Abstract
Alveolar bone resorption generally occurs during healing after tooth extraction. This study aimed to evaluate the effects of platelet-poor plasma (PPP), platelet-rich plasma (PRP), and platelet-rich fibrin (PRF) on healing in a ridge-augmentation model of the canine socket with dehiscence of the buccal wall. The third mandibular premolars of 12 beagle dogs were extracted and a 3 mm buccal dehiscence from the alveolar crest to the buccal wall of the extraction socket was created. These sockets were then divided into four groups on the basis of the material used to fill the sockets: PPP, PRP, PRF, and control (no graft material) groups. Results were evaluated at 4 and 8 weeks after surgery. The ultrastructural morphology and constructs of each blood product were studied by a scanning electron microscope (SEM) or calculating concentrations of platelets, fibrinogen, platelet-derived growth factor, and transforming growth factor-β. A total of five microcomputed tomography images of specimens were selected for measurement, and the area occupied by the newly formed bone as well as the horizontal bone width were measured. Moreover, decalcified tissue specimens from each defect were analyzed histologically. The median area of new bone at 4 and 8 weeks and median horizontal bone width at 8 weeks were the highest in the PPP group. However, bone maturation in the PRF and the PRP groups was more progressed than that in the PPP and control groups. By SEM findings, the PRF group showed a more highly condensed fibrin fiber network that was regularly arranged when compared with the PPP and PRP groups. The growth factors released from platelets in PRP indicated higher concentrations than that in PRF. Under more severe conditions for bone formation, as in this experiment, the growth factors released from platelets had a negative effect on bone formation. This study showed that PPP is an effective material for the preservation of sockets with buccal dehiscence.
Introduction
Alveolar bone resorption generally occurs during healing after tooth extraction.1–5 In particular, the resorption of the buccal plate is more significant compared with lingual or palatal bone.2,4 Bone resorption after tooth extraction can make dental implant treatment difficult and impairs the long-term functional stability of the implant and the esthetic results of prosthodontic treatment. Therefore, the socket preservation concept was introduced to minimize bone resorption after tooth extraction and preserve the alveolar bone by means of bone graft materials filled into the socket immediately after extraction. Many studies on the effectiveness of various bone graft materials in socket preservation, including demineralized freeze-dried bone allograft,6,7 bovine bone mineral,8,9 hydroxyapatite,10,11 and β-tricalcium phosphate, have been published.12,13 However, bone graft materials may inhibit the normal healing process in some cases; therefore, use has been questioned.14
In a study conducted in 1998 by Marx et al.,15 platelet-rich plasma (PRP) was reported to promote new bone formation in mandibular continuity defects and cause faster maturation of autologous bone grafts. PRP contains a high concentration of platelets and is an autologous source of platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), and vascular endothelial growth factor.2,16 Many reports15–21 have suggested the suitability of PRP for enhancing bone regeneration in autologous bone grafts and other bone substitutes, although others have shown no benefit of PRP on bone formation.19,22–28 With regard to socket preservation, some reports17,29,30 have suggested the benefits of using PRP alone to increase the rate of bone formation and decrease the healing time following socket extraction. However, Alissa et al. reported that bone healing and regeneration in sockets created after the extraction of mandibular third molars were similar between a PRP-treated group and an untreated group.31
Recently, platelet-rich fibrin (PRF) was introduced by Dohan et al. as a second-generation platelet concentrate.32 In a simple preparation technique, blood is collected without anticoagulant or thrombin and immediately centrifuged only once. The growth factors present in PRF are the same as those in PRP. The same group also reported that PRF can promote bone regeneration and epithelialization in postextraction sockets as well as bone maturation after sinus lift procedures.32–34 On the other hand, Gürbüzer reported that scintigraphically detectable enhanced bone healing in sockets created by the extraction of impacted mandibular third molars was not different between a PRP-treated group and an untreated group.35
Platelet-poor plasma (PPP) is the layer of plasma that contains few platelets. Few studies have attempted to evaluate the effects of bone regeneration using PPP. We reported that PPP with bone marrow stromal cells (MSCs) and β-TCP scaffolds promoted bone formation to a greater extent than PRP with MSCs did.36
The amount and rate of bone regeneration depend on the type of material to be used, the size of the socket to be filled, and the condition of the remaining bone walls; therefore, careful consideration should be paid to the use of each material. However, no report has evaluated sockets with buccal dehiscence, which makes preservation of the alveolar ridge difficult. In addition, no studies have evaluated the effects of PPP, PRP, and PRF alone on the healing of extraction sockets. This study aimed to evaluate the effects of PPP, PRP, and PRF on the healing of extraction sockets with buccal dehiscence in dogs.
Materials and Methods
Animals
Twelve 1-year-old beagle dogs weighing ∼10 kg each and possessing a complete set of teeth were included in this study. All animal procedures were performed in accordance with the guidelines of the Tokyo Medical and Dental University for the care and use of laboratory animals. The protocol was approved by the Institutional Animal Care and Use Committee of the Tokyo Medical and Dental University.
