Abstract
Background: We have previously shown that in healthy young men, a less favorable body composition is associated with higher free triiodothyronine (fT3) levels within the euthyroid range. Besides, a higher free-triiodothyronine-to-free-thyroxin (fT3-to-fT4) ratio has been related to a less favorable metabolic phenotype and more placental growth in pregnant women. In the present study, we therefore investigated whether serum thyrotropin (TSH), thyroid hormone levels, and the fT3-to-fT4 ratio are associated with metabolic and adiposity-related cardiovascular risk markers in a healthy population of middle-aged euthyroid men and women.
Methods: Thyroid parameters were measured in 2524 generally healthy subjects from the Asklepios Study (35–55 years, mean age 46 years). Analyses were restricted to 2315 subjects (1138 women and 1177 men), not using thyroid medication, not having anti-TPO levels above clinical cutoff values or TSH levels outside the reference range (0.27–4.2 mU/L). Twenty-seven percent of the women and 47.5% of the men were overweight, while 13% of women and 17% of men were obese. Twenty percent of the subjects were active smokers. Serum thyroid function parameters were determined by electrochemiluminescence.
Results: fT3 and the fT3-to-fT4 ratio were positively related to body mass index (BMI), waist circumference, and components of metabolic syndrome, that is, triglycerides, systolic and diastolic blood pressure, and fasting plasma glucose, and negatively with HDL-cholesterol levels, whereas fT4 was negatively associated with BMI, waist circumference, and triglycerides (p<0.001). TSH related positively with total cholesterol levels (p<0.01), triglycerides, and systolic and diastolic blood pressure (p<0.001). The fT3-to-fT4 ratio was further positively associated with the adiposity-related inflammation markers interleukin-6 and high-sensitivity C-reactive protein and to pulse wave velocity. All associations were adjusted for sex, age, height, and smoking, and most associations persisted after additional adjustment for weight or waist circumference.
Conclusion: In healthy euthyroid middle-aged men and women, higher fT3 levels, lower fT4 levels, and thus a higher fT3-to-fT4 ratio are consistently associated with various markers of unfavorable metabolic profile and cardiovascular risk.
Introduction
The interrelation of thyroid hormones (THs) and weight status is widely recognized. Thyroid hormone increases basal metabolic rate, which generally leads to weight loss in overt hyperthyroidism and might cause weight gain in hypothyroidism (1). Weight status could also exert an influence on circulating TH, since elevations in thyrotropin (TSH) and free triiodothyronine (fT3), generally without effects on free thyroxine (fT4), were shown in obese subjects (2–5). Also, in a more general (nonobese) euthyroid population, associations of TSH and thyroid hormones with body composition and/or metabolic parameters have been found (2,6–12). Higher TSH levels within the normal range have been related to blood pressure (13–17) and different lipid parameters (14,18–20).
Regarding free thyroid hormones, results might appear contradictory at first sight. On the one hand, lower fT4 levels have been related to (components of ) metabolic syndrome (7,11,12), whereas higher fT3 levels have been linked to body fat and different metabolic parameters (2,6,8–10). A higher fT3-to-fT4 ratio has been related to a less favorable metabolic phenotype and more placental growth in pregnant women (21), which led us to hypothesize that this ratio could also be associated with metabolic markers in the general population. However, to our present knowledge, this has not yet been investigated.
Finally, evidence exists that both subclinical (SC) hypothyroidism as well as SC hyperthyroidism are associated with an increased cardiovascular risk and mortality (22–29). Some studies have also reported on positive associations of TSH and/or TH concentrations, even within the euthyroid range, with more specific cardiovascular risk parameters (30–32). One study showed a positive association between excess fT3 and the prevalence and incidence of coronary events (33), whereas another study reported an inverse association of fT4 levels with coronary artery calcifications in healthy euthyroid subjects (34).
Therefore, in this population of 2315 healthy, euthyroid middle-aged men and women, we aimed to assess associations of fT3, fT4, and TSH levels, and the fT3-to-fT4 ratio with different metabolic indices. Furthermore, we investigated whether these thyroid parameters are associated with other adiposity-related cardiovascular risk markers, namely high sensitivity C-reactive protein (hs-CRP), interleukin 6 (IL-6), and pulse wave velocity, and whether these associations were dependent on body weight.
Methods
Study population
The Asklepios Study is an ongoing population study focused on the interplay of aging, cardiovascular hemodynamics and the emergence of cardiovascular disease. Subjects were randomly sampled from the twinned Belgian communities of Erpe-Mere and Nieuwerkerken (Europe). Study design, in- and exclusion criteria, study components, and baseline characteristics of the population have been described in detail before (35). The cohort (n=2524; 50.5% female) is a population-representative random sample between 35 and 55 years, free from overt cardiovascular disease when entering the study. The cross-sectional data of the present investigation were acquired during the first measuring round (2002–2004). The study was approved by the medical ethical committee of Ghent University Hospital. All participants gave written informed consent prior to enrollment. Data collection and measurements have been described previously (35–37).
Individuals receiving thyroid hormone substitution or antithyroid drugs, having TPO antibodies above clinical cutoff for positivity (i.e., 35 U/L for the assay used), or with TSH levels outside the reference range of our laboratory (0.27–4.2 mU/L) were excluded from the analyses, leaving 2315 subjects (1138 women and 1177 men) for inclusion in the present investigation.
Measurements
Body weight (kg) was measured in light indoor clothing. Standing height (m) was measured using a wall-mounted Harpenden stadiometer. Body mass index (BMI) is defined as the body weight divided by the square of the body height. Waist circumference (cm) is defined as the abdominal circumference located in the middle between the lower rib and the iliac crest, hip circumference (cm) as the widest part of the buttocks or hips. The waist:hip ratio is the waist circumference divided by the hip circumference.
Blood pressure was recorded using bilateral triplicate measurements (1 min intervals) on a rested, sitting subject using a validated and calibrated oscillometric Omron HEM-907 device (Omron Healthcare Co. Ltd., Kyoto, Japan). Blood pressure values of these six readings were averaged, and the mean value is used throughout the study.
The International Diabetes Federation's (IDF) definition of metabolic syndrome is defined as central obesity (i.e., waist circumference ≥94 cm in men or ≥80 cm in women) plus any two of the following four factors: (i) high triglycerides (i.e., ≥150 mg/dL) or specific treatment for this lipid abnormality; (ii) low HDL cholesterol (i.e., <40 mg/dL in men or <50 mg/dL in women) or specific treatment for this lipid abnormality; (iii) high blood pressure (i.e., ≥130/≥85 mmHg) or antihypertensive treatment; (iv) high fasting plasma glucose concentration (i.e., ≥100 mg/dL) or previously diagnosed type 2 diabetes (38).
All subjects underwent an ECG-gated echographic examination of the carotid and femoral arteries (VIVID 7; GE Vingmed Ultrasound, Horten, Norway). Flow was recorded with a pulse wave Doppler using a 10–12 MHz vascular linear probe, and the flow measurement site was marked and recorded. Carotid–femoral pulse wave velocity (PWV) was estimated as (ΔLS-F−ΔLS-C)/(ΔTQ-F−ΔTQ-C) with ΔLS-F and ΔLS-C the distance measured from sternum notch to femoral and carotid measuring sites respectively. ΔTQ-F and ΔTQ-C are the time delay between the start of the QRS complex and the upstroke of flow measured with Doppler echography in the femoral and carotid artery (39,40).
Biochemical determinations
Blood samples were obtained throughout the day after six hours fasting and refraining from smoking. All serum samples were stored at −80°C until batch analysis. Thyroid hormone function tests (TSH, fT3, fT4, and TT3) and TPO antibodies were determined using immunoelectrochemiluminescence (Roche reagents) on Cobas 411 (Roche Diagnostics GmbH, Mannheim, Germany). The intra- and interassay CV % were below 10% for all measurements. The fT3-to-fT4 ratio was calculated by dividing fT3 (pg/dL) by fT4 (ng/dL) and further dividing this value by 1000.
Serum glucose was assayed by a standard hexokinase enzymatic method. Total serum cholesterol was assayed by the enzymatic colorimetric CHOD-PAP method of Allain et al. (41). Serum HDL cholesterol was determined by the homogenous enzymatic method that uses dextrans sulfate and polyethylene glycol-modified cholesterol esterase and cholesterol oxidase. For serum triglycerides, the lipase kinetic colorimetric reaction without glycerol correction was used. LDL cholesterol was calculated using the classical Friedewald formula LDL-C=(total C−HDL-C−TG)/5. When triglycerides exceeded 350 mg/dL, a value for LDL-C was not calculated. High sensitive serum C-reactive protein was measured by a high-sensitive, particle-enhanced immunoturbidimetric method on an Integra 400 analyzer (Roche Diagnostics). CV was <3.0%. Serum interleukin-6 (IL-6) concentrations were measured by an automated solid-phase, enzyme-labeled, chemiluminescent sequential immunometric assay (IMMULITE 2000; Diagnostic Products Corp., Los Angeles, CA), making use of murine monoclonal anti-IL-6 coated beads and alkaline phosphatase-conjugated polyclonal sheep anti-IL-6 antibodies. Total CV was <5.6%.
Statistical analysis
TSH, fT3, fT4, TT3, and the fT3-to-fT4 ratio were analyzed both as a continuous variable and, for TSH and the FT3-to-FT4 ratio, also using quartiles (quartiles TSH women: 0.27–1.08, 1.09–1.54, 1.55–2.08, 2.09–4.2 mU/L; quartiles TSH men: 0.27–1.03, 1.04–1.47, 1.48–1.99, 2.00–4.2 mU/L; quartiles fT3-to-FT4 ratio in women: <0.22, 0.22–0.24, 0.24–0.26, >0.26; quartiles fT3-to-fT4 ratio in men: <0.23, 0.23–0.25, 0.25–0.28, >0.28).
All statistical analyses (linear regression analysis, one-way analysis of variance [ANOVA], and logistic regression analysis) were performed using SPSS v19 (SPSS Inc., Chicago, IL). Since men and women were analyzed together to increase the power, analyses were first adjusted for sex. Additional adjustment for age, height, and current smoking status was performed. A separate adjustment for weight or waist circumference was made in a second model. Statistical significance was assumed for p-values <0.05. Data are given as mean±SD for normally distributed data or median (interquartile range) for skewed data.
Results
Baseline characteristics of the study population
Characteristics of the study population, together with descriptives for thyroid hormones, metabolic parameters, and adiposity-related cardiovascular predictors are shown in Table 1. Twenty-seven percent of the women and 47.5% of the men were overweight (25 kg/m2<BMI≤29.9 kg/m2), and 12.8% of the women and 17% of the men were obese (BMI≥30 kg/m2). After adjustment for sex, age, and smoking, TSH related negatively to fT4 (β=− 0.14, p<0.0001) and positively to the fT3-to-fT4 ratio (β=0.10, p<0.0001). fT3 was not significantly associated with TSH.
Table 1.
Patient Characteristics
| Women (n=1138) | Men (n=1177) | |
|---|---|---|
| General characteristics | ||
| Age (years) | 46 (41–51) | 46 (41–51) |
| Height (cm) | 163±6 | 176±7 |
| Weight (kg) | 65.4±11.6 | 81.1±12.2 |
| BMI (kg/m2) | 24.5±4.2 | 26.2±3.6 |
| Smoking (active/former/never) (%) | 19/21/60 | 22/36/42 |
| Overweight (25≤BMI≤29.9), n (%) | 309 (27%) | 559 (47.5%) |
| Obesity (BMI≥30), n (%) | 146 (12.8%) | 200 (17%) |
| TSH and thyroid hormones | ||
| TSH (mU/L) | 1.53 (1.07–2.08) | 1.47 (1.03–1.98) |
| fT4 (ng/dL) | 1.25 (1.15–1.35) | 1.34 (1.23–1.44) |
| fT3 (pg/dL) | 300 (280–320) | 338 (310–3.60) |
| TT3 (ng/dL) | 120 (105–140) | 118 (107–129) |
| fT3-to-fT4 ratio | 0.24 (0.22–0.26) | 0.25 (0.23–0.28) |
| Metabolic parameters | ||
| Fasting blood glucose (mg/dL) | 88 (83–93) | 92 (87–98) |
| Triglycerides (mg/dL) | 78 (59–111) | 104 (76–153) |
| Total cholesterol (mg/dL) | 212 (189–235) | 217 (192–242) |
| LDL-cholesterol (mg/dL) | 121 (101–144) | 136 (113–159) |
| HDL-cholesterol (mg/dL) | 71±17 | 56±14 |
| Systolic BP (mmHg) | 121±13 | 130±12 |
| Diastolic BP (mmHg) | 77±9 | 82±9 |
| Waist circumference (cm) | 80±11 | 94±10 |
| Waist:hip ratio | 0.77 (0.73–0.82) | 0.92 (0.87–0.96) |
| Other cardiovascular risk markers | ||
| hs-CRP (mg/L) | 1.4 (0.59–3.51) | 1.03 (0.53–2.00) |
| IL-6 (pg/mL) | 0.75 (0–1.5) | 0.78 (0–1.56) |
| Pulse wave velocity (m/s) | 6.3 (5.6–7.3) | 6.3 (5.7–7.2) |
Subjects on thyroid medication with TPO antibodies above clinical cutoff and with TSH levels outside the reference range were excluded from further analyses. Data are mean±SD or medians (first–third quartiles) in case of non-Gaussian distribution. Conversion factor for fT3 from pg/dL to pmol/L and for TT3 from ng/dL to nmol/L is ×0.0154; conversion factor for fT4 from ng/dL to pmol/L is ×12.87.
BMI, body mass index; TSH, thyrotropin, BP, blood pressure, hs-CRP, high sensitivity C-reactive protein; IL-6, interleukin 6.
Associations of TSH and thyroid hormones with body composition and metabolic parameters
Analyses regarding the association between thyroid function indices and metabolic parameters and adiposity-related cardiovascular risk markers pooled for men and women are shown in Table 2. All analyses were adjusted for sex, age, height, and current smoking status. Subjects taking lipid-lowering (n=142, 6%) or antihypertensive drugs (n=237, 10%) were excluded from the analyses on lipid parameters and blood pressure respectively.
Table 2.
Associations Between Thyroid Hormones and Metabolic Parameters (Pooled Men and Women)
| TSH | fT3 | TT3 | fT4 | fT3:fT4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dependent variable | β | p | β | p | β | p | β | p | β | p |
| BMI | 0.03 | 0.1 | 0.12 | <0.0001‡ | 0.18 | <0.0001‡ | −0.10 | <0.0001‡ | 0.19 | <0.0001‡ |
| Waist circumference | 0.02 | 0.2 | 0.13 | <0.0001‡ | 0.16 | <0.0001‡ | −0.08 | <0.0001‡ | 0.18 | <0.0001‡ |
| Waist:hip ratio | 0.02 | 0.2 | 0.10 | <0.0001‡ | 0.10 | <0.0001‡ | −0.05 | <0.0001‡ | 0.08 | <0.0001‡ |
| Systolic RR | 0.10 | <0.0001‡ | 0.14 | <0.0001‡ | 0.16 | <0.0001‡ | −0.02 | 0.4 | 0.12 | <0.0001‡ |
| Diastolic RR | 0.10 | <0.0001‡ | 0.12 | <0.0001‡ | 0.12 | <0.0001‡ | 0.01 | 0.6 | 0.09 | <0.0001‡ |
| Triglycerides | 0.08 | <0.0001‡ | 0.07 | 0.005† | 0.12 | <0.0001‡ | −0.07 | 0.002† | 0.11 | <0.0001‡ |
| Total cholesterol | 0.06 | 0.008† | −0.05 | 0.045 | −0.03 | 0.2 | −0.01 | 0.6 | −0.02 | 0.3 |
| LDL-cholesterol | 0.3 | 0.2 | −0.01 | 0.8 | −0.05 | 0.01† | 0.01 | 0.8 | −0.01 | 0.7 |
| HDL-cholesterol | −0.01 | 0.5 | −0.12 | <0.0001‡ | −0.06 | 0.003† | 0.03 | 0.1 | −0.12 | <0.0001‡ |
| Fasting blood glucose | −0.01 | 0.6 | 0.12 | <0.0001‡ | 0.04 | 0.04* | −0.03 | 0.1 | 0.12 | <0.0001‡ |
Reported values are betas and results from linear regression analysis with metabolic parameters as dependent variables and thyroid parameters as independent variables. Betas are scaled; an adjustment for age, height, current smoking, and sex was performed (except for BMI, which was only adjusted for age, sex, and smoking). For all lipid parameters, an exclusion of subjects on lipid-lowering drugs, and for blood-pressure parameters, an exclusion of subjects on antihypertensive treatment, was made. Significant associations are indicated in bold.
0.01<p≤0.05; †0.001<p≤0.01; ‡p≤0.001.
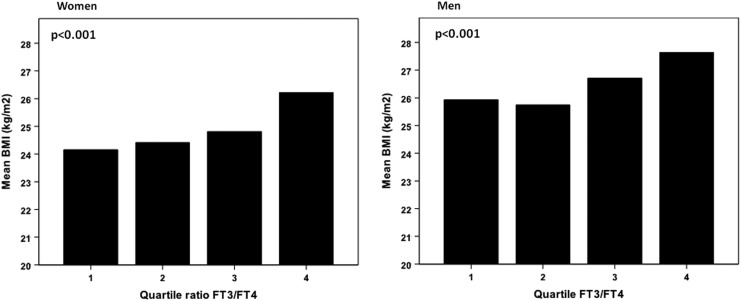
fT3 and the fT3-to-fT4 ratio were positively related and fT4 was negatively related to BMI (Fig. 1), waist circumference, and the waist:hip ratio (p<0.0001). Blood pressure (both systolic and diastolic) was also positively related to fT3 and the fT3-to-fT4 ratio (p<0.0001). The same was true for fasting glucose levels, although the significance level for association with TT3 was much lower (p=0.04). With regard to lipid parameters, we observed a positive association between triglycerides and fT3 and the fT3-to-fT4 ratio, whereas a negative association with fT4 was seen. The mirror image was observed for HDL cholesterol, which was negatively associated with fT3 and the fT3-to-fT4 ratio. No associations were seen for total cholesterol, and there was only a rather marginal association between LDL cholesterol and TT3 (p=0.01).
FIG. 1.
Mean BMI according to quartile of the FT3-to-FT4 ratio in women and in men. p-Value results from ANOVA (analysis of variance, between categories).
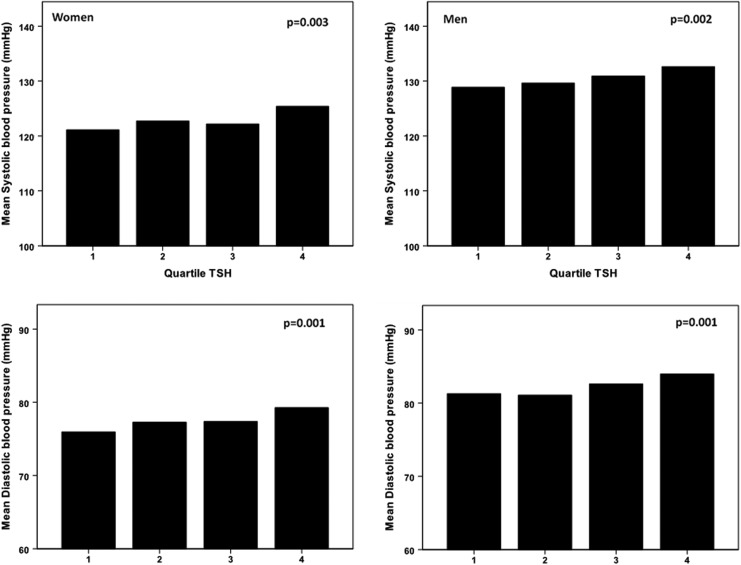
TSH was positively associated with triglycerides, blood pressure (both systolic and diastolic), and less significantly with total cholesterol levels. Mean systolic and diastolic blood pressure according to quartiles of TSH in men and women are illustrated in Figure 2.
FIG. 2.
Mean systolic and diastolic blood pressure according to quartiles of TSH (within reference range). p-Values result from overall ANOVA.
When an additional adjustment for weight or waist circumference was performed, most associations kept their significance, except for the associations between fT3 and triglycerides, between TT3 and HDL, and between the fT3-to-fT4 ratio and diastolic blood pressure. Also, excluding the obese subjects from the analyses did not alter the significance of most associations, although the beta coefficients decreased. Analyzing men and women separately, most associations remained unchanged in the separate groups, except for associations of fT3 and fT4 with triglycerides, which were not present in men.
Associations of thyroid hormones with the presence of metabolic syndrome as a whole
Additionally, fT3 and fT4 and the fT3-to-fT4 ratio were related to the presence of metabolic syndrome as a whole (according to the IDF criteria) (fT3: standardized OR=1.3, p<0.0001; fT4: standardized OR=0.9, p=0.008; fT3-to-fT4 ratio: standardized OR=1.5, p<0.0001). All associations were adjusted for sex, age, height, and current smoking status.
Associations between thyroid hormones and other cardiovascular risk markers
The IL-6 levels were negatively related to fT4 levels and positively to TT3, TSH, and to the fT3-to-fT4 ratio in a linear regression model (Table 3). The associations with fT4 and the fT3-to-fT4 ratio remained significant when additionally adjusting for weight or waist circumference.
Table 3.
Associations Between Throid Hormones and Other Cardiovascular Risk Parameters
| TSH | fT3 | TT3 | fT4 | fT3:fT4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dependent variable | β | p | β | p | β | p | β | p | β | p |
| IL-6 | 0.02 | 0.04* | 0.04 | 0.07 | 0.11 | <0.0001‡ | −0.06 | 0.004† | 0.09 | <0.0001‡ |
| hs-CRP | 0.04 | 0.07 | 0.07 | 0.001‡ | 0.35 | <0.0001‡ | −0.12 | <0.0001‡ | 0.16 | <0.0001‡ |
| Pulse wave velocity | 0.06 | 0.1 | 0.09 | <0.0001‡ | 0.09 | <0.0001‡ | 0.02 | 0.4 | 0.07 | <0.0001‡ |
Reported values are betas and results from linear regression analysis with cardiovascular risk parameters as dependent variables and thyroid parameters as independent variables. Betas are scaled; an adjustment for age, height, current smoking, and sex was performed. Significant associations are indicated in bold.
0.01<p≤0.05; †0.001<p≤0.01; ‡p≤0.001.
For hs-CRP, the findings were essentially similar, that is, a negative association with fT4 and a positive association with the fT3-to-fT4 ratio. In addition, a positive relation with fT3 levels was observed, although the association with fT3 was lost when additionally adjusting for weight.
Carotid-femoral pulse wave velocity (reflecting vascular stiffening) displayed positive associations with fT3 as well as with the fT3-to-fT4 ratio, even after adjustment for weight or waist circumference.
Exclusion of obese subjects from the analyses did not alter the observed associations with IL-6, hs-CRP, and pulse wave velocity. When men and women were analyzed separately, only the positive associations of hs-CRP and IL-6 with the fT3-to-fT4 ratio remained significant in both sexes.
Discussion
The main finding from this cross-sectional study in 2315 euthyroid middle-aged men and women is that in the general population a higher conversion of fT4 to fT3, as inferred from the fT3-to-fT4 ratio, is a marker of an unfavorable metabolic profile, with a higher BMI, waist circumference, waist:hip ratio, blood pressure, triglycerides, fasting glycemia, and lower HDL levels. Besides, this fT3-to-fT4 ratio was positively related to IL6, hs-CRP, and carotid-femoral pulse wave velocity. These observations remained significant after additional adjustment for weight or waist circumference and after exclusion of obese subjects (BMI >30 kg/m2), which shows that the associations are not driven by the obese subjects solely. Finally, most associations were present in both women and men when considered separately.
Our observations of positive associations between fT3 and metabolic and cardiovascular parameters, while fT4 was negatively related to the latter parameters, might seem counterintuitive. However, the interrelation of THs within normal range with body composition and metabolic status is complex, and apparently discordant results have been reported.
On the one hand, inverse associations between fT4 and different metabolic parameters have been observed (7,8,11,12,42,43). Shon et al. observed negative associations between fT4 within the normal range and BMI (43). Roos et al. have also reported on associations between low normal fT4 levels and increased insulin resistance and an unfavorable lipid profile in a cross-sectional study of 2703 euthyroid adults (11). In another cross-sectional study in 3148 subjects, fT4 was positively related to HDL and inversely to waist circumference, HOMA-IR, and insulin (12). Kim et al. reported that subjects in the highest fT4 quintiles had a significantly lower prevalence of metabolic syndrome than those in the lowest fT4 quintile, but such differences disappeared after adjustment for age (7). Finally, Alevizaki et al. showed that fT4 levels were negatively associated with the abdominal subcutaneous fat layer thickness as well as with the subcutaneous fat/periperitoneal fat ratio (42). However, other studies observed no associations between fT4 and body weight or BMI (8,44).
On the other hand, fT3 has been related positively to body fat mass and various metabolic parameters (2,5,8–10). In obese subjects, positive associations between fT3 and BMI, waist circumference, and insulin were observed (2,5,9). Also in a more general population, positive associations between fT3 and metabolic status have been observed (8,10). We previously reported on positive associations of BMI, fat mass, and insulin resistance with fT3 in healthy euthyroid young men (10). Recently, Kithara et al. also described positive associations between body fatness and fT3 (8).
These findings, together with observations of Bassols et al. (21), who described a less favorable metabolic phenotype and more placental growth in pregnant women with lower fT4 levels and a higher fT3-to-fT4 ratio, have led us to hypothesize that a higher conversion of fT4 to fT3, as inferred from changes in the fT3-to-fT4 ratio, might be an indicator of an unfavorable metabolic profile.
Several mechanisms can be postulated. First, prior studies have suggested changes in deiodinase (DIO) 1 and/or 2 activity in subjects with a relatively higher fat mass and/or a less favorable metabolic profile, hereby leading to higher conversion of fT4 to fT3. Indeed, under conditions of increasing adiposity, a higher DIO1 activity in white adipose tissue, through stimulation by leptin, has been shown in mice (45). Also in humans, Ortega et al. have shown an increase in DIO1-activity in hypertrophic white adipose tissue (46). DIO2 contributes to the activation of UCP-1 and thermogenesis and is typically present in brown adipose tissue (47). We did not find any studies reporting changes in DIO2 activity in healthy subjects with a relatively higher fat mass. Nevertheless, DIO2 expression was shown to be increased in the dorso-cervical subcutaneous adipose tissue among patients with HIV lipodystrophy, particularly those with increased visceral adiposity, and this increased DIO2 expression was positively associated with energy expenditure (48).
Second, an adverse metabolic profile could theoretically also lead to an altered thyroid hormone secretion by the thyroid gland itself, with a relatively higher T3-secretion. However, to the best of our knowledge, there are presently no literature data in support of this hypothesis.
Third, since both higher TSH levels as well as a higher fT3-to-fT4 ratio can indicate a lower iodine pool, and since TSH was also positively related to the fT3-to-fT4 ratio, another mechanism underlying the higher fT3-to-fT4 ratio (and the higher TSH levels) in subjects with a less favorable metabolic profile could be a lower iodine intake in the diet or a decreased absorption. Nevertheless, we found no studies indicative of a lower iodine intake in obesity.
Our findings of associations between TSH and blood pressure and lipid parameters are in line with several large epidemiological studies that also showed a positive association between TSH within the reference range and (systolic and diastolic) blood pressure (13,15–17). Itterman et al. (16) suggested that the effect of variation in TH levels on blood pressure is only a direct short-term effect, since only associations with current and not incident hypertension were found in their study. Concerning the lipid status, overt hypothyroidism is associated with hyperlipidemia, and even subclinical hypothyroidism has been related to alterations in plasma lipids (49–52). Large observational studies evaluating associations between normal-range TSH and lipids have also shown positive relations with triglycerides, total and LDL cholesterol (12,18), and a negative association with HDL (18). Finally, Wang et al. (20) demonstrated a positive linear association between increasing levels of TSH within the normal range and total cholesterol and triglycerides, which according to the authors might be caused both by a direct effect of TSH as well as through an indirect effect via circulating THs. A proposed mechanism for direct effects of TSH involves TSH receptors on hepatocytes and the upregulation of hepatic 3-hydroxy-3-methyl glutaryl coenzymeA reductase, hereby promoting cholesterol synthesis in the liver (20). Remarkable in our study is that TSH was related to total but not to LDL-C or HDL-C, suggesting an association with remnant cholesterol, or non-HDL, non-LDL cholesterol. (Subclinical) hypothyroidism has indeed been associated with elevated serum concentrations of remnant lipoproteins, an effect that is reversible with L-T4 treatment (52). Of course, the associations of TSH and lipid levels in our study may also be coincidental and not causally related.
We further observed positive associations of the fT3-to-fT4 ratio with the adiposity-related markers of inflammation—IL-6 and hs-CRP—and with carotid-femoral pulse wave velocity, a marker of arterial stiffness, which persisted after additional adjustment for weight or waist circumference. Basically, our results suggest that a higher TSH, fT3, and fT3-to-fT4 ratio, within the euthyroid range, are associated with a higher cardiovascular risk. Peters et al. (33) showed earlier an excess of coronary events in subjects with elevated fT3 levels, whereas fT4 levels were inversely associated with coronary artery calcifications in euthyroid healthy men, independent from conventional CV risk factors in another study (34). We propose that a relatively higher fT3-to-fT4 ratio might also be considered as a potential indicator of increased cardiovascular risk, although we presume that this effect results at least in part from the less favorable body composition and metabolic parameters associated with this higher fT3-to-fT4 ratio, and, of course, this concept needs validation in a prospective setting.
The strengths of this study are the large number of subjects, together with the extensive phenotypic characterization, which includes both an elaborated set of metabolic cardiovascular parameters, as well as a complete thyroid status assessment with measurements of TSH, free thyroid hormones, TT3, and thyroid antibodies.
The novelty of our results is that we extend the findings of Bassols et al. (21)—who stated that the fT3-to-fT4 ratio is associated with an unfavorable metabolic profile in pregnant women—to a population of middle-aged euthyroid women and men. Besides, the associations with different cardiovascular risk markers reinforce our observations.
The limitations of our study include the lack of a precise assessment on body composition, such as by dual X-ray absorptiometry (DXA). Besides, the fact that blood samples were taken throughout the day after only six hours of fasting instead of an entire night fasting may have influenced increased levels of TG and therefore LDL-C as well. Also, exercise during the day could have influenced lipid levels, but this would rather have led to a decrease of TG and an increase in HDL. An important limitation consists in the cross-sectional design, which does not allow us to make causal inferences from the observed associations. Longitudinal follow-up is presently ongoing. Finally, it must be emphasized that this study was performed in a relatively young population (mean age 46 years) in the context of evaluating parameters associated with cardiovascular disease, and it might not be possible to extrapolate all our findings to an older population.
In conclusion, in this population of 2315 euthyroid middle-aged men and women, we have shown robust positive associations of TSH, fT3, and the fT3-to-fT4 ratio, and negative associations of fT4 with different components of an unfavorable metabolic profile and other (adiposity-related) cardiovascular risk markers. Whether the observed associations merely reflect metabolic effects on the regulation of thyroid hormone levels, or might represent an adaptive reaction to a less favorable metabolic profile, is presently unknown.
Acknowledgments
The Asklepios Study is supported by a Fonds voor Wetenschappelijk Onderzoek-Vlaanderen FWO research grant G.0427.03 and G.0838.10 (Asklepios Study), and for this study, also by grant G0867.11. The Asklepios Study is indebted to Frida Brusselmans, Femke Van Hoeke, and Bianca Leydens, and the residents and general practitioners of Erpe-Mere and Nieuwerkerken for their invaluable help in completing the study.
The authors also wish to thank Magda Becqué, Eric Vandersypt, Kathelyne Mertens, and Kaatje Toye for the determinations of free thyroid hormones, TSH, and anti-TPO.
Author Disclosure Statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Hoogwerf BJ, Nuttall FQ.1984Long-term weight regulation in treated hyperthyroid and hypothyroid subjects. Am J Med 76:963–970 [DOI] [PubMed] [Google Scholar]
- 2.De Pergola G, Ciampolillo A, Paolotti S, Trerotoli P, Giorgino R.2007Free triiodothyronine and thyroid stimulating hormone are directly associated with waist circumference, independently of insulin resistance, metabolic parameters and blood pressure in overweight and obese women. Clin Endocrinol (Oxf ) 67:265–269 [DOI] [PubMed] [Google Scholar]
- 3.Diez JJ, Iglesias P.2011Relationship between thyrotropin and body mass index in euthyroid subjects. Exp Clin Endocrinol Diabetes 119:144–150 [DOI] [PubMed] [Google Scholar]
- 4.Reinehr T, Isa A, de Sousa G, Dieffenbach R, Andler W.2008Thyroid hormones and their relation to weight status. Horm Res 70:51–57 [DOI] [PubMed] [Google Scholar]
- 5.Reinehr T.2010Obesity and thyroid function. Mol Cell Endocrinol 316:165–171 [DOI] [PubMed] [Google Scholar]
- 6.Fox CS, Pencina MJ, D'Agostino RB, Murabito JM, Seely EW, Pearce EN, Vasan RS.2008Relations of thyroid function to body weight: cross-sectional and longitudinal observations in a community-based sample. Arch Intern Med 168:587–592 [DOI] [PubMed] [Google Scholar]
- 7.Kim BJ, Kim TY, Koh JM, Kim HK, Park JY, Lee KU, Shong YK, Kim WB.2009Relationship between serum free T4 (FT4) levels and metabolic syndrome (MS) and its components in healthy euthyroid subjects. Clin Endocrinol (Oxf ) 70:152–160 [DOI] [PubMed] [Google Scholar]
- 8.Kitahara CM, Platz EA, Ladenson PW, Mondul AM, Menke A, Berrington de González A.2012Body fatness and markers of thyroid function among U.S. men and women. PLoS One 7:e34979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortega E, Koska J, Pannacciulli N, Bunt JC, Krakoff J.2008Free triiodothyronine plasma concentrations are positively associated with insulin secretion in euthyroid individuals. Eur J Endocrinol 158:217–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roef G, Lapauw B, Goemaere S, Zmierczak HG, Toye K, Kaufman JM, Taes Y.2012Body composition and metabolic parameters are associated with variation in thyroid hormone levels among euthyroid young men. Eur J Endocrinol 167:719–726 [DOI] [PubMed] [Google Scholar]
- 11.Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH.2007Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab 92:491–496 [DOI] [PubMed] [Google Scholar]
- 12.Garduno-Garcia JJ, Alvirde-Garcia U, Lopez-Carrasco G, Padilla Mendoza ME, Mehta R, Arellano-Campos O, Choza R, Sauque L, Garay-Sevilla ME, Malacara JM, Gomez-Perez FJ, Aguilar-Salinas CA.2010TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol 163:273–278 [DOI] [PubMed] [Google Scholar]
- 13.Asvold BO, Bjoro T, Nilsen TI, Vatten LJ.2007Association between blood pressure and serum thyroid-stimulating hormone concentration within the reference range: a population-based study. J Clin Endocrinol Metab 92:841–845 [DOI] [PubMed] [Google Scholar]
- 14.Boekholdt SM, Titan SM, Wiersinga WM, Chatterjee K, Basart DC, Luben R, Wareham NJ, Khaw KT.2010Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf ) 72:404–410 [DOI] [PubMed] [Google Scholar]
- 15.Gumieniak O, Perlstein TS, Hopkins PN, Brown NJ, Murphey LJ, Jeunemaitre X, Hollenberg NK, Williams GH.2004Thyroid function and blood pressure homeostasis in euthyroid subjects. J Clin Endocrinol Metab 89:3455–3461 [DOI] [PubMed] [Google Scholar]
- 16.Ittermann T, Tiller D, Meisinger C, Agger C, Nauck M, Rettig R, Hofman A, Franco O, Joergensen T, Linneberg A, Witteman J, Greiser H, Werdan K, Doring A, Kluttig A, Stricker B, Volzke H.2013High serum TSH levels are associated with current but not with incident hypertension. Thyroid 23:955–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu D, Jiang F, Shan Z, Wang B, Wang J, Lai Y, Chen Y, Li M, Liu H, Li C, Xue H, Li N, Yu J, Shi L, Bai X, Hou X, Zhu L, Lu L, Wang S, Xing Q, Teng W.2010. A cross-sectional survey of relationship between serum TSH level and blood pressure. J Hum Hypertens 24:134–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asvold BO, Vatten LJ, Nilsen TI, Bjoro T.2007The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT Study. Eur J Endocrinol 156:181–186 [DOI] [PubMed] [Google Scholar]
- 19.Lu L, Wang B, Shan Z, Jiang F, Teng X, Chen Y, Lai Y, Wang J, Xue H, Wang S, Li C, Liu H, Li N, Yu J, Shi L, Hou X, Xing Q, Bai X, Teng W.2011The correlation between thyrotropin and dyslipidemia in a population-based study. J Korean Med Sci 26:243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Tan Y, Wang C, Zhang X, Zhao Y, Song X, Zhang B, Guan Q, Xu J, Zhang J, Zhang D, Lin H, Yu C, Zhao J.2012Thyroid-stimulating hormone levels within the reference range are associated with serum lipid profiles independent of thyroid hormones. J Clin Endocrinol Metab 97:2724–2731 [DOI] [PubMed] [Google Scholar]
- 21.Bassols J, Prats-Puig A, Soriano-Rodriguez P, Garcia-Gonzalez MM, Reid J, Martinez-Pascual M, Mateos-Comeron F, de Zegher F, Ibanez L, Lopez-Bermejo A.2011Lower free thyroxin associates with a less favorable metabolic phenotype in healthy pregnant women. J Clin Endocrinol Metab 96:3717–3723 [DOI] [PubMed] [Google Scholar]
- 22.Biondi B.2012How could we improve the increased cardiovascular mortality in patients with overt and subclinical hyperthyroidism? Eur J Endocrinol 167:295–299 [DOI] [PubMed] [Google Scholar]
- 23.Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW.2006Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 295:1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duggal J, Singh S, Barsano CP, Arora R.2007Cardiovascular risk with subclinical hyperthyroidism and hypothyroidism: pathophysiology and management. J Cardiometab Syndr 2:198–206 [DOI] [PubMed] [Google Scholar]
- 25.Gencer B, Collet TH, Virgini V, Bauer DC, Gussekloo J, Cappola AR, Nanchen D, den Elzen WP, Balmer P, Luben RN, Iacoviello M, Triggiani V, Cornuz J, Newman AB, Khaw KT, Jukema JW, Westendorp RG, Vittinghoff E, Aujesky D, Rodondi N.2012Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation 126:1040–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gencer B, Collet TH, Virgini V, Auer R, Rodondi N.2013Subclinical thyroid dysfunction and cardiovascular outcomes among prospective cohort studies. Endocr Metab Immune Disord Drug Targets 13:4–12 [DOI] [PubMed] [Google Scholar]
- 27.Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC.2000Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med 132:270–278 [DOI] [PubMed] [Google Scholar]
- 28.Walsh JP, Bremner AP, Bulsara MK, O'Leary P, Leedman PJ, Feddema P, Michelangeli V.2005Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med 165:2467–2472 [DOI] [PubMed] [Google Scholar]
- 29.Westerink J, van der GY, Faber DR, Spiering W, Visseren FL.2012Relation between thyroid-stimulating hormone and the occurrence of cardiovascular events and mortality in patients with manifest vascular diseases. Eur J Cardiovasc Prev Rehabil 19:864–873 [DOI] [PubMed] [Google Scholar]
- 30.Dullaart RP, de Vries R, Roozendaal C, Kobold AC, Sluiter WJ.2007Carotid artery intima media thickness is inversely related to serum free thyroxine in euthyroid subjects. Clin Endocrinol (Oxf ) 67:668–673 [DOI] [PubMed] [Google Scholar]
- 31.Lambrinoudaki I, Armeni E, Rizos D, Georgiopoulos G, Kazani M, Alexandrou A, Deligeoroglou E, Livada A, Psychas C, Creatsa M, Bouboulis G, Alevizaki M, Stamatelopoulos K.2012High normal thyroid-stimulating hormone is associated with arterial stiffness in healthy postmenopausal women. J Hypertens 30:592–599 [DOI] [PubMed] [Google Scholar]
- 32.Takamura N, Akilzhanova A, Hayashida N, Kadota K, Yamasaki H, Usa T, Nakazato M, Maeda T, Ozono Y, Aoyagi K.2009Thyroid function is associated with carotid intima-media thickness in euthyroid subjects. Atherosclerosis 204:e77–e81 [DOI] [PubMed] [Google Scholar]
- 33.Peters A, Ehlers M, Blank B, Exler D, Falk C, Kohlmann T, Fruehwald-Schultes B, Wellhoener P, Kerner W, Fehm HL.2000Excess triiodothyronine as a risk factor of coronary events. Arch Intern Med 160:1993–1999 [DOI] [PubMed] [Google Scholar]
- 34.Kim ES, Shin JA, Shin JY, Lim DJ, Moon SD, Son HY, Han JH.2012Association between low serum free thyroxine concentrations and coronary artery calcification in healthy euthyroid subjects. Thyroid 22:870–876 [DOI] [PubMed] [Google Scholar]
- 35.Rietzschel ER, De Buyzere ML, Bekaert S, Segers P, De Bacquer D, Cooman L, Van Damme P, Cassiman P, Langlois M, van Oostveldt P, Verdonck P, De Backer G, Gillebert TC.2007Rationale, design, methods and baseline characteristics of the Asklepios Study. Eur J Cardiovasc Prev Rehabil 14:179–191 [DOI] [PubMed] [Google Scholar]
- 36.Rietzschel ER, Langlois M, De Buyzere ML, Segers P, De Bacquer D, Bekaert S, Cooman L, van Oostveldt P, Verdonck P, De Backer GG, Gillebert TC.2008Oxidized low-density lipoprotein cholesterol is associated with decreases in cardiac function independent of vascular alterations. Hypertension 52:535–541 [DOI] [PubMed] [Google Scholar]
- 37.Roef GL, Taes YE, Kaufman JM, Van Daele C, De Buyzere ML, Gillebert TC, Rietzschel ER.2013Thyroid hormone levels within reference range are associated with heart rate, cardiac structure and function in middle-aged men and women. Thyroid 23:947–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr2009Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 39.Segers P, Rietzschel ER, De Buyzere ML, Vermeersch SJ, De Bacquer D, Van Bortel LM, De Backer G, Gillebert TC, Verdonck PR.2007Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension 49:1248–1255 [DOI] [PubMed] [Google Scholar]
- 40.Vermeersch SJ, Rietzschel ER, De Buyzere ML, De Bacquer D, De Backer G, Van Bortel LM, Gillebert TC, Verdonck PR, Segers P.2008Age and gender related patterns in carotid-femoral PWV and carotid and femoral stiffness in a large healthy, middle-aged population. J Hypertens 26:1411–1419 [DOI] [PubMed] [Google Scholar]
- 41.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC.1974Enzymatic determination of total serum cholesterol. Clin Chem 20:470–475 [PubMed] [Google Scholar]
- 42.Alevizaki M, Saltiki K, Voidonikola P, Mantzou E, Papamichael C, Stamatelopoulos K.2009Free thyroxine is an independent predictor of subcutaneous fat in euthyroid individuals. Eur J Endocrinol 161:459–465 [DOI] [PubMed] [Google Scholar]
- 43.Shon HS, Jung ED, Kim SH, Lee JH.2008Free T4 is negatively correlated with body mass index in euthyroid women. Korean J Intern Med 23:53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manji N, Boelaert K, Sheppard MC, Holder RL, Gough SC, Franklyn JA.2006Lack of association between serum TSH or free T4 and body mass index in euthyroid subjects. Clin Endocrinol (Oxf ) 64:125–128 [DOI] [PubMed] [Google Scholar]
- 45.Macek JZ, Pavelka S, Flachs P, Hensler M, Kus V, Kopecky J.2010Modulation of type I iodothyronine 5′-deiodinase activity in white adipose tissue by nutrition: possible involvement of leptin. Physiol Res 59:561–569 [DOI] [PubMed] [Google Scholar]
- 46.Ortega FJ, Jilkova ZM, Moreno-Navarrete JM, Pavelka S, Rodriguez-Hermosa JI, Kopeck YJ, Fernandez-Real JM.2012Type I iodothyronine 5′-deiodinase mRNA and activity is increased in adipose tissue of obese subjects. Int J Obes (Lond) 36:320–324 [DOI] [PubMed] [Google Scholar]
- 47.Bianco AC, Kim BW.2006Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest 116:2571–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torriani M, Fitch K, Stavrou E, Bredella MA, Lim R, Sass CA, Cypess AM, Grinspoon S.2012Deiodinase 2 expression is increased in dorsocervical fat of patients with HIV-associated lipohypertrophy syndrome. J Clin Endocrinol Metab 97:E602–E607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duntas LH, Wartofsky L.2007Cardiovascular risk and subclinical hypothyroidism: focus on lipids and new emerging risk factors. What is the evidence? Thyroid 17:1075–1084 [DOI] [PubMed] [Google Scholar]
- 50.Duntas LH, Brenta G.2012The effect of thyroid disorders on lipid levels and metabolism. Med Clin North Am 96:269–281 [DOI] [PubMed] [Google Scholar]
- 51.Fabbrini E, Magkos F, Patterson BW, Mittendorfer B, Klein S.2012Subclinical hypothyroidism and hyperthyroidism have opposite effects on hepatic very-low-density lipoprotein-triglyceride kinetics. J Clin Endocrinol Metab 97:E414–E418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pearce EN.2012Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab 97:326–333 [DOI] [PubMed] [Google Scholar]