9±2.2 | 0.566 | 0.234 |
| PTC:FTC (FTC %) | 9:2 (18.2) | 65:14 (17.7) | 51:11 (17.7) | 0.999 | 0.997 |
| Multiplicity, present % | 55.6 | 37.5 | 52.2 | 0.380 | 0.243 |
| Extrathyroidal invasion, gross, present % | 42.9 | 20.4 | 47.4 | 0.063 | 0.028 |
| LN metastasis, present % | 44.4 | 40.0 | 67.7 | 0.043 | 0.013 |
| Pattern of lung uptake in WBS | <0.001 | <0.001 | |||
| Bilateral diffuse, n (%) | 2 (25.0) | 29 (42.6) | 8 (16.0) | ||
| Focal, n (%) | 4 (50.0) | 29 (42.6) | 14 (28.0) | ||
| Nonavid, n (%) | 2 (25.0) | 10 (14.7) | 28 (56.0) | ||
| Size of lung lesions from radiologic exam | |||||
| Micro:macro (macro %) | 2:6 (75.0) | 9:17 (65.4) | 10:39 (79.6) | 0.414 | 0.184 |
| Prognosis | <0.001 | <0.001 | |||
| Good:poor (poor %) | 3:8 (72.7) | 53:26 (32.9) | 22:40 (64.5) | ||
p-Value by one-way analysis of variance, chi-square test, or Fisher's exact test between three groups.
p-value by t-test, chi-square test, or Fisher's exact test between immediate and late groups.
Among patients who had no metastasis at the time of the diagnosis of the primary cancer (n=141), the immediate (n=79) and late (n=62) metastasis groups clearly showed different clinical characteristics and prognoses. The pathologic findings of the primary tumors showed that the late metastasis group had higher rates of extrathyroidal invasion (47.4% vs. 20.4%, p=0.028) and LN metastasis (40.0% vs. 67.7%, p=0.013). In terms of metastasis-related factors, the late metastasis group showed higher rates of 131I nonavidity (56.0% vs. 14.7%, p<0.001) and poor prognosis (64.5% vs. 32.9%, p<0.001) than that of the immediate metastasis group.
Progression-free survival of lung metastasis
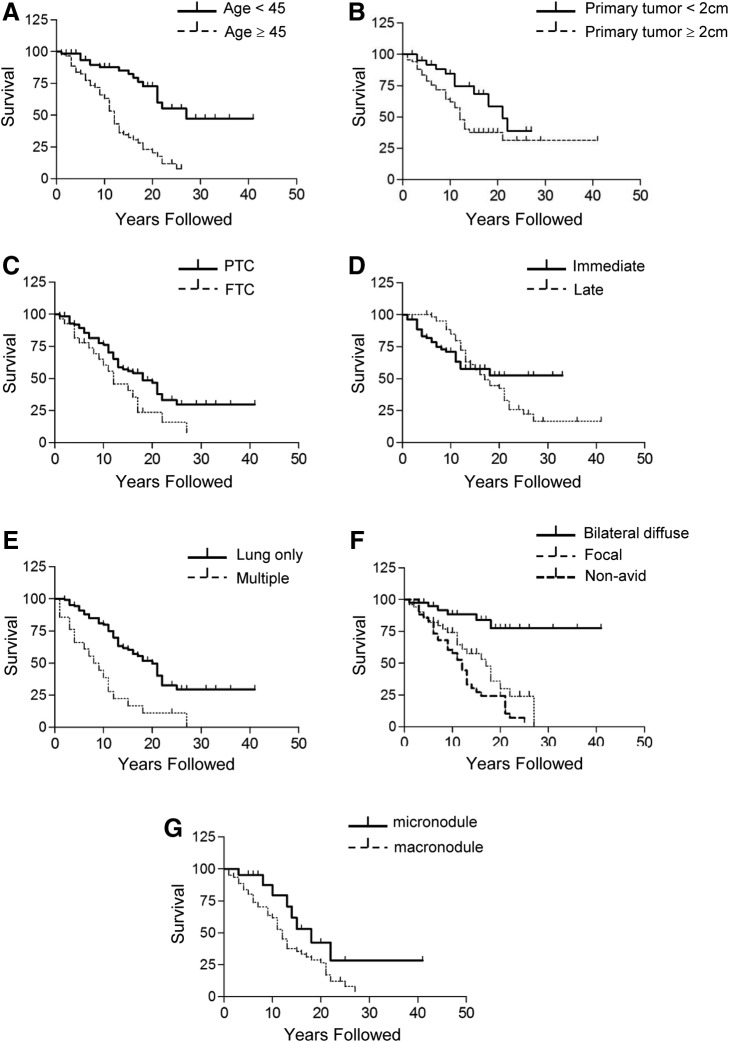
Further analysis evaluating prognostic factors was performed with the 141 patients who were diagnosed with lung metastasis after surgery (immediate or late metastasis groups), excluding the pre-op metastasis group because of their distinct characteristics, having markedly higher rates of poor prognosis. Data on progression-free survival for the 141 patients were further analyzed by Kaplan–Meier survival analysis. Survival curves for age at diagnosis over 45 years (Fig. 1A; p<0.001, by log-rank test), primary tumor size larger than 2 cm (Fig. 1B; p=0.037, by log-rank test), FTC (Fig. 1C; p=0.030, by log-rank test), late metastasis group (Fig. 1D; p<0.001, by log-rank test), multiple metastases (Fig. 1E; p<0.001, by log-rank test), focal uptake or nonavidity of radioactive iodine in WBS (Fig. 1F; p<0.001, by log-rank test), and macronodule group (Fig. 1G; p=0.040, by log-rank test) showed markedly poor progression-free survival.