Preparation of PPP, PRP, and PRF
Under general anesthesia, the dogs were anesthetized by an intramuscular injection of ketamine hydrochloride (0.2 mL/kg, Veterinary Ketalar®; Daiichi Sankyo Propharma) and medetomidine hydrochloride (0.1 mL/kg, Domitor™; Orion), and whole blood (10 mL) was collected into sterile syringes containing 1 mL of the anticoagulant citrate phosphate dextrose (CPD; TERUMO). The whole blood was first centrifuged at 800 g for 10 min, and 2 mL of the upper layer that contained a few platelets was collected as PPP. Next, another sample of whole blood (10 mL) containing 1 mL of CPD was collected by the same method used for the first sample. This sample of whole blood was centrifuged at 700 g for 8 min, and the upper layers, including the buffy coat, were transferred to a new centrifuge tube to remove the lowest layer that contained most of the red blood cells (RBCs). After further centrifugation of the transferred layers at 1600 g for 8 min, 2 mL of PRP was collected from the bottom layer, which was rich in platelets. This PPP and PRP were activated for experimental use with 2% calcium chloride (Fig. 1A, B).
FIG. 1.

Platelet-poor plasma (PPP) (A) and platelet-rich plasma (PRP) (B) after activation with a calcium chloride–thrombin mixture. Platelet-rich fibrin (PRF) clot after centrifugation (C).
PRF was prepared according to the technique described by Dohan et al.32 Blood samples were obtained in 10-mL tubes without an anticoagulant. The tubes were immediately centrifuged at 400 g for 10 min, following which the blood in the tubes separated into two visible layers: an RBC layer that occupied the lower part of the tube and a PRF layer that occupied the upper part of the tube (Fig. 1C). The PRF was thus collected for experimental use.
Quantification of platelet, fibrinogen, TGF-β1, and PDGF-AB concentration
The concentration of platelets and fibrinogen in the whole blood, PPP, and PRP was measured by a blood cell counter (KX-21NV; Sysmex) and coagulation analyzer (CA-50; Sysmex). In addition, the TGF-β1 and PDGF-AB concentrations of the PPP, PRP, and PRF were assayed using an enzyme-linked immunosorbent assay (ELISA) kit.
Scanning electron microscopy observations
The graft samples (PPP, PRP, and PRF) were fixed in 2.5% glutaraldehyde, divided into two halves, dehydrated through a graded series of ethanol, dried by a critical point dryer, mounted onto aluminum stubs, and sputter-coated with platinum. The samples were observed from the cut plane using a scanning electron microscope.
Surgical procedures
Local anesthesia (lidocaine HCl, 2% with epinephrine, 1:80,000) was administered by infiltration. An intrasulcular incision was placed in the third mandibular premolar region, followed by elevation of mucoperiosteal flaps. The third premolar was carefully extracted, and a 3 mm dehiscence from the alveolar crest to the buccal wall was created in two roots of each extraction socket (Fig. 2). Therefore, a total of four experimental bone defects, two each on the right and left, were created in the mandible of each dog. These bone defect sites were then randomly divided into four groups on the basis of the material used to fill their sockets: PPP, PRF, PRP, and control groups. These materials were filled into each root of the extraction sockets. The extraction sockets of the control group were left unfilled. After grafting, the flap was repositioned and sutured, leaving the socket as an open wound. Cefazolin sodium (Cefamezin®) and butorphanol tartrate injections (Stadol®) were administered for 3 days after surgery. We observed wound dehiscence until the tooth extraction socket of the dog epithelized. All 12 animals were euthanized after 4- and 8-week healing periods (n=6 each). Subsequently, the mandibles were block resected and the segments were immediately immersed in a 10% formaldehyde solution.
FIG. 2.
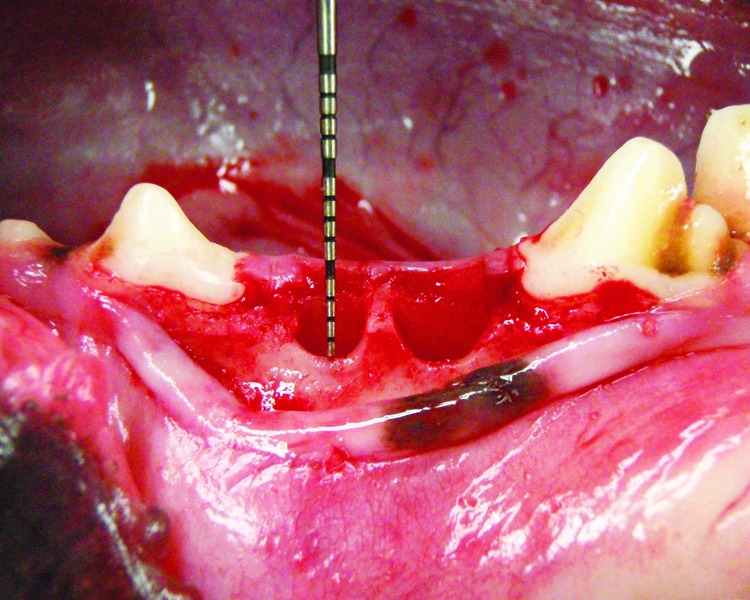
Clinical photograph illustrating the 3 mm dehiscence from the alveolar crest to the buccal wall was created in two roots of the extraction socket.
Radiographic analysis
The specimens, including the bone defects were scanned by microcomputed tomography (micro-CT; X-ray CT System SMX100CT; Shimazu Corp.), with a voltage of 85 kV, a current of 100 μA, a slice thickness of 0.14 mm, and a field of view of 40.0 mm, at 4 and 8 weeks after surgery. Measurements were performed for five areas of each grafted site: the midline, 0.2 and 1.2 mm medial to the midline, and 0.2 and 1.2 mm lateral to the midline. The region of interest (ROI) was set as an area of 5×3 mm in the alveolar crest perpendicular to the long axis of the extraction socket (Fig. 3). The ROI image within a selected complete image was extracted and the ridge width at a point 1.5 and 3 mm below the tip of the alveolar crest was measured in addition to the newly formed bone area using image analysis software (ImageJ; National institutes of Health).
FIG. 3.
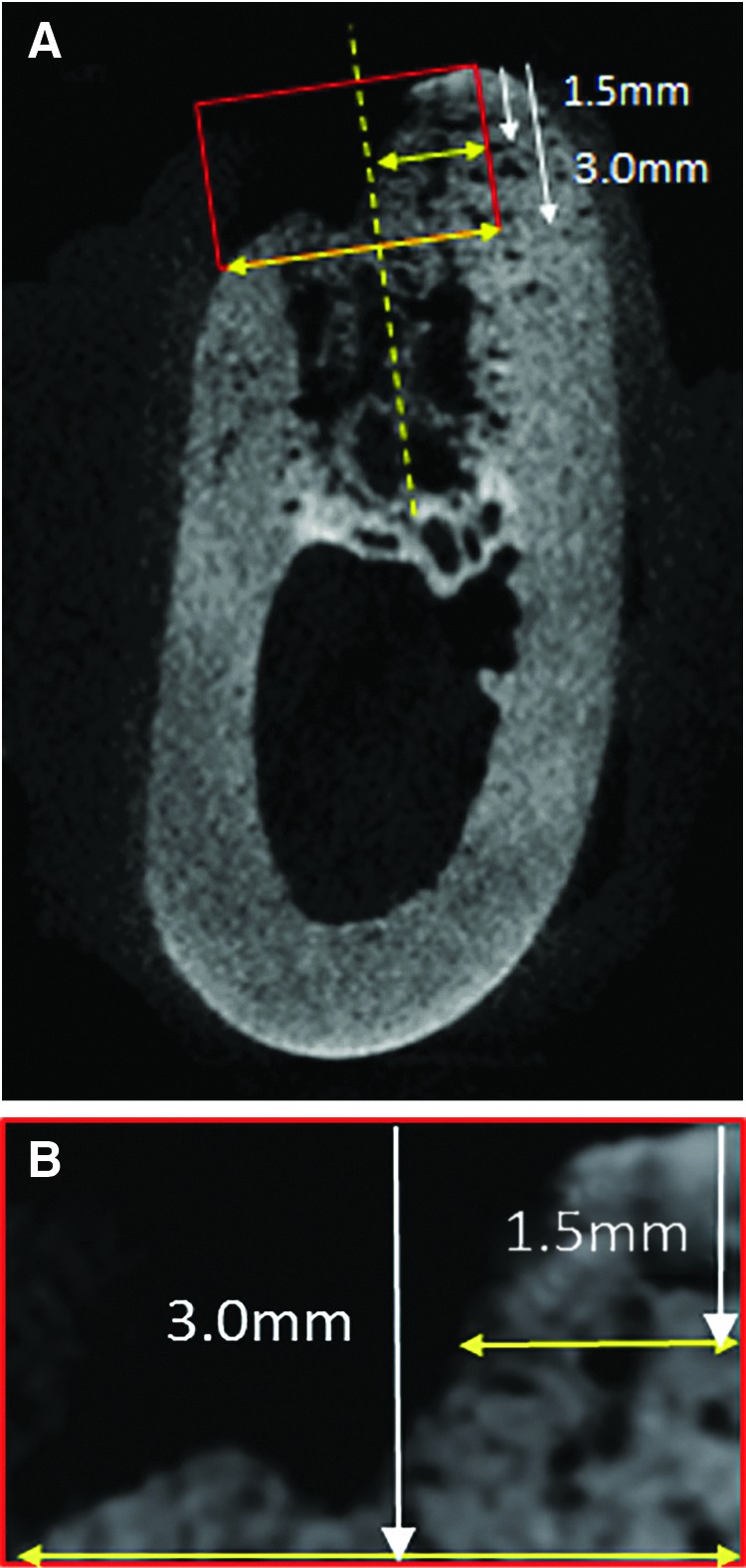
Microcomputed tomography (CT) images of the midline area (A). The region of interest (ROI) is set at the size of 5×3 mm in the alveolar crest (B). The major axis of the ROI is set to be perpendicular to the long axis of the extraction socket. The horizontal bone widths at a point 1.5 mm and 3.0 below the tip of the alveolar crest were measured.
Histological analysis
Decalcified specimens were prepared by immersing the formalin-fixed tissues in a Plank–Rychlo solution for 2 weeks. Following decalcification, the specimens were dehydrated in increasing concentrations of ethanol, embedded in paraffin, and cut in the buccal–palatal plane. The paraffin blocks were sliced into thin sections at a thickness of ∼5 μm using a sliding microtome, and the sliced sections were stained with hematoxylin–eosin and examined under a light microscope.
Statistics
Data analysis was performed using PASW Statistics 18 for Windows. The endpoint differences between the groups were analyzed using the Kruskal–Wallis test and the Mann–Whitney U test (p<0.05). The data of platelet, fibrinogen, PDGF-AB, and TGF-β1 concentrations are expressed as mean±standard deviation. Other data are expressed as median±standard deviation.
Results
Platelet, fibrinogen, PDGF-AB, and TGF-β1concentration (Table 1)
Table 1.
The Average Concentration of Platelets, Fibrinogen, PDGF-AB, and TGF-β1 in Whole Blood, PPP, PRP, and PRF (±Standard Deviation)
| Platelet (×104/μL) | Fibrinogen (mg/dL) | PDGF-AB (pg/mL) | TGF-β1 (ng/mL) | |
|---|---|---|---|---|
| Whole blood | 18.6±2.2 | 244.8±103.6 | (−) | (−) |
| PPP | 1.8±0.8 | 304.5±60.4 | 16.6±7.8 | 12.5±5.3 |
| PRP | 55.6±7.4 | 234.3±25.9 | 474.6±201.6 | 91.9±9.3 |
| PRF | (−) | (−) | 364.2±93.0 | 63.7±13.7 |
(−), the value did not measure; PPP, platelet-poor plasma; PRP, platelet-rich plasma; PRF, platelet-rich fibrin; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor-β.
The mean concentrations of platelet, PDGF-AB, and TGF-β1 were all higher in PRP, with platelets about 3-fold higher, and platelet-released growth factors about 5-30-fold higher than in PPP, and about 1.3–1.4-fold higher than in PRF. The average fibrinogen concentration among whole blood, PPP, and PRP was not significantly different, although it was slightly higher in PPP than in whole blood and PRP.
Scanning electron microscopy findings
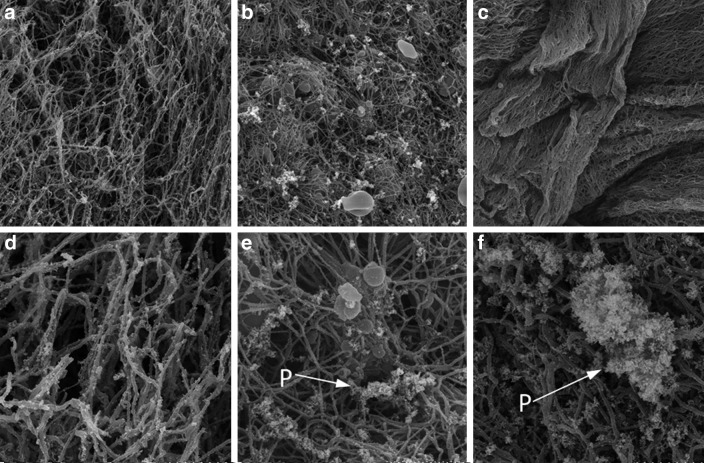
The number of fibrin fibers in bundle form was greater in PPP than in PRP (Fig. 4a). The same field as in Figure 4a, but at higher magnification, shows platelets on the surface of the fibrin network (Fig. 4d). The fibrin fiber network in PRP was sparse, with many platelet aggregates or cells (RBCs and leucocytes) in the space between the fibrin fibers (Fig. 4b, e). PRF showed more highly condensed fibrin bundles with platelet aggregates embedded within the fibrin network (Fig. 4c, f) when compared with PPP and PRP.
FIG. 4.
Scanning electron microscopy images of PPP, PRP, and platelet-rich fibrin (PRF) show platelet aggregates and fibrin fibers. (a) PPP×3000; (b) PRP×3000; (c) PRF×3000; (d) PPP×10,000; (e) PRP×10,000; (f ) PRF×10,000. P, platelet aggregates.
Postoperative course
Clinically, all 48 extraction sockets healed uneventfully with no occurrence of complications. The average number of days until epithelialization after tooth extraction was 9.0±2.1, 8.0±1.1, 6.3±1.6, and 8.6±2.1, in the PPP, PRP, PRF, and control groups, respectively. There was a tendency that soft-tissue healing was the fastest in the PRF group and slowest in the PPP group.
Radiographical findings
Four weeks after surgery, bone formation was late in the center part of the alveolar crest, the center part became dented in four of six cases in the PRF group and in all cases in the PRP and control groups, but the sockets were filled with the newly formed bone up to the alveolar crest in all cases in the PPP group (Fig. 5). At 8 weeks, bone recess on the buccal plate was observed in four of six cases in the PRP and control groups, but in none of the cases in the PPP and PRF groups (Fig. 6). The alveolar crests in the PPP group had retained the original mandible form, but that in the PRF and PRP groups the form showed a shaped edge, and the preservation of the crest width was not sufficient.
FIG. 5.
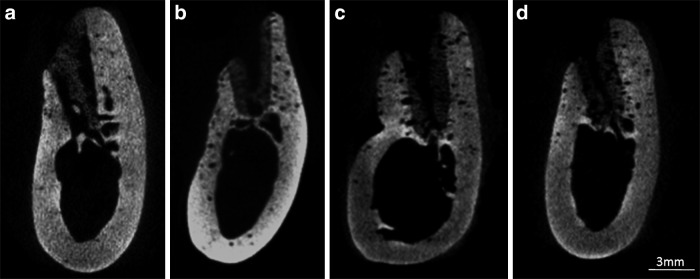
Microcomputed tomography (CT) images in the midline area of each defect at 4 weeks: (a) PPP group, (b) PRP group, (c) PRF group, and (d) control group.
FIG. 6.
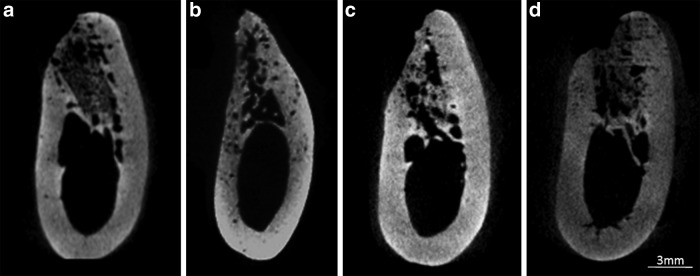
Microcomputed tomography (CT) images in the midline area of each defect at 8 weeks. (a) PPP group, (b) PRP group, (c) PRF group, and (d) control group.
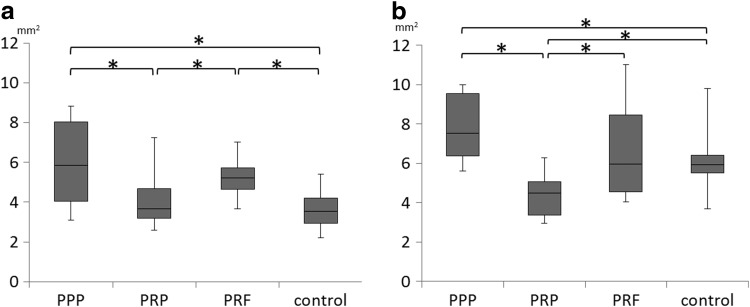
The median area of newly formed bone at 4 weeks after surgery was 5.85, 3.66, 5.22, and 3.55 mm2 in the PPP, PRP, PRF, and control groups, respectively (Fig. 7). The PPP and PRF groups showed significantly higher median values compared with the PRP and control groups.
FIG. 7.
Median area of the newly formed bone at the alveolar crest measured by postoperative computed tomography (CT) at (a) 4 weeks and (b) 8 weeks. *Statistically significant differences with p<0.05.
The median area of newly formed bone at 8 weeks after surgery was 7.52, 4.50, 5.98, and 5.93 mm2 in the PPP, PRP, PRF, and control groups, respectively (Fig. 7). The PPP and PRF groups showed significantly higher median values compared with the PRP group, whereas the PRP group showed significantly lower median values compared with the control group. Significant differences were not observed between the PPP and PRF groups at both 4 and 8 weeks.
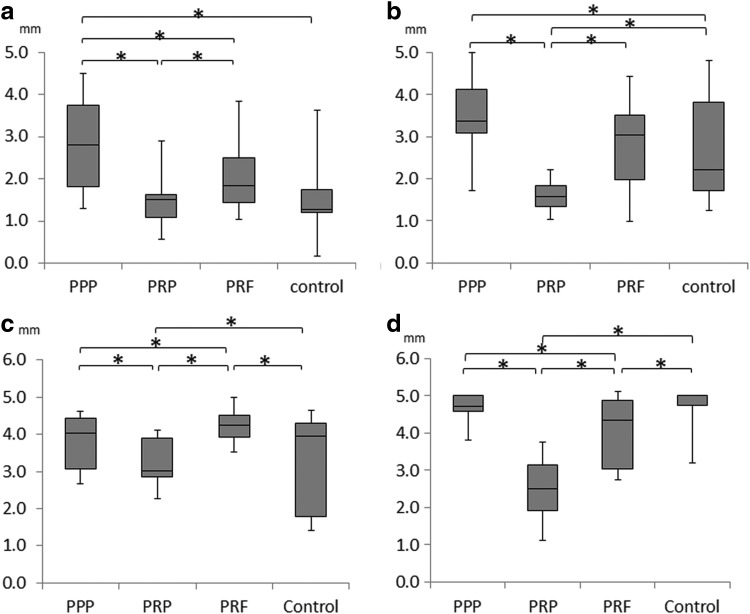
Regarding the width of the ridge at a point 1.5 and 3 mm below the tip of the alveolar crest, all of the PPP group other than the PRF group of 4 weeks at a point 3.0 mm showed the largest ridge width, while the PRP group showed the smallest ridge width at both 4 and 8 weeks (Fig. 8). At a point 1.5 mm, significant differences were observed between the PPP group and the control groups, but there was no significant difference between the PRF and control groups.
FIG. 8.
Median horizontal bone width at a point 1.5 mm below the tip of the alveolar crest at (a) 4 weeks and (b) 8 weeks, a point below 3.0 mm at (c) 4 weeks, (d) 8 weeks. *Statistically significant differences with p<0.05.
Histological evaluation
Four weeks after surgery, the amount of new bone formation in the extraction sockets was the highest in the PPP group (Fig. 9a), but bone trabeculae were thin and sparse. The PRP, PRF, and control groups exhibited the unmineralized connective tissue forming in the sockets (Fig. 9b–d). The new formation of woven bone originating from the existing bone in the PRP, PRF, and control groups was more progressed than in the PPP group.
FIG. 9.
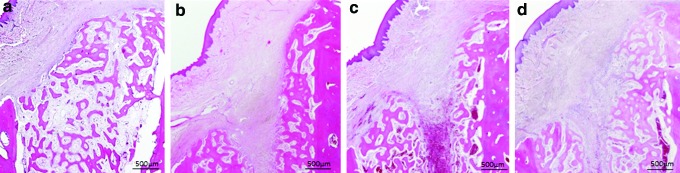
Hematoxylin–eosin (HE)-stained specimens of each sample at 4 weeks: (a) PPP group, (b) PRP group, (c) PRF group, and (d) control group. Color images available online at www.liebertpub.com/tea
Eight weeks after surgery, bone maturation had progressed in all groups (Fig. 10a–d). The volume of new bone augmentation in the sockets was the highest in the PPP group, although the contour of the alveolar bone crest had regained its original form. Bone maturation was more progressed in the PRP and PRF groups than in the PPP and control groups. The external layer of the buccal alveolar bone in the PRP and PRF groups showed corticalization and that lamellar bone had formed. (Fig. 10b, c). The bone trabeculae on the buccal lateral aspect had started to coalesce with each other in the PPP and control groups, reflecting the maturation of the newly formed bone (Fig. 10a, d).
FIG. 10.
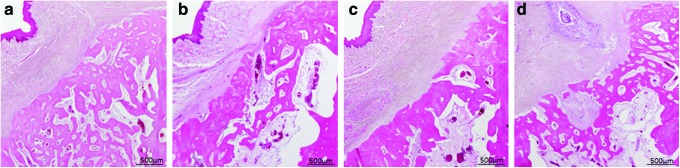
Hematoxylin–eosin (HE)-stained specimens of each sample at 8 weeks: (a) PPP group, (b) PRP group, (c) PRF group, and (d) control group. Color images available online at www.liebertpub.com/tea
Discussion
The present study demonstrated the difference of components and structure in PPP, PRP, and PRF, and the effect of these blood products on the bone healing of extraction sockets in dogs.
As an evaluation of components in PPP, PRP, and PRF, we applied the concentration of platelets, fibrinogen, and growth factors released from platelets. The concentration of growth factors released from platelets in the PRP and PRF groups was much higher than in the PPP group according to theory. In this study, the concentration of PRP was significantly higher compared with PRF. The fibrin network structure was formed in all three blood products, and we found that PPP and PRP contained an irregularly formed, sparse fibrin network, whereas PRF contained a regularly formed, dense fibrin network. As to the reason, it was thought that gelation of fibrin was different, because PRF did not need 2% calcium chloride for gelation and the activation of platelets. Furthermore, PPP and PRP was shown to be different in that the concentration of fibrinogen in PPP was slightly higher than in PRP, and fibrin fibers in PPP formed bundles to some extent. We speculated that the strength of the PPP gel was stronger than the PRP gel.
The role of platelets in the wound healing process has been extensively studied. These processes are mediated by various factors, including cytokines and growth factors that stimulate cell activity.37 Growth factors released from platelets, such as PDGF and TGF-β1, have been shown to promote the regeneration of mucosa and bones.15,16 PDGF has been reported to inhibit osteoblastic differentiation and stimulate cell proliferation in vitro. The effects of TGF-β on osteoblastic differentiation depend on the dose and differentiation stage of the osteoblastic lineage.38–44 We have demonstrated in a previous in vitro study that PRP promotes cell proliferation and inhibits osteoblast differentiation of MSCs in a dose-dependent manner.36 Furthermore, the results of the in vivo study showed that artificial bone materials enhanced bone formation with a combination of PPP and MSCs, whereas those with a combination of PRP and MSCs had an inhibitory effect on bone formation.
In the present study, the best results in terms of bone volume and width retention at the area of alveolar crest with buccal dehiscence were observed in the PPP group. The PRP and PRF group did not show sufficient alveolar bone retention, with an edge-shaped alveolar crest. These results seemed to accord with our results mentioned above. The area of the alveolar crest with buccal dehiscence had fewer osteogenic cells and was not surrounded by preexisiting bone. In the PRP and PRF groups, bone regeneration was not promoted in these areas compared with that in the PPP group. Growth factors can stimulate the proliferation of any cells, irrespective of whether or not the cells are involved in bone differentiation. However, the PRP and PRF groups showed a higher rate of bone maturation compared with the PPP group in areas with abundant osteogenic cells surrounded by preexisting bone within the socket. PRP and PRF, which include a large amount of platelets, increased the numbers of osteogenic cells, and exhibited a faster rate of bone maturation.
The PRF promoted a greater amount of bone formation compared with PRP in the current study. As for the reasons, there were thought to be attributed to the lower concentrations of PDGF and TGF-β released from PRF than those from PRP, and that the strength of PRF supported a denser fibrin network. Kawase et al. suggested that a fibrous network of insoluble fibrin provides a scaffold for cells and serves as a substrate for the sustained release of growth factors.45,46 Findings from our previous study demonstrated that fibrin gels provide a matrix for cell growth and differentiation and provide a preferable environment for osteoblastic differentiation, during which cells contact fibrin molecules and exhibit three-dimensional cell–cell interactions.36 The fibrin network strength of especially PRF is strong, it be considered that PRF has played a role as space making for bone regeneration.
A great number of studies6–13 on socket preservation using various bone graft materials have been presented in the literature. However, only a limited number of studies have used PPP, PRP, or PRF alone for socket preservation. Yeo and Ong claimed that socket preservation should only be performed in selected cases.47 Whether synthetic bone, PPP, or PRF should be used, and whether these should be used alone or in combination, should be determined on the basis of the socket size, number of remaining bone walls, and other such factors. Further studies are required to address this issue, as outcomes will vary depending on the selection of materials.
The present study suggested the usefulness of PPP in the preservation of sockets with buccal dehiscence. The preparation of PPP is simple, with an uncomplicated setting, and has a minimal error rate among manufacturers compared with PRP. Furthermore, although the form of PRF was decided at the time of preparation, the form and timing of PPP can be adjusted for use through gelation, and mix easily synthetic materials in gel form. With few studies having been carried out for bone regeneration with PPP,48,49 this study is of great significance as it demonstrates the effectiveness of PPP on bone formation.
Conclusion
This study showed that the PPP could sufficiently maintain bone width and height with no depression for the preservation of socket with buccal dehiscence. PRP and PRF promote bone maturation in the presence of abundant osteogenic cells, whereas PPP plays a significant role in the presence of fewer osteogenic cells. The fibrin network of PPP and PRF has played a role as space making for bone regeneration and would be stimulatory to bone formation.
Acknowledgments
We thank Dr. Shizuko Ichinose for help with scanning electron microscopy analyses. This work was supported by a Grant-in-Aid for Young Scientists in the Japan Society for the Promotion of Science (23792324) and Takeda Science Foundation.
Disclosure Statement
No competing financial interests exist.
References
- 1.Amler M.H.The time sequence of tissue regeneration in human extraction wounds. Oral Surg Oral Med Oral Pathol 27,309, 1969 [DOI] [PubMed] [Google Scholar]
- 2.Pietrokovski J., and Massler M.Alveolar ridge resorption following tooth extraction. J Prosthet Dent 17,21, 1967 [DOI] [PubMed] [Google Scholar]
- 3.Schropp L., Wenzel A., Kostopoulos L., and Karring T.Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent 23,313, 2003 [PubMed] [Google Scholar]
- 4.Araújo M.G., and Lindhe J.Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol 32,212, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Fickl S., Zuhr O., Wachtel H., Stappert C.F., Stein J.M., and Hürzeler M.B.Dimensional changes of the alveolar ridge contour after different socket preservation techniques. J Clin Periodontol 35,906, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Becker W., Becker B.E., and Caffesse R.A comparison of demineralized freeze-dried bone and autologous bone to induce bone formation in human extraction sockets. J Periodontol 65,1128, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Iasella J.M., Greenwell H., Miller R.L., Hill M., Drisko C., Bohra A.A., and Scheetz J.P.Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol 74,990, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Artzi Z., Tal H., and Dayan D.Porous bovine bone mineral in healing of human extraction sockets. Part 1: histomorphometric evaluations at 9 months. J Periodontol 71,1015, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Carmagnola D., Adriaens P., and Berglundh T.Healing of human extraction sockets filled with Bio-Oss. Clin Oral Implants Res 14,137, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Nemcovsky C.E., and Serfaty V.Alveolar ridge preservation following extraction of maxillary anterior teeth. Report on 23 consecutive cases. J Periodontol 67,390, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Sàndor G.K., Kainulainen V.T., Queiroz J.O., Carmichael R.P., and Oikarinen K.S.Preservation of ridge dimensions following grafting with coral granules of 48 post-traumatic and post-extraction dento-alveolar defects. Dent Traumatol 19,221, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Horowitz R.A., Mazor Z., Miller R.J., Krauser J., Prasad H.S., and Rohrer M.D.Clinical evaluation alveolar ridge preservation with a beta-tricalcium phosphate socket graft. Compend Contin Educ Dent 30,588, 2009 [PubMed] [Google Scholar]
- 13.Araújo M.G., Liljenberg B., and Lindhe J.beta-Tricalcium phosphate in the early phase of socket healing: an experimental study in the dog. Clin Oral Implants Res 21,445, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Lekovic V., Kenney E.B., Weinlaender M., Han T., Klokkevold P., Nedic M., and Orsini M.A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. J Periodontol 68,563, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Marx R.E., Carlson E.R., and Eichstaedt R.M.Platelet rich plasma. Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol 85,638, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Marx R.E.Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 62,489, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Aghaloo T.L., Moy P.K., and Freymiller E.G.Investigation of platelet-rich plasma in rabbit cranial defects: a pilot study. J Oral Maxillofac Surg 60,1176, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Wiltfang J., Schlegel K.A., Schultze-Mosgau S., Nkenke E., Zimmermann R., and Kessler P.Sinus floor augmentation with beta-tricalcium phosphate (beta-TCP): does platelet-rich plasma promote its osseous integration and degradation? Clin Oral Implants Res 14,213, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Wiltfang J., Kloss F.R., Kessler P., Nkenke E., Schultze-Mosgau S., Zimmermann R., and Schlegel K.A.Effects of platelet-rich plasma on bone healing in combination with autogenous bone and bone substitutes in critical-size defects. An animal experiment. Clin Oral Implants Res 15,187, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Schlegel K.A., Donath K., Rupprecht S., Falk S., Zimmermann R., Felszeghy E., and Wiltfang J.De novo bone formation using bovine collagen and platelet-rich plasma. Biomaterials 25,5387, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Hokugo A., Ozeki M., Kawakami O., Sugimoto K., Mushimoto K., Morita S., and Tabata Y.Augmented bone regeneration activity of platelet-rich plasma by biodegradable gelatin hydrogel. Tissue Eng 11,1224, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Choi B.H., Im C.J., Huh J.Y., Suh J.J., and Lee S.H.Effect of platelet-rich plasma on bone regeneration in autogenous bone graft. Int J Oral Maxillofac Surg 33,56, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Aghaloo T.L., Moy P.K., and Freymiller E.G.Evaluation of platelet-rich plasma in combination with freeze-dried bone in the rabbit cranium. A pilot study. Clin Oral Implants Res 16,250, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Raghoebar G.M., Schortinghuis J., Liem R.S., Ruben J.L., van der Wal J.E., and Vissink A.Does platelet-rich plasma promote remodeling of autologous bone grafts used for augmentation of the maxillary sinus floor? Clin Oral Implants Res 16,349, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Klongnoi B., Rupprecht S., Kessler P., Thorwarth M., Wiltfang J., and Schlegel K.A.Influence of platelet-rich plasma on a bioglass and autogenous bone in sinus augmentation. An explorative study. Clin Oral Implants Res 17,312, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Plachokova A.S., van den Dolder J., Stoelinga P.J., and Jansen J.A.The bone regenerative effect of platelet-rich plasma in combination with an osteoconductive material in rat cranial defects. Clin Oral Implants Res 17,305, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Sarkar M.R., Augat P., Shefelbine S.J., Schorlemmer S., Huber-Lang M., Claes L., Kinz l.L., and Ignatius A.Bone formation in a long bone defect model using a platelet-rich plasma-loaded collagen scaffold. Biomaterials 27,1817, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Thorwarth M., Wehrhan F., Schultze-Mosgau S., Wiltfang J., and Schlegel K.A.PRP modulates expression of bone matrix proteins in vivo without long-term effects on bone formation. Bone 38,30, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Sammartino G., Tia M, Marenzi G., di , Lauro A.E., D'Agostino E., and Claudio P.P.Use of autologous platelet-rich plasma (PRP) in periodontal defect treatment after extraction of impacted mandibular third molars. J Oral Maxillofac Surg 63,766, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Célio-Mariano R., de Melo W.M., and Carneiro-Avelino C.Comparative radiographic evaluation of alveolar bone healing associated with autologous platelet-rich plasma after impacted mandibular third molar surgery. J Oral Maxillofac Surg 70,19, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Alissa R., Esposito M., Horner K., and Oliver R.The influence of platelet-rich plasma on the healing of extraction sockets: an explorative randomised clinical trial. Eur J Oral Implantol 3,121, 2010 [PubMed] [Google Scholar]
- 32.Dohan D.M., Choukroun J., Diss A., et al. . Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101,37, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Choukroun J., Diss A., Simonpieri A., et al. . Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101,299, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Choukroun J., Diss A., Simonpieri A., et al. . Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101,e56, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Gürbüzer B., Pikdöken L., Tunali M., Urhan M., Küçükodaci Z., and Ercan F