Abstract
Purpose: Delayed healing is a common problem whenever tendon allografts are used for tendon or ligament reconstruction. Repopulating the allograft with host cells may accelerate tendon regeneration, but cell penetration into the allograft tendon is limited. Processing the tendon surface with slits that guide cells into the allograft substrate may improve healing. The purpose of this study was to describe a surface modification of allograft tendon that includes slits to aid cell repopulation and lubrication to enhance tendon gliding.
Methods: Canine flexor digitorum profundus tendons were used for this study. Cyclic gliding resistance was measured over 1000 cycles. Tensile stiffness was assessed for normal tendon, tendon decellularized with trypsin and Triton X-100 (decellularized group), tendon decellularized and perforated with multiple slits (MS group) and tendon decellularized, perforated with slits and treated with a carbodiimide-derivatized hyaluronic acid and gelatin (cd-HA-gelatin) surface modification (MS-SM group). To assess tendon repopulation, bone marrow stromal cells (BMSCs) were used in the decellularized and MS groups. DNA concentration and histology were evaluated and compared to normal tendons and nonseeded decellularized tendons.
Results: The gliding resistance of the decellularized and MS groups was significantly higher compared with the normal group. There was no significant difference in gliding resistance between the decellularized and MS group. Gliding resistance of the normal group and MS-SM group was not significantly different. The Young's modulus was not significantly different among the four groups. The DNA concentration in the MS group was significantly lower than in normal tendons, but significantly higher than in decellularized tendons, with or without BMSCs. Viable BMSCs were found in the slits after 2 weeks in tissue culture.
Conclusions: Tendon slits can successfully harbor BMSCs without compromising their survival and without changing tendon stiffness. Surface modification restores normal gliding function to the slit tendon.
Clinical Relevance: A multislit tendon reseeded with BMSCs, with a surface treatment applied to restore gliding properties, may potentially promote tendon revitalization and accelerate healing for tendon or ligament reconstruction applications.
Introduction
Functional repair of flexor tendon injury, especially in zone II, remains a great challenge for hand surgeons.1–3 Advances in suture materials,4–6 suture techniques,7,8 and postoperative rehabilitation protocols9,10 have improved clinical outcomes.11 Nevertheless, many complications, such as a rupture at the repair site and the formation of restrictive adhesions, still occur and require further operations.
In such cases, tendon grafts are often indicated to restore normal digit function. Currently, the palmaris longus, plantaris, and toe extensor autografts are most often used as sources of tendon grafts in the hand.12–14 However, these donor tendons are of extrasynovial origin and do not entirely match the anatomic and biomechanical characteristics of the intrasynovial flexor tendons they are intended to replace. The intrasynovial tendon surface has an elliptical cross section, and is lined with a thin layer of epitenon cells, which secrete lubricants such as hyaluronic acid (HA) and lubricin. Extrasynovial tendons are flat, and they are surrounded by a multilayer paratenon with an irregular surface,15,16 with a much higher gliding resistance than intrasynovial tendons.17 These differences in shape and surface structure may account for the poor clinical outcomes that commonly occur after extrasynovial tendon grafting.18 Intrasynovial tendon grafts cause fewer adhesions and scarring than extrasynovial tendon grafts in experimental animals,16,19,20 but intrasynovial tendon autografts are rarely available in the clinical setting, or come at the cost of donor-site morbidity and prolonged operative time.21 In extensive hand injuries, the demand for tendon grafts might exceed the autologous supply, regardless of tolerance for donor-site problems.22,23 Thus, there is a clinical need for alternatives to conventional tendon autografts.
An ideal tissue-engineered tendon scaffold should possess histological and mechanical properties similar to the native tendon, and be compatible with the recipient's tissues and cells. In that regard, an intrasynovial tendon allograft is a desirable scaffold, because its material properties closely match the host need.24 Problems associated with immune reaction can be reduced by decellularization of the scaffold; decelluarization may also facilitate reseeding with host cells such as tenocytes,25 dermal fibroblasts,26 bone marrow stromal cells (BMSCs),27,28 adipose-derived mesenchymal stem cells,29,30 or tendon-derived stem cells.31 However, cell penetration into an allograft tendon is limited, due to the tendon's dense collagen structure, and thus, seeded cells are typically present only on the scaffold surface.25 A method to enhance the penetration of seeded host cells into the allograft that did not affect its mechanical properties could potentially be valuable, since increased graft cellularity could accelerate intrinsic healing and tissue regeneration. Mechanically perforating the tendon surface might be a practical method for greater cell penetration.
In this study, we used a mechanical perforation technique to create multiple slits (MS) longitudinally along the tendon to aid cell repopulation and survival. In addition, to further enhance the allograft by reducing the gliding friction, we used surface lubricants to modify the MS tendon surface. The purpose of this study was to investigate the effect of these mechanical perforations on tendon mechanical properties, surface friction, and viability of the seeded cells. We hypothesized that making multiple slits in the decellularized tendon would not affect the tendon tensile properties, but would increase the tendon gliding resistance as compared with nonperforated tendons. We further hypothesized that this increased friction would be reversed by surface modification with carbodiimide-derivatized hyaluronic acid and gelatin (cd-HA-gelatin). Finally, we hypothesized that making slits on the tendon allograft would improve cell migration into the core of the scaffold compared to nonperforated tendons.
Materials and Methods
Flexor tendon harvest
Tissue was obtained from 11 mixed-breed dogs, weighing 21 to 26 kg, euthanized for other Institutional Animal Care and Use Committee (IACUC) approved studies. The studies were unrelated to the tendons and treatments had no relevant effect on tendon tissue. A total of 84 flexor digitorum profundus (FDP) tendons from the 2nd through 5th hindpaw digits were divided distally at the bony insertion and proximally just distal to the common FDP tendon, at which level the four FDP tendons fuse together. Respective digits were disarticulated and prepared for friction testing, with each tendon sliding against its own proximal pulley.32 Tendons were assigned to four groups: (1) untreated, unprocessed normal tendons, to serve as a control (normal group), (2) tendons decellularized with trypsin and Triton X-100 (decellularized group), (3) tendons decellularized as in group 2 and perforated with multiple, short slits (MS group), and (4) tendons decellularized and slit as in group 3 and treated with a surface modification of cd-HA-gelatin (MS-SM group). Normal tendons were used immediately. The remaining tendons were frozen at −80°C until further use.
Decellularization and slit of tendon
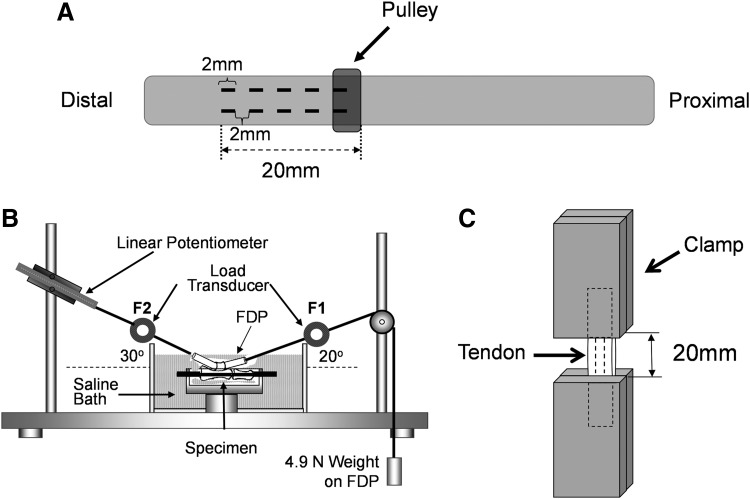
Frozen tendons were thawed at room temperature before use. In the MS and MS-SM groups, two rows of 2-mm full thickness slits at 2-mm intervals were made with a #11 scalpel (Fig. 1A). The tendons were then immersed in trypsin 0.05%/0.53 mM ethylenediaminetetraacetic acid (EDTA) for 24 h at 37°C followed by 0.5% Triton X-100 (Alfa Aesar, Ward Hill, MA) for 24 h at room temperature.27,30 Decellularized tendons were washed in PBS for 24 h.
FIG. 1.
Illustration of experimental setup and testing apparatus. (A) In the MS and MS-SM groups, two rows of 2-mm full thickness slits were made on the tendon at 2-mm intervals over a 2-cm length. (B) Testing apparatus for measurement of gliding resistance between FDP tendon and proximal pulley. (C) The tendon was attached to the clamps at a gauge length of 20 mm, including the region of the slits. FDP, flexor digitorum profundus; HA, hyaluronic acid; MS, multiple slits; MS-SM, surface modification of cd-HA-gelatin.
Treatment of tendons with cd-HA-gelatin
For the cd-HA-gelatin treatment group, after perforation, the tendons were immersed in a solution of 1% sodium hyaluronate (95%; Acros, Geel, Belgium), 1% 1-ethyl 1-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC; Sigma Chemical, St. Louis, MO), 1% N-hydroxysuccinimide (NHS; Pierce Biotechnology, Rockford, IL), and 10% gelatin (from porcine skin; Sigma Chemical) in 0.1 M Mes (2-[morpholino]ethanesulfonic acid)-buffered saline solution (Sigma Chemical), pH 6.0, for 1 min. After surface treatment, tendons were wrapped in aluminum foil for 10 min at 37°C for gelation and to maintain hydration until friction testing.33 The excess reagent was removed with a saline wash, and friction testing was then performed.
Measurement of gliding resistance (n=8)
Gliding resistance between each FDP and its respective intact proximal pulley was measured using methods that have been described previously.34,35 The friction measurement system consists of a mechanical actuator instrumented with a linear potentiometer and two tensile load transducers, a mechanical pulley, and a resistance weight36 (Fig. 1B). The tendon was pulled by the actuator at a rate of 2 mm/s. The excursion distance was set at 14 mm, which is the normal canine FDP excursion.37 The gliding resistance was calculated as previously described.38 The first two cycles of friction testing preconditioned the tendons. A complete cycle of data was recorded, every 50 cycles up to 500 cycles, and then every 100 cycles up to 1000 cycles.39
Biomechanical assessment (n=8)
After the gliding resistance measurements were obtained, the Young's modulus was assessed. The tendon was mounted on a servohydraulic testing machine (MTS 858 Mini Bionix II, Eden Prairie, MN). A region of the tendon, including a point just proximal to the proximal pulley and 2 cm distal to this point, was included in the gauge length with tissue adjacent to the region gripped in the clamps with sandpaper (Fig. 1C). Before testing, the cross-sectional dimensions of each tendon were measured with a digital caliper (RS 232; Brantford, Canada). The caliper has a rated accuracy of 0.02 mm and a resolution of 0.01 mm. Measurements were obtained at three different levels (proximal end, distal end, and midpoint). The area was calculated based on the assumption that the tendon cross section was elliptical. The cross-sectional area of the tendon was averaged over the three levels.
At the start of each test, the tendon was preconditioned with 10 cycles of loading from 10 to 50 N at a rate of 20 mm/min. Following the 10th cycle, the tendon was distracted until failure, at a rate of 20 mm/min. Tendons were moistened with a saline mist throughout testing. Tensile force and displacement data were continuously recorded at a sample rate of 20 Hz. The Young's modulus was calculated from the slope of the linear region of the stress–strain curve.
BMSC harvest and suspension
To assess the effectiveness of cell seeding, we used BMSCs harvested in other IACUC approved studies. About 8.0 mL of bone marrow was aspirated aseptically from the tibia, using a 15-mL syringe containing 2.0 mL of heparin solution. The heparin was removed by centrifugation at 1500 rpm for 5 min at room temperature, and the bone marrow pellet was resuspended in a cell culture medium and divided into three 100-mm dishes in 10 mL of standard medium. The medium consisted of the minimal essential medium with Earle's salts (GIBCO, Grand Island, NY), 10% fetal bovine serum, and 1% antibiotics (antibiotic–antimycotic; GIBCO). The bone marrow cells were incubated at 37°C with 5% CO2 and 95% air at 100% humidity. After 3 days, the medium containing floating cells was removed and a new medium was added to the remaining adherent cells. These adherent cells were defined as BMSCs. The medium was changed every 3 days. When reaching 70% to 80% confluence, the BMSCs were harvested with 0.25% trypsin-EDTA and subcultured. Cells between passage 2 and 4 were used for the experiments.
BMSC seeding
Thirty-nine decellularized tendons were cut into 20-mm lengths and assigned to three groups: intact tendon without BMSCs (decellularized group); intact tendon with BMSCs (decellularized+cell group); and slit tendon with BMSCs (MS+cell group). Tendons were lyophilized, gas sterilized, and rehydrated in PBS for 24 h before seeding BMSCs. Normal tendons served as the control group.
Confluent plates of BMSCs were washed twice with sterile PBS and then trypsinized. The cells were counted with a hemocytometer and centrifuged to remove the trypsin and to create a cell suspension in the culture medium at a density of 2×107cells/1 mL. About 100 μL of this cell suspension was seeded on the surface of the tendon or directly into the slits with a micropipette. After 1 h of incubation at 37°C in a 5% CO2 humidified incubator, the culture medium was added and the BMSC-seeded tendons were cultured for 2 weeks. The culture medium was changed every 3 days.
Cell viability assessment and histology (n=3)
The BMSCs were labeled with the Vybrant® DiI cell labeling solution (Molecular Probes, Eugene, OR) according to the manufacturer's instruction before seeding on the decellularized tendon scaffold. After tissue culture, the tendon segments were embedded in the optical cutting temperature compound (Tissue-Tek, Sakura Finetek, Japan) and cut into 7-μm slices with a cryostat (Leica CM 1850, Wetzlar, Germany). Three tendons in each group were observed with a confocal microscope (LSM510; Zeiss, Oberkochen, Germany). Sections were also stained with hematoxylin and eosin using standard techniques. The gross appearance was noted at 100× magnification in eight randomly selected sections from each segment. To evaluate cell viability, a Live/Dead Viability/Cytotoxicity kit (Life Technologies, Grand Island, NY) was used for the MS+cell group, according to the manufacturer's instructions. After the tissue culturing period, the tendons were labeled with Live/Dead stain (7.7 μL calcein AM and 15.4 μL ethidium homodimer-1 dissolved in 10 mL of serum-free medium) and incubated for 30 min before viewing with the confocal microscope.
DNA concentration (n=10)
The tendon cellularity was assessed by quantifying the tissue concentration of DNA using a PicoGreen DNA assay (Life Technologies) according to the manufacturer's instructions.40 Briefly, the tendon segments were lyophilized and cut on both ends to avoid including cells attached to the end of the tendon. A 7- to 10-mg sample of tissue was solubilized by incubation for 48 h in 1.0 mL of papain solution (300 μg/mL papain and 2 mM Dithiothreitol) at 65°C. After incubation, digested samples were centrifuged at 14,000 rpm for 5 min. The supernatant was placed in a fresh tube and incubated with the reagent. Fluorescence was measured at an excitation/emission wavelength of 480 nm/520 nm with a fluorometer (FLUOstar Omega; BMG Labtech, Ortenberg, Germany). Results were expressed as nanograms of DNA per milligram of dry tissue.
Statistical analysis
The mean±SD of the gliding resistance, cross-sectional area, Young's modulus, and DNA concentration were determined for each group. The gliding resistance, cross-sectional area, Young's modulus, and DNA concentration were compared among the four groups with one-way factorial analysis of variance. The Tukey-Kramer post hoc test for each pairwise comparison was performed if a significant difference was detected. The significance level was set at p<0.05 in all cases. All statistical analyses were performed using JMP software, version 9.0.1 (SAS Institute, Cary, NC).
Results
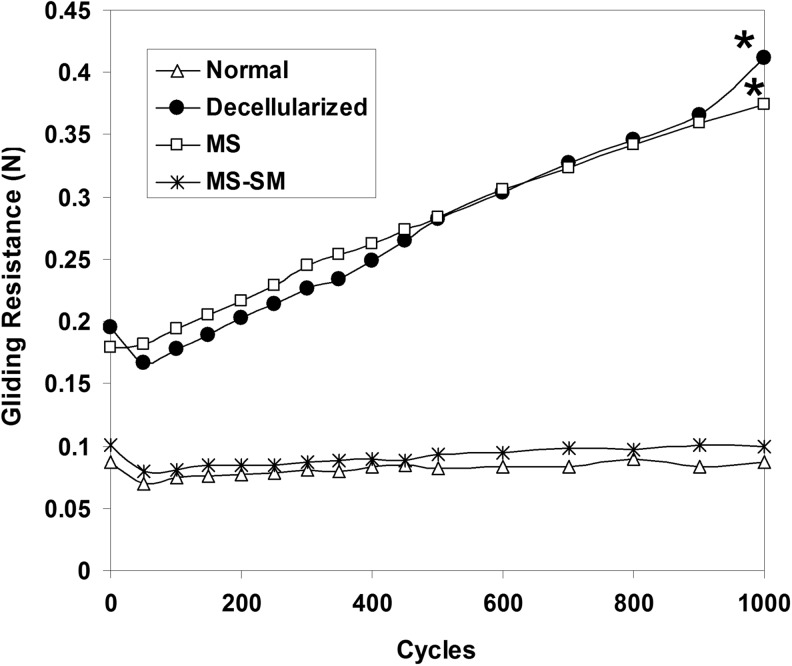
The gliding resistance of the decellularized and MS groups gradually increased as the number of cycles increased, and there was a significant difference in the gliding resistance between the first cycle and the 1000th cycle in both. However, the gliding resistance of the normal tendon group and the MS-SM group did not change significantly over 1000 cycles (Fig. 2).
FIG. 2.
Mean gliding resistance of the normal group, decellularized group, MS group, and MS-SM group over 1000 cycles of tendon motion. An asterisk indicates a significant difference between the first cycle and 1000th cycle (p<0.05).
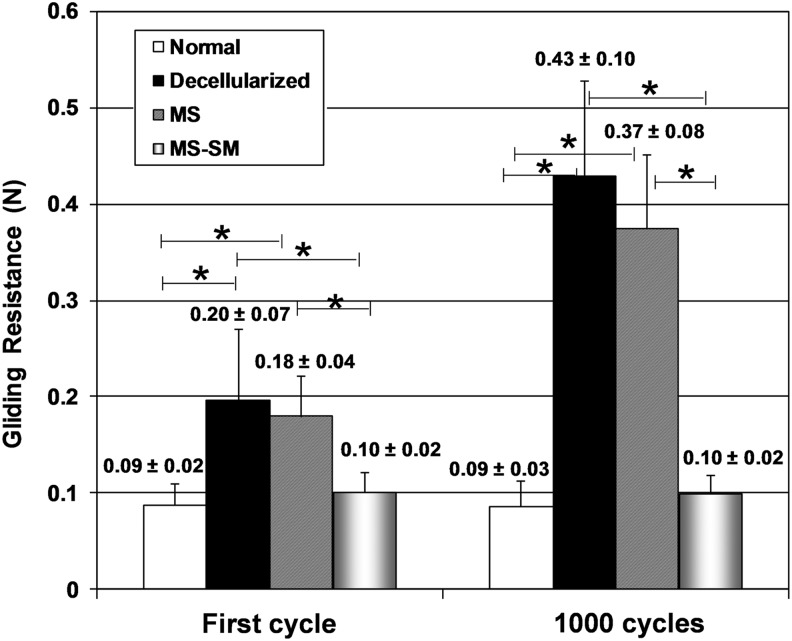
After the first cycle, the gliding resistance of the decellularized and MS groups was significantly higher compared with the normal group (p<0.05). After 1000 cycles, the gliding resistance of the decellularized and MS groups remained significantly higher compared with the normal group (p<0.05). The gliding resistance of the MS group was significantly reduced by surface modification with cd-HA-gelatin at both the first cycle and the 1000th cycle (p<0.05). There was no significant difference in gliding resistance between the normal group and MS-SM group, or between the decellularized group and MS group at either the first cycle or 1000th cycle (Fig. 3).
FIG. 3.
Mean gliding resistance of first cycle and 1000th cycle for the normal group, decellularized group, MS group, and MS-SM group. Error bars represent standard deviation. An asterisk indicates a significant difference (p<0.05).
The cross-sectional area of the decellularized group was 3.98±0.65 mm2. This did not significantly differ from either the MS or MS-SM groups (3.57±0.47 mm2 and 3.83±0.38 mm2, respectively). However, there was a significant difference between the cross-sectional areas of the decellularized and normal groups (p<0.05), the cross-sectional area of the latter was 3.27±0.45 mm2.
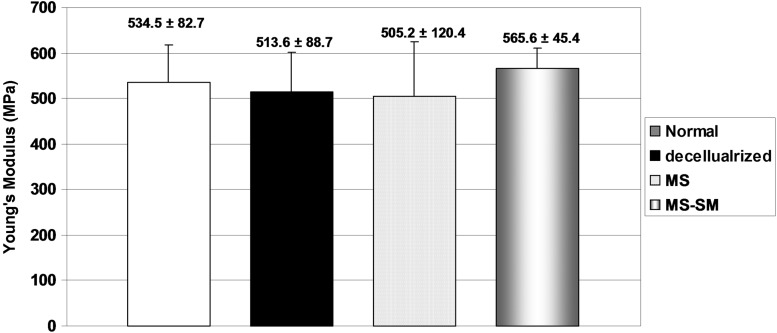
All failures occurred at the clamping site. Rupture of the tendon at the grip site occurred in 6 tendons and slippage occurred in the remaining tendons. Slipping/breaking did not compromise our ability to calculate the Young's modulus, which was not significantly different among the four groups (Fig. 4).
FIG. 4.
The mean Young's modulus of normal group, decellularized group, MS group, and MS-SM group. Error bars represent standard deviation.
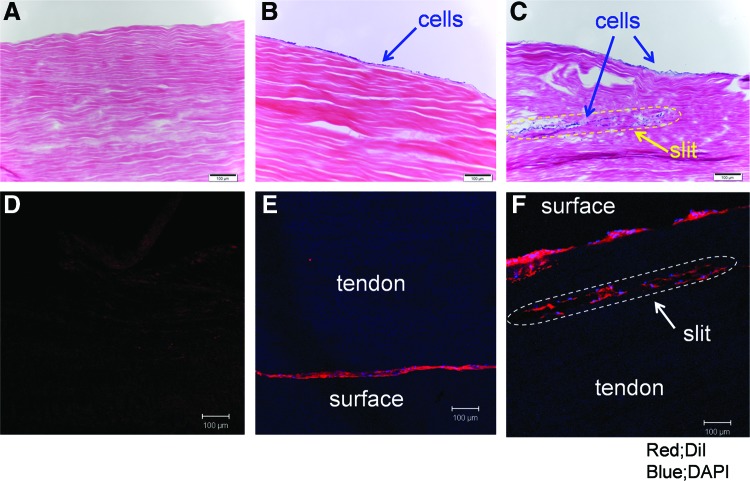
Representative histologic findings are noted in Figure 5. No cells were noted in the decellularized group tendons (Figs. 5A, D). Labeled cells attached only to the surface of the decellularized+cell group tendons (Fig. 5B, E). Labeled BMSCs were identified in the slits of the MS+cell group tendons (Fig. 5C, F). Some labeled cells also spread in between the collagen fibers. Most BMSCs were still alive in the slits after 2 weeks in culture (Fig. 6).
FIG. 5.
Histology of longitudinal sections of tendon scaffolds. (A–C; H&E staining, D–F; DiI and DAPI staining. Original magnification ×100. Scale bar represents 100 μm). There were no cells seen in the decellularized group tendon (A, D). Seeded cells were present only on the surface of the decellularized+cell group tendon (B, E). Note cells in the slits of the MS+cell group tendon (C, F). H&E, hematoxylin and eosin. Color images available online at www.liebertpub.com/tea
FIG. 6.
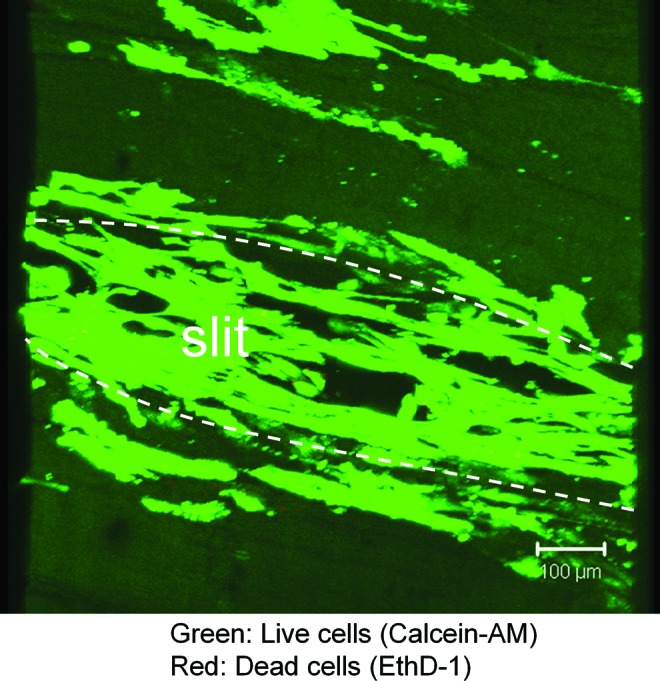
Live/Dead cell viability assay showing live cells stained with Calcein-AM and dead cells with EthD-1 (Original magnification ×100. Scale bar represents 100 μm). The viability of most seeded BMSCs was maintained in the slits of the decellularized tendon. BMSCs, bone marrow stromal cells. Color images available online at www.liebertpub.com/tea
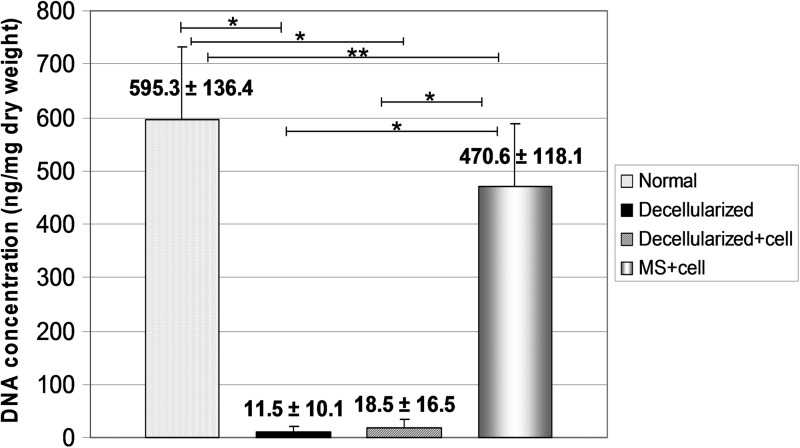
The DNA concentration results are shown in Figure 7. The DNA concentrations of the decellularized group tendons and decellularized+cell group tendons were significantly decreased compared with the normal tendons (p<0.05). The DNA concentration of the MS+cell group tendons was also significantly lower than the normal tendons, but significantly higher than the decellularized and decellularized+cell group tendons (p<0.05).
FIG. 7.
Mean DNA concentration of normal group, decellularized group, decellularized+cell group, and MS+cell group. Error bars represent standard deviation. An asterisk indicates significant difference (*p<0.001, **p=0.02).
Discussion
Our study demonstrates that making multiple short slits improved the ability of BMSCs to penetrate into a decellularized tendon scaffold without compromising the tendon stiffness, and that these cells remain viable.
The process of healing after tendon grafting is slow. After implantation, soft tissue grafts are degraded and remodeled by host cells.41 Dustmann et al. reported that free soft-tissue allografts showed delayed remodeling of their extracellular matrix compared to autografts in ACL reconstruction, and that mechanical properties of allografts were significantly reduced at 1 year.42 In addition, when the tendon grafting is performed after a tendon injury in zone II, the distal repair site usually requires tendon to bone healing as well.43
The presence of host cells inside the decellularized tendon scaffold has many potential advantages for tendon healing. Increased cellularity may speed healing and reduce the risk of tendon rupture. A shorter period of immobilization may reduce the risk of adhesions and permit the patient to return to normal activities sooner. Cell seeded tendon scaffolds have been shown to exhibit superior strength over unseeded tendon scaffolds,44 even when cell attachment is limited to the surface of the tendon.26 To improve cell penetration, Woon et al. used peracetic acid to increase tendon porosity; however, cell penetration was still limited.45 The same team also showed that tendon surface scoring increased the surface area and improved cell seeding without diminishing the tendon's biomechanical properties.46
In the current study, the gliding resistance was increased, compared to the normal tendon, in the tendons with multiple short slits. However, there was no significant difference in gliding resistance between the decellularized group and the MS group, suggesting that the effect on gliding resistance was due to the decellularization processing rather than the slits themselves. This is comparable to the findings of previous studies, which demonstrated that intrasynovial tendons treated with trypsin also had increased gliding resistance, presumably due to the removal of surface proteins with a lubricating function or enzymatic roughening of the tendon surface.47 The lyophilization process that we used to prepare the tendon grafts, although commonly done clinically, may also have increased the gliding resistance in our tendons, and this has also been observed in other studies.33
We found that the increase in gliding resistance was reversed through surface modification with cd-HA-gelatin. The efficacy of this modification has been reported in both in vitro33,48 and in vivo39,49 models. Karabekmez and Zhao reported that surface modification with cd-HA decreased the adhesion formation without altering the cellularity in either the autologous extrasynovial tendon or the intrasynovial allograft in an in vivo canine model.50 In the current study, the surface modification also restored tendon gliding resistance to that of normal tendon over 1000 cycles.
We found seeded cells inside the slits of the decellularized tendon scaffold. Although the DNA content of the MS+cell group tendons was less compared with the normal tendons, it reached 79% of normal tendon levels. This was higher than the level observed both in this study and by others in decellularized intact tendons cultured with cells.46
Introducing cells at high density into small, tight spaces may affect viability. Erickson et al. seeded bovine MSCs in a HA hydrogel for chondrogenic differentiation at a density of 60 million cells/mL and demonstrated high viability.51 Our seeding density was less compared with their study and our seeded BMSCs were still alive in the slits after 2 weeks in tissue culture. In vivo, we believe that this mechanical perforation might work as a nutrient foramen. The fact that our seeded cells were viable at 2 weeks also suggests that the chemical decellularization process used in this study successfully eliminated host cells without causing any residual cytotoxity.
There are several limitations to this study. First, we did not confirm the postseeding phenotype of the implanted BMSCs, and thus, we do not know if they expressed any tendon-specific markers, such as scleraxis or tenomodulin, after implantation. There is strong evidence, though, to suggest that when mesenchymal stem cells are seeded into tendon scaffolds and exposed to the appropriate environment and stimuli, the cells can differentiate into tenocytes.52,53 We also did not measure the matrix synthesis of the seeding cells. However, other studies have shown that seeded cells can produce collagens 1 and 3, which contribute to tendon matrix remodeling in vivo.30 Second, we chose BMSCs as a donor cell for simplicity, and did not test cells of other tissue origins. Tendon-derived stem cells are particularly difficult to harvest.25,54 Third, we did not attempt to stimulate the seeded cells in any way postimplantation. Several growth factors are thought to play a role in cell migration. Schmidt et al. demonstrated that bFGF is able to attract MSCs and control the direction of migration.55 The next logical step would be to culture the seeded tendon under mechanical stimulation, such as repetitive tensile loaded, with or without exposure to various cytokines. Fourth, we did not test the cell viability with cd-HA gelatin treatment. As a result, it is clear if this treatment would affect cell seeding and fate, although Karabekmez reported that this surface treatment did not impair the cellularity of either the autograft or allograft in an in vivo canine model.50 Fifth, we were unable to successfully evaluate all tensile properties to midsubstance failure, as some specimens slipped at the grips before tensile failure. Tensile properties were analyzed from the linear portion of the stress–strain curve variable failure mode. Ng et al. reported that “an analysis of specimens that failed at the grip-specimen interface versus those that failed at mid-substance shows that there was no significant difference in their tensile properties.”56 Therefore, we believe that tensile properties (Young modulus) were reliable. Lastly, we did not analyze the mechanical properties of the tendon scaffold after cell seeding. This would be a necessary analysis in a future in vivo study, to assess the effect of cellularity on graft degradation and remodeling.57
In conclusion, making multiple short slits in an intrasynovial tendon decellularized for potential allograft use enhances the penetration and survival of seeded cells. The process does not change the tendon's tensile stiffness. Increased gliding resistance, related to the decellularization process, was reversed with cd-HA-gelatin treatment. Further study will be required to investigate utilization of this mechanical perforation technique in tendon allograft reconstruction in vivo.
Acknowledgment
This study was supported by grants from NIH/NIAMS (AR57745).
Disclosure Statement
All the authors report no conflicts of interest.
References
- 1.Amadio P.C.What's new in hand surgery. J Bone Joint Surg Am 89,460, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Silfverskiold K.L., and May E.J.Gap formation after flexor tendon repair in zone II. Results with a new controlled motion programme. Scand J Plast Reconstr Surg Hand Surg 27,263, 1993 [PubMed] [Google Scholar]
- 3.Tang J.B.Clinical outcomes associated with flexor tendon repair. Hand Clin 21,199, 2005 [DOI] [PubMed] [Google Scholar]
- 4.McDonald E., Gordon J.A., Buckley J.M., and Gordon L.Comparison of a new multifilament stainless steel suture with frequently used sutures for flexor tendon repair. J Hand Surg Am 36,1028, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Miller B., Dodds S.D., deMars A., Zagoreas N., Waitayawinyu T., and Trumble T.E.Flexor tendon repairs: the impact of fiberwire on grasping and locking core sutures. J Hand Surg Am 32,591, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Su B.W., Protopsaltis T.S., Koff M.F., Chang K.P., Strauch R.J., Crow S.A., et al. . The biomechanical analysis of a tendon fixation device for flexor tendon repair. J Hand Surg Am 30,237, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Al-Qattan M.M., Al-Rakan M.A., and Al-Hassan T.S.A biomechanical study of flexor tendon repair in zone II: Comparing a combined grasping and locking core suture technique to its grasping and locking components. Injury 42,1300, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Peltz T.S., Haddad R., Scougall P.J., Nicklin S., Gianoutsos M.P., and Walsh W.R.Influence of locking stitch size in a four-strand cross-locked cruciate flexor tendon repair. J Hand Surg Am 36,450, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Chesney A., Chauhan A., Kattan A., Farrokhyar F., and Thoma A.Systematic review of flexor tendon rehabilitation protocols in zone II of the hand. Plast Reconstr Surg 127,1583, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Trumble T.E., Vedder N.B., Seiler J.G., 3rd, Hanel D.P., Diao E., and Pettrone S.Zone-II flexor tendon repair: a randomized prospective trial of active place-and-hold therapy compared with passive motion therapy. J Bone Joint Surg Am 92,1381, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Strickland J.W.Development of flexor tendon surgery: twenty-five years of progress. J Hand Surg Am 25,214, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Bertelli J.A., Santos M.A., Kechele P.R., Rost J.R., and Tacca C.P.Flexor tendon grafting using a plantaris tendon with a fragment of attached bone for fixation to the distal phalanx: a preliminary cohort study. J Hand Surg Am 32,1543, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hwang M.D., Pettrone S., and Trumble T.E.Work of flexion related to different suture materials after flexor digitorum profundus and flexor digitorum superficialis tendon repair in zone II: a biomechanical study. J Hand Surg Am 34,700, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Sun S., Ding Y., Ma B., and Zhou Y.Two-stage flexor tendon reconstruction in zone II using Hunter's technique. Orthopedics 33,880, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Gelberman R.H., Seiler J.G., 3rd, Rosenberg A.E., Heyman P., and Amiel D.Intercalary flexor tendon grafts. A morphological study of intrasynovial and extrasynovial donor tendons. Scand J Plast Reconstr Surg Hand Surg 26,257, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Seiler J.G., 3rd, Chu C.R., Amiel D., Woo S.L., and Gelberman R.H.The Marshall R. Urist Young Investigator Award. Autogenous flexor tendon grafts. Biologic mechanisms for incorporation. Clin Orthop 239, 1997 [PubMed] [Google Scholar]
- 17.Momose T., Amadio P.C., Zobitz M.E., Zhao C., and An K.N.Effect of paratenon and repetitive motion on the gliding resistance of tendon of extrasynovial origin. Clin Anat 15,199, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Finsen V.Two-stage grafting of digital flexor tendons: a review of 43 patients after 3 to 15 years. Scand J Plast Reconstr Surg Hand Surg 37,159, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Abrahamsson S.O., Gelberman R.H., Amiel D., Winterton P., and Harwood F.Autogenous flexor tendon grafts: fibroblast activity and matrix remodeling in dogs. J Orthop Res 13,58, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Hasslund S., Jacobson J.A., Dadali T., Basile P., Ulrich-Vinther M., Soballe K., et al. . Adhesions in a murine flexor tendon graft model: autograft versus allograft reconstruction. J Orthop Res 26,824, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaSalle W.B., and Strickland J.W.An evaluation of the two-stage flexor tendon reconstruction technique. J Hand Surg Am 8,263, 1983 [DOI] [PubMed] [Google Scholar]
- 22.Hashizume H., Nishida K., Fujiwara K., and Inoue H.Spontaneous “spaghetti” flexor tendon ruptures in the rheumatoid wrist. Mod Rheumatol 14,257, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Matsuzaki H., Narisawa H., Miwa H., and Toishi S.Predicting functional recovery and return to work after mutilating hand injuries: usefulness of Campbell's Hand Injury Severity Score. J Hand Surg Am 34,880, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Cartmell J.S., and Dunn M.G.Development of cell-seeded patellar tendon allografts for anterior cruciate ligament reconstruction. Tissue Eng 10,1065, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Chong A.K., Riboh J., Smith R.L., Lindsey D.P., Pham H.M., and Chang J.Flexor tendon tissue engineering: acellularized and reseeded tendon constructs. Plast Reconstr Surg 123,1759, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Pridgen B.C., Woon C.Y., Kim M., Thorfinn J., Lindsey D., Pham H., et al. . Flexor tendon tissue engineering: acellularization of human flexor tendons with preservation of biomechanical properties and biocompatibility. Tissue Eng Part C Methods 17,819, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Issa R.I., Engebretson B., Rustom L., McFetridge P.S., and Sikavitsas V.I.The effect of cell seeding density on the cellular and mechanical properties of a mechanostimulated tissue-engineered tendon. Tissue Eng Part A 17,1479, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Omae H., Sun Y.L., An K.N., Amadio P.C., and Zhao C.Engineered tendon with decellularized xenotendon slices and bone marrow stromal cells: an in vivo animal study. J Tissue Eng Regen Med 6,238, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angelidis I.K., Thorfinn J., Connolly I.D., Lindsey D., Pham H.M., and Chang J.Tissue engineering of flexor tendons: the effect of a tissue bioreactor on adipoderived stem cell-seeded and fibroblast-seeded tendon constructs. J Hand Surg Am 35,1466, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Kryger G.S., Chong A.K., Costa M., Pham H., Bates S.J., and Chang J.A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J Hand Surg Am 32,597, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Zhang J., Li B., and Wang J.H.The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials 32,6972, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuda Y., Gorski J.P., An K.N., and Amadio P.C.Biochemical, histological, and biomechanical analyses of canine tendon. J Orthop Res 5,60, 1987 [DOI] [PubMed] [Google Scholar]
- 33.Ikeda J., Zhao C., Sun Y.L., An K.N., and Amadio P.C.Carbodiimide-derivatized hyaluronic acid surface modification of lyophilized flexor tendon: a biomechanical study in a canine in vitro model. J Bone Joint Surg Am 92,388, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolodzinskyi M.N., Zhao C., Sun Y.L., An K.N., Thoreson A.R., Amadio P.C., et al. . The effects of hylan g-f 20 surface modification on gliding of extrasynovial canine tendon grafts in vitro. J Hand Surg Am 38,231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchiyama S., Coert J.H., Berglund L., Amadio P.C., and An K.N.Method for the measurement of friction between tendon and pulley. J Orthop Res 13,83, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Silva J.M., Zhao C., An K.N., Zobitz M.E., and Amadio P.C.Gliding resistance and strength of composite sutures in human flexor digitorum profundus tendon repair: an in vitro biomechanical study. J Hand Surg Am 34,87, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao C., Amadio P.C., Zobitz M.E., Momose T., Couvreur P., and An K.N.Effect of synergistic motion on flexor digitorum profundus tendon excursion. Clin Orthop 223,2002 [DOI] [PubMed] [Google Scholar]
- 38.Zhao C., Amadio P.C., Zobitz M.E., and An K.N.Gliding characteristics of tendon repair in canine flexor digitorum profundus tendons. J Orthop Res 19,580, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Zhao C., Sun Y.L., Amadio P.C., Tanaka T., Ettema A.M., and An K.N.Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic Acid. An in vivo canine model. J Bone Joint Surg Am 88,2181, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ning L.J., Zhang Y., Chen X.H., Luo J.C., Li X.Q., Yang Z.M., et al. . Preparation and characterization of decellularized tendon slices for tendon tissue engineering. J Biomed Mater Res A 100,1448, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Olender E., Uhrynowska-Tyszkiewicz I., and Kaminski A.Revitalization of biostatic tissue allografts: new perspectives in tissue transplantology. Transplant Proc 43,3137, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Dustmann M., Schmidt T., Gangey I., Unterhauser F.N., Weiler A., and Scheffler S.U.The extracellular remodeling of free-soft-tissue autografts and allografts for reconstruction of the anterior cruciate ligament: a comparison study in a sheep model. Knee Surg Sports Traumatol Arthrosc 16,360, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Green S.M., and Posner M.A.Intraosseous and extraosseous attachments of flexor tendon to bone: a biomechanical in vivo study in rabbits. Am J Orthop 38,E170, 2009 [PubMed] [Google Scholar]
- 44.Woon C.Y., Kraus A., Raghavan S.S., Pridgen B.C., Megerle K., Pham H., et al. . Three-dimensional-construct bioreactor conditioning in human tendon tissue engineering. Tissue Eng Part A 17,2561, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Woon C.Y., Pridgen B.C., Kraus A., Bari S., Pham H., and Chang J.Optimization of human tendon tissue engineering: peracetic acid oxidation for enhanced reseeding of acellularized intrasynovial tendon. Plast Reconstr Surg 127,1107, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Woon C.Y., Farnebo S., Schmitt T., Kraus A., Megerle K., Pham H., et al. . Human flexor tendon tissue engineering: revitalization of biostatic allograft scaffolds. Tissue Eng Part A 18,2406, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Sun Y., Chen M.Y., Zhao C., An K.N., and Amadio P.C.The effect of hyaluronidase, phospholipase, lipid solvent and trypsin on the lubrication of canine flexor digitorum profundus tendon. J Orthop Res 26,1225, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka T., Sun Y.L., Zhao C., Zobitz M.E., An K.N., and Amadio P.C.Optimization of surface modifications of extrasynovial tendon to improve its gliding ability in a canine model in vitro. J Orthop Res 24,1555, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Zhao C., Sun Y.L., Ikeda J., Kirk R.L., Thoreson A.R., Moran S.L., et al. . Improvement of flexor tendon reconstruction with carbodiimide-derivatized hyaluronic acid and gelatin-modified intrasynovial allografts: study of a primary repair failure model. J Bone Joint Surg Am 92,2817, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karabekmez F.E., and Zhao C.Surface treatment of flexor tendon autograft and allograft decreases adhesion without an effect of graft cellularity: a pilot study. Clin Orthop 470,2522, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erickson I.E., Kestle S.R., Zellars K.H., Farrell M.J., Kim M., Burdick J.A., et al. . High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta Biomater 8,3027, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caplan A.I.The mesengenic process. Clin Plast Surg 21,429, 1994 [PubMed] [Google Scholar]
- 53.Zhang A.Y., and Chang J.Tissue engineering of flexor tendons. Clin Plast Surg 30,565, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Costa M.A., Wu C., Pham B.V., Chong A.K., Pham H.M., and Chang J.Tissue engineering of flexor tendons: optimization of tenocyte proliferation using growth factor supplementation. Tissue Eng 12,1937, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Schmidt A., Ladage D., Schinkothe T., Klausmann U., Ulrichs C., Klinz F.J., et al. . Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells 24,1750, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Ng B.H., Chou S.M., Lim B.H., and Chong A.The changes in the tensile properties of tendons after freeze storage in saline solution. Proc Inst Mech Eng [H] 219,387, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Cousineau-Pelletier P., and Langelier E.Relative contributions of mechanical degradation, enzymatic degradation, and repair of the extracellular matrix on the response of tendons when subjected to under- and over- mechanical stimulations in vitro. J Orthop Res 28,204, 2010 [DOI] [PubMed] [Google Scholar]