FIG. 1.
Progression-free survival for the patients with lung metastasis by (A) age at diagnosis, (B) tumor size of primary cancer, (C) type of histology, (D) timing of metastasis, (E) sites of metastasis, (F) the pattern of lung uptakes in WBS, and (G) the presence of macronodules in the lungs on radiological examination. Immediate, metastases that were diagnosed during the time of 131I remnant ablation; late, metastases that were diagnosed during the follow-up period after remnant ablation; micronodule, the largest lung lesions <1 cm; macronodule, the largest lung lesions ≥1 cm. FTC, follicular thyroid carcinoma; PTC, papillary thyroid carcinoma; WBS, whole-body scan.
Independent risk factors for prognosis of lung metastasis
To further analyze the predictive variables for a poor prognosis, Cox proportional HR for progression-free survival was used (Table 3). Variables were selected from factors that showed a significant difference between the good and poor prognostic groups except for the largest size of lung lesions (macro- or micronodules) from the radiologic examination, which had many missing values. Cox analysis with age at diagnosis for primary cancer, primary tumor size, pathology, sites of metastasis, timing of metastasis, and the pattern of lung uptake in WBS showed 131I nonavidity (HR 10.44 [CI 2.59–45.05], p=0.001) was the only independent predictive variable for a poor prognosis.
Table 3.
Cox Models of Prognostic Factors in Patients with Lung Metastasis
| HR | [95% CI] | p | |
|---|---|---|---|
| Age at diagnosis of primary cancer ≥45 years | 1.94 | [0.77–4.88] | 0.160 |
| Primary tumor size ≥2 cm | 2.05 | [0.95–4.43] | 0.069 |
| Follicular pathology | 1.49 | [0.47–4.68] | 0.500 |
| Multiple site of metastasis | 2.47 | [0.81–7.55] | 0.113 |
| Late metastasis | 1.76 | [0.74–4.22] | 0.204 |
| Pattern of lung uptake in WBS | |||
| Bilateral diffuse | 1 | Reference | |
| Focal | 2.09 | [0.51–8.56] | 0.304 |
| Nonavid | 10.44 | [2.59–42.05] | 0.001 |
HR and 95% CI were based on Cox proportional hazard models for risk prediction. HR, hazard ratio; CI, confidential interval.
131I avidity and its predictive factors
Since 131I nonavidity was the most predictable value for a poor prognosis, clinical characteristics according to the pattern of lung uptakes in WBS were further analyzed (Table 4). Of the 38 patients with 131I nonavid lung lesions, 34 (89.5%) patients showed a poor prognosis, whereas only 22 of 80 (24.4%) patients showed a poor prognosis in the 131I-avid group. The 131I nonavid group showed higher rates of initial LN metastasis, the presence of macronodules, and later timing of metastasis compared to the 131I-avid group (Table 4).
Table 4.
Comparison of Clinical Characteristics According to the Pattern of Lung Uptake in Whole-Body Scan
| Bilateral diffuse(n=37) | Focal(n=43) | Nonavid(n=38) | p | |
|---|---|---|---|---|
| Mean age at diagnosis (years) | 32.7±17.1 | 49.4±14.3 | 51.7±11.7 | <0.001 |
| Male:female (male %) | 7:30 (18.9) | 6:36 (14.0) | 11:27 (28.9) | 0.531 |
| Pathology of primary tumor | ||||
| Size (cm) | 2.8±1.9 | 2.7±1.9 | 2.9±1.8 | 0.905 |
| PTC: FTC (FTC %) | 33:4 (10.8) | 32:11 (25.6) | 35:3 (7.9) | 0.080 |
| Multiplicity, present % | 40.0 | 44.0 | 42.9 | 0.958 |
| Extrathyroidal invasion, gross, present % | 36.4 | 20.6 | 33.3 | 0.335 |
| LN metastasis, present % | 33.3 | 46.7 | 70.8 | 0.020 |
| Time of metastasis | ||||
| Immediate:late (late %) | 29:8 (21.6) | 29:14 (32.6) | 10:28 (73.7) | <0.001 |
| Sites of metastasis | ||||
| Lung only:multiple (multiple %) | 35:2 (5.4) | 32:11 (25.6) | 33:5 (13.2) | 0.044 |
| Size of lung lesions from radiologic exam | ||||
| Micro:macro (macro %) | 6:2 (25.0) | 8:14 (63.6) | 5:32 (86.5) | 0.002 |
| Prognosis | ||||
| Good:poor (poor %) | 35:2 (5.4) | 23:20 (46.5) | 4:34 (89.5) | <0.001 |
Within the 131I-avid groups, the bilateral diffuse group was related to a good prognosis (94.6% vs. 53.5%, p<0.001) compared to the focal group. Younger patients and micronodular lung lesions were more frequent in the bilateral diffuse group than in the focal or nonavid group. Among patients less than 45 years of age, bilateral diffuse uptake (28/43, 65.1%) was more frequent, whereas focal uptake (32/43, 74.4%) was more frequent among patients over 45 years of age (p<0.001).
T4-off Tg concentrations and prognosis
Bone metastasis is a well-known major determinant factor for elevating T4-off Tg concentrations. T4-off Tg concentrations in the multiple metastasis group (median 932; range 22–25,000 μg/L) showed a marked elevation compared to the lung-metastasis-only group (median 66; range 0–8150 μg/L, p<0.001). Therefore, subgroup analyses were performed within the lung-metastasis-only patients (n=125). The T4-off Tg levels at the time of lung metastasis were significantly different based on the different times of metastasis groups. The median T4-off Tg values of the immediate, late, and pre-op metastasis groups were 30 (range 0–3000), 82 (range 2–8150), and 407 (range 121–7850), respectively (p<0.01). Among the 125 patients, 76 patients who had serial T4-off Tg levels over the different times were further evaluated using the delta values of the T4-off Tg levels [ΔTg=Tg (at the time of last 131I WBS)−Tg (at the time of diagnosis of lung metastasis)]. Nineteen (25%) of them showed a ΔTg≥1.0 ng/mL, and this group had significantly higher rates of poor prognosis than that of the ΔTg<1.0 ng/mL group (58% vs. 28%, p<0.05).
One of the notable findings was that 9 patients showed undetectable T4-off Tg levels without elevation of the Tg-Ab levels over the different times, although 19 patients had undetectable Tg levels with increased Tg-Ab (range 41–7690 IU/mL). These 9 patients were diagnosed with lung metastasis immediately after thyroid surgery by WBS with multiple micronodular lesions (3–5 mm) in the chest CT (n=6) or positive mediastinal (n=7) lesions on WBS. All lesions were ablated after at least 3 sessions of 131I therapy with 160–450 mCi of cumulative 131I doses and the patients have currently an NED status.
Discussion
In this study, we show that Korean DTC patients with lung metastasis have very good long-term survival. Older age at diagnosis, a larger size of the primary cancer, follicular thyroid cancer, later timing of metastasis, multiple sites of metastasis, 131I nonavidity in metastatic lung lesions, and the presence of macronodules in radiologic examination predicted a poor prognosis. Among them, 131I nonavidity was proven as the only independent factor of predicting a poor prognosis.
The 5- and 10-year survival rates of DTC lung metastasis patients in this study were 93% and 85%, whereas they were 99% and 97% in all the DTC patients at our hospital (n=4393) (32). The survival rates in this study were much higher than those demonstrated in previous studies (Table 5) (9,14,16,33). The higher rate of PTC (82%) among DTC in this study is one possible explanation. This rate is consistent with other Korean data (26), but much higher than those in other countries (75–51%) (Table 5). Therapeutic factors, including initial total thyroidectomy and postoperative 131I ablation therapy, showed no differences (Table 5). The 10- and 20-year survival rates of the PTC subgroup from DTC with lung metastasis in an Italian study (33) were 81.1% and 70.7%, which were very similar to our results. These similarities in the survival rates from two different ethnic groups suggest that histologic type should be a critical factor in determining the survival of DTC patients with lung metastasis. PTC has been known to be more commonly associated with lung metastasis than with FTC (36% vs. 25%), and inversely, FTC has been identified to be more frequent in bone metastasis than in PTC (44% vs. 19%) (34). In association with the higher frequency of PTC (82%), the present study also showed higher rates of metastasis limited to the lungs (86.2%) compared to other studies (45–51%) (9,14,16), while patients with both lung and bone metastases had a relatively higher frequency of FTC than patients with lung metastasis only (55.6% vs. 11.1%). Collectively, we deduced that our favorable long-term prognosis is due to the higher frequency of PTC with consequently much less bone metastasis in Korean patients than in patients from other countries. However, one previous study with patients who had lung-only metastasis showed poor survival (44% for 20-year survival rate) (33) compared to the present study, indicating that other factors could be involved in long-term outcomes.
Table 5.
Comparison of Long-Term Survival of Metastatic Differentiated Thyroid Cancer
| Survival rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Follow-up duration (median, year) | Lung only % | Follicular % | Total thyroidectomy % | 131I ablation | 5 years | 10 years | 20 years | |
| Cho et al. (present study) | 90 | 12.0 | 90 | 84 | 89 | 88 | 93 | 85 | 71 |
| Sampson et al.16 | 49 | 3.5 | 45 | 51 | 82 | 88 | 50 | − | − |
| Huang et al.14 | 126 | 9.6 | 42 | 75 | 100 | 85 | − | 71 | − |
| Mihailovic et al.9 | 77 | 6.0 | 51 | 71 | − | − | 58 | 48 | 40 |
| Ronga et al.33 | 96 | 4.1 | 100 | 67 | 100 | 100 | 79 | 60 | 44 |
Since our patients showed high survival rates, we then analyzed the Cox model with progression-free survival instead of disease-free survival to investigate the prognostic factors for DTC with lung metastasis. Among the factors that were significantly different between the good- and poor-prognosis groups, size of the lung lesions (macro- or micronodules) was excluded because of its many missing values. As a result, the Cox proportional HR identified 131I nonavidity as the only independent factor for a poor prognosis. 131I avidity is one of the well-characterized prognostic factors in lung metastasis of DTC. Sampson et al. (16) showed that patients with 131I nonavid lung metastasis had a higher risk of death (HR 3.4 [CI 1.2–9.2], p=0.02) and 3-year survival rates were much lower in the 131I nonavid group (57% vs. 82%) than in the131I-avid group. Several other studies also reported that DTC patients with 131I avidity showed longer survival rates than those with nonavid lung metastasis (35–37). In agreement with the previous studies, we show that 131I nonavidity is the strongest predictive factor for a poor prognosis and related to older age at the initial cancer diagnosis, later metastasis, and more frequent macronodules compared to 131I-avid lung lesions. The reason for 131I nonavidity has been explained by the dedifferentiation of DTC cells, which is accompanied by decreased expression of the sodium iodide symporter NIS, the TSH receptor, and thyroid peroxidase (38–40). The ability of Tg production is also reduced. Because of these molecular changes, they are more difficult to monitor than 131I-avid lesions, resulting in later diagnosis and poor prognosis.
One of our limitations in accessing 131I-avid lesions was that we could not distinguish the mixed responses from homogenous responses. We defined 131I nonavidity only in the cases that had a negative WBS image despite lung lesions in the chest CT/X-ray and elevated Tg levels. Although 131I-avid and nonavid lesions could co-exist in one patient, we could not find any of those mixed responses in this study. The limited sensitivity of our WBS used in this study is one possible explanation for this finding. Currently, we are using single-photon emission computed tomography–CT and can easily determine mixed responses; hence, further study is needed. Another limitation consists in the diagnosis of lung metastasis in 131I nonavid patients. Among 40 patients, 20 of them could not be evaluated with a lung biopsy because of the location or small size of the lesions. Therefore, they were clinically diagnosed based on their CT images (miliary or multiple lung lesions in both lobes) and elevated T4-off/on Tg levels. However, we cannot completely rule out the possibility of other conditions, such as incidental primary lung cancers or benign lung diseases such as inflammations or bronchiectasis.
In addition, our study shows that among patients with 131I-avid lung metastasis, the pattern of lung uptake in WBS could also predict the prognosis; bilateral diffuse uptakes had better prognoses than the focal uptakes. Previously, Casara et al. (15) showed that micronodular metastasis susceptible to 131I therapy had a good prognosis, whereas macronodular metastasis of 131I nonavid lesions had a poor prognosis. In the present study, our goal was to predict the prognosis of lung metastasis by the initial WBS images. We clearly proved that within the 131I-avid groups, focal uptake had a worse prognosis than bilateral diffuse uptake even though it had a better prognosis than the 131I nonavid group. Since micronodular lesions as well as macronodular lesions with focal uptakes had a poor prognosis, we suggest that the prognosis of lung metastasis could be predicted by the pattern of lung uptake in WBS instead of the size of the metastatic lesions.
To provide useful information for clinical decisions such as the dose of 131I therapy, it is important to analyze the predictive factors for 131I avidity. In our data, younger age and micronodular lesions tended to have bilateral diffuse uptake, whereas late metastasis had 131I nonavidity more frequently. Taken together, we suggest that patient age over 45 years, macronodular lesions, or late metastasis can be associated with poor uptake of 131I; thus, a higher dose of 131I or the use of differentiating agents such as retinoic acid (41–43) or Selumetinib (44), which could increase the susceptibility of 131I therapy, might be helpful in increasing the efficacy of the 131I therapy.
One of the aims of this study was to evaluate the prognostic values of the pattern of lung lesions, such as single-macronodule or multiple-macronodule or multiple-miliary micronodular lesions. However, to our surprise, all patients with lung metastasis had multiple lesions. We could further divide these lung lesions into three different groups: (i) multiple miliary micronodules (n=21), or (ii) single or several macronodules with multiple micronodules (n=56), or (iii) multiple macronodules without any micronodules (n=6). However, the number or the size (the longest diameter) of the macronodular lesions had no effect on disease progression. Only the presence of a macronodule was associated with poor prognosis. Most of our macronodules were sized between 1 and 2 cm except two patients, who had 6- or 10-cm-sized macronodules with tiny multiple micronodules. Their initial tumor burden of the lung was comparably bigger than that of the others; however, they were currently in an NED status with surgical management (lobectomy) of the lung lesions, following 131I therapy for the remaining lesions. Although this is a very small number of patients, in combination with other studies (16,45), we could carefully deduce that large lung lesions could achieve good prognosis with optimal surgical intervention.
The age at diagnosis of the initial cancer is known to be a valuable prognostic factor for the recurrence and mortality of DTC (15,46). Our data showing a higher rate of bilateral diffuse uptake in younger patients than in older patients could explain the reason for the particularly high survival rates in young patients with DTC lung metastasis.
As with the 131I avidity, timing of metastasis was a valuable prognostic factor. Among 3 metastasis groups classified according to the timing of metastasis detection, 11 patients in the pre-op metastasis group had markedly higher rates of poor prognosis (73%), mortality (30%), and macronodular lesions (75%). Although early lung metastasis before the diagnosis of the primary tumor is a strong predictable factor for a poor prognosis, it is still hard to recommend an evaluation of metastatic lesions at the initial diagnosis of thyroid cancer because it is very rare and there is no effective treatment other than 131I therapy until now. Late metastasis was found in ∼40% of the DTC patients during a median of 11 (range 2–41) years of follow-up, and was significantly related to a poor prognosis compared to immediate metastasis (64.5% vs. 32.9%) in association with a higher frequency of 131I nonavidity (53.7% vs. 15.3%).
Interestingly, we found that 9 patients diagnosed with lung metastasis by 131I WBS lesions immediately after the operations had undetectable Tg and Tg-Ab levels. Because 6 of them had multiple (3–8) micronodular (3–5 mm) lesions in the chest CT and 7 of them had positive uptake of 131I in the mediastinum, we considered them as having lung metastasis. All 9 lesions were ablated after at least 3 sessions of 131I therapy with 160–450 mCi of cumulative 131I doses (none had false-positive lesions). Consistent with our data, Huang et al. (14) also reported on five patients with thyroid cancer lung metastasis who showed undetectable Tg and Tg-Ab levels and who had favorable outcomes. One of the possible explanations for the good prognosis of 131I WBS–positive, Tg-negative lesions is that this is early detection of a very small cancer load with a minute secretion of Tg can be associated with a good prognosis.
One of the critical limitations of this study is its retrospective design. During the four decades of the study period, some important clinical data were missing, which might cause a bias in the data selection. Indeed, WBS images acquired in the early 1980s were not available to check for the pattern of lung lesions (∼17%), and chest CT and/or chest X-ray were performed more frequently in the 131I nonavid group (99%) than in the 131I-avid group (∼40%). Further prospective studies are needed.
In conclusion, lung metastasis from DTC showed good overall and progression-free survival rates in patients from Korea. The higher prevalence of PTC with less frequent bone metastasis in our patients might be one of the reasons for this favorable long-term prognosis. The age at diagnosis or tumor size for the primary cancer, type of histology, timing of metastasis, site of metastasis, 131I avidity, and size of lung lesions could predict the prognosis. Among them, 131I nonavidity was the only independent factor for a poor prognosis.
Acknowledgments
This study was supported by the Research Grant Number CB-2011-03-01 from the Korean Foundation for Cancer Research.
Author Disclosure Statement
The authors have no conflicts of interest.
References
- 1.Massin JP, Savoie JC, Garnier H, Guiraudon G, Leger FA, Bacourt F.1984Pulmonary metastases in differentiated thyroid carcinoma. Study of 58 cases with implications for the primary tumor treatment. Cancer 53:982–992 [DOI] [PubMed] [Google Scholar]
- 2.Ruegemer JJ, Hay ID, Bergstralh EJ, Ryan JJ, Offord KP, Gorman CA.1988Distant metastases in differentiated thyroid carcinoma: a multivariate analysis of prognostic variables. J Clin Endocrinol Metab 67:501–508 [DOI] [PubMed] [Google Scholar]
- 3.Brown AP, Greening WP, McCready VR, Shaw HJ, Harmer CL.1984Radioiodine treatment of metastatic thyroid carcinoma: the Royal Marsden Hospital experience. Br J Radiol 57:323–327 [DOI] [PubMed] [Google Scholar]
- 4.Casara D, Rubello D, Saladini G, Gallo V, Masarotto G, Busnardo B.1991Distant metastases in differentiated thyroid cancer: long-term results of radioiodine treatment and statistical analysis of prognostic factors in 214 patients. Tumori 77:432–436 [DOI] [PubMed] [Google Scholar]
- 5.Samaan NA, Schultz PN, Haynie TP, Ordonez NG.1985Pulmonary metastasis of differentiated thyroid carcinoma: treatment results in 101 patients. J Clin Endocrinol Metab 60:376–380 [DOI] [PubMed] [Google Scholar]
- 6.Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis M, Maxon HR, Sherman SI.2006Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 16:1229–1242 [DOI] [PubMed] [Google Scholar]
- 7.Lang BH, Wong KP, Cheung CY, Wan KY, Lo CY.2013Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol 20:1329–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlumberger M, Challeton C, De Vathaire F, Travagli JP, Gardet P, Lumbroso JD, Francese C, Fontaine F, Ricard M, Parmentier C.1996Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J Nucl Med 37:598–605 [PubMed] [Google Scholar]
- 9.Mihailovic J, Stefanovic L, Malesevic M, Markoski B.2009The importance of age over radioiodine avidity as a prognostic factor in differentiated thyroid carcinoma with distant metastases. Thyroid 19:227–232 [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Soh EY.2010Differentiated thyroid carcinoma presenting with distant metastasis at initial diagnosis clinical outcomes and prognostic factors. Ann Surg 251:114–119 [DOI] [PubMed] [Google Scholar]
- 11.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M.2006Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 91:2892–2899 [DOI] [PubMed] [Google Scholar]
- 12.Shoup M, Stojadinovic A, Nissan A, Ghossein RA, Freedman S, Brennan MF, Shah JP, Shaha AR.2003Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. J Am Coll Surg 197:191–197 [DOI] [PubMed] [Google Scholar]
- 13.Benbassat CA, Mechlis-Frish S, Hirsch D.2006Clinicopathological characteristics and long-term outcome in patients with distant metastases from differentiated thyroid cancer. World J Surg 30:1088–1095 [DOI] [PubMed] [Google Scholar]
- 14.Huang IC, Chou FF, Liu RT, Tung SC, Chen JF, Kuo MC, Hsieh CJ, Wang PW.2012Long-term outcomes of distant metastasis from differentiated thyroid carcinoma. Clin Endocrinol 76:439–447 [DOI] [PubMed] [Google Scholar]
- 15.Casara D, Rubello D, Saladini G, Masarotto G, Favero A, Girelli ME, Busnardo B.1993Different features of pulmonary metastases in differentiated thyroid cancer: natural history and multivariate statistical analysis of prognostic variables. J Nucl Med 34:1626–1631 [PubMed] [Google Scholar]
- 16.Sampson E, Brierley JD, Le LW, Rotstein L, Tsang RW.2007Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer 110:1451–1456 [DOI] [PubMed] [Google Scholar]
- 17.Olaleye O, Ekrikpo U, Moorthy R, Lyne O, Wiseberg J, Black M, Mitchell D.2011Increasing incidence of differentiated thyroid cancer in South East England: 1987–2006. Eur Arch Otorhinolaryngol 268:899–906 [DOI] [PubMed] [Google Scholar]
- 18.Davies L, Welch HG.2006Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 19.Reynolds RM, Weir J, Stockton DL, Brewster DH, Sandeep TC, Strachan MW.2005Changing trends in incidence and mortality of thyroid cancer in Scotland. Clin Endocrinol 62:156–162 [DOI] [PubMed] [Google Scholar]
- 20.Hayashi Y, Lagarde F, Tsuda N, Funamoto S, Preston DL, Koyama K, Mabuchi K, Ron E, Kodama K, Tokuoka S.2010Papillary microcarcinoma of the thyroid among atomic bomb survivors: tumor characteristics and radiation risk. Cancer 116:1646–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rego-Iraeta A, Perez-Mendez LF, Mantinan B, Garcia-Mayor RV.2009Time trends for thyroid cancer in northwestern Spain: true rise in the incidence of micro and larger forms of papillary thyroid carcinoma. Thyroid 19:333–340 [DOI] [PubMed] [Google Scholar]
- 22.Rumyantsev PO, Saenko VA, Ilyin AA, Stepanenko VF, Rumyantseva UV, Abrosimov AY, Lushnikov EF, Rogounovitch TI, Shibata Y, Mitsutake N, Tsyb AF, Yamashita S.2011Radiation exposure does not significantly contribute to the risk of recurrence of Chernobyl thyroid cancer. J Clin Endocrinol Metab 96:385–393 [DOI] [PubMed] [Google Scholar]
- 23.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, Lee JS.2012Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat 44:11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, Rothman N.2009International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control 20:525–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park YJ, Ahn HY, Choi HS, Kim KW, Park do J, Cho BY.2012The long-term outcomes of the second generation of familial nonmedullary thyroid carcinoma are more aggressive than sporadic cases. Thyroid 22:356–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim WG, Yoon JH, Kim WB, Kim TY, Kim EY, Kim JM, Ryu JS, Gong G, Hong SJ, Shong YK.2008Change of serum antithyroglobulin antibody levels is useful for prediction of clinical recurrence in thyroglobulin-negative patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 93:4683–4689 [DOI] [PubMed] [Google Scholar]
- 27.Park SY, Park YJ, Lee YJ, Lee HS, Choi SH, Choe G, Jang HC, Park SH, Park do J, Cho BY.2006Analysis of differential BRAF(V600E) mutational status in multifocal papillary thyroid carcinoma: evidence of independent clonal origin in distinct tumor foci. Cancer 107:1831–1838 [DOI] [PubMed] [Google Scholar]
- 28.Kim TH, Park YJ, Lim JA, Ahn HY, Lee EK, Lee YJ, Kim KW, Hahn SK, Youn YK, Kim KH, Cho BY, Park do J.2012The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer 118:1764–1773 [DOI] [PubMed] [Google Scholar]
- 29.Jo YS, Li S, Song JH, Kwon KH, Lee JC, Rha SY, Lee HJ, Sul JY, Kweon GR, Ro HK, Kim JM, Shong M.2006Influence of the BRAF V600E mutation on expression of vascular endothelial growth factor in papillary thyroid cancer. J Clin Endocrinol Metab 91:3667–3670 [DOI] [PubMed] [Google Scholar]
- 30.Stanojevic B, Dzodic R, Saenko V, Milovanovic Z, Pupic G, Zivkovic O, Markovic I, Djurisic I, Buta M, Dimitrijevic B, Rogounovitch T, Mitsutake N, Mine M, Shibata Y, Nakashima M, Yamashita S.2011Mutational and clinico-pathological analysis of papillary thyroid carcinoma in Serbia. Endocr J 58:381–393 [DOI] [PubMed] [Google Scholar]
- 31.Kim JY, Moon SJ, Kim KR, Sohn CY, Oh JJ.1998Dietary iodine intake and urinary iodine excretion in normal Korean adults. Yonsei Med J 39:355–362 [DOI] [PubMed] [Google Scholar]
- 32.Cho BY, Choi H, Park YJ, Lim JA, Ahn HY, Lee EK, Kim KW, Yi KH, Chung JK, Youn YK, Cho NH, Park DJ, Koh CS.2013Changes in the clinicopathological characteristics and outcomes of thyroid cancer in Korea over the past 4 decades. Thyroid 23:797–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ronga G, Filesi M, Montesano T, Di Nicola AD, Pace C, Travascio L, Ventroni G, Antonaci A, Vestri AR.2004Lung metastases from differentiated thyroid carcinoma. A 40 years' experience. Q J Nucl Med Mol Imaging 48:12–19 [PubMed] [Google Scholar]
- 34.Lin JD, Huang MJ, Juang JH, Chao TC, Huang BY, Chen KW, Chen JY, Li KL, Chen JF, Ho YS.1999Factors related to the survival of papillary and follicular thyroid carcinoma patients with distant metastases. Thyroid 9:1227–1235 [DOI] [PubMed] [Google Scholar]
- 35.Pelizzo MR, Boschin IM, Toniato A, Piotto A, Pagetta C, Gross MD, Al-Nahhas A, Rubello D.2007Papillary thyroid carcinoma: 35-year outcome and prognostic factors in 1858 patients. Clin Nucl Med 32:440–444 [DOI] [PubMed] [Google Scholar]
- 36.Sisson JC, Giordano TJ, Jamadar DA, Kazerooni EA, Shapiro B, Gross MD, Zempel SA, Spaulding SA.1996131-I treatment of micronodular pulmonary metastases from papillary thyroid carcinoma. Cancer 78:2184–2192 [DOI] [PubMed] [Google Scholar]
- 37.Dinneen SF, Valimaki MJ, Bergstralh EJ, Goellner JR, Gorman CA, Hay ID.1995Distant metastases in papillary thyroid carcinoma: 100 cases observed at one institution during 5 decades. J Clin Endocrinol Metab 80:2041–2045 [DOI] [PubMed] [Google Scholar]
- 38.Castro MR, Bergert ER, Goellner JR, Hay ID, Morris JC.2001Immunohistochemical analysis of sodium iodide symporter expression in metastatic differentiated thyroid cancer: correlation with radioiodine uptake. J Clin Endocrinol Metab 86:5627–5632 [DOI] [PubMed] [Google Scholar]
- 39.Filetti S, Bidart JM, Arturi F, Caillou B, Russo D, Schlumberger M.1999Sodium/iodide symporter: a key transport system in thyroid cancer cell metabolism. Eur J Endocrinol 141:443–457 [DOI] [PubMed] [Google Scholar]
- 40.Hansen LA, Sigman CC, Andreola F, Ross SA, Kelloff GJ, De Luca LM.2000Retinoids in chemoprevention and differentiation therapy. Carcinogenesis 21:1271–1279 [PubMed] [Google Scholar]
- 41.Coelho SM, Corbo R, Buescu A, Carvalho DP, Vaisman M.2004Retinoic acid in patients with radioiodine non-responsive thyroid carcinoma. J Endocrinol Invest 27:334–339 [DOI] [PubMed] [Google Scholar]
- 42.Fernandez CA, Puig-Domingo M, Lomena F, Estorch M, Camacho Marti V, Bittini AL, Marazuela M, Santamaria J, Castro J, Martinez de Icaya P, Moraga I, Martin T, Megia A, Porta M, Mauricio D, Halperin I.2009Effectiveness of retinoic acid treatment for redifferentiation of thyroid cancer in relation to recovery of radioiodine uptake. J Endocrinol Invest 32:228–233 [DOI] [PubMed] [Google Scholar]
- 43.Courbon F, Zerdoud S, Bastie D, Archambaud F, Hoff M, Eche N, Berry I, Caron P.2006Defective efficacy of retinoic acid treatment in patients with metastatic thyroid carcinoma. Thyroid 16:1025–1031 [DOI] [PubMed] [Google Scholar]
- 44.Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, Pentlow KS, Zanzonico PB, Haque S, Gavane S, Ghossein RA, Ricarte-Filho JC, Domínguez JM, Shen R, Tuttle RM, Larson SM, Fagin JA.2013Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 368:623–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pak H, Gourgiotis L, Chang WI, Guthrie LC, Skarulis MC, Reynolds JC, Merino MJ, Schrump DS, Libutti SK, Alexander HR, Jr, Sarlis NJ.2003Role of metastasectomy in the management of thyroid carcinoma: the NIH experience. J Surg Oncol 82:10–18 [DOI] [PubMed] [Google Scholar]
- 46.Brierley J, Tsang R, Panzarella T, Bana N.2005Prognostic factors and the effect of treatment with radioactive iodine and external beam radiation on patients with differentiated thyroid cancer seen at a single institution over 40 years. Clin Endocrinol 63:418–427 [DOI] [PubMed] [Google Scholar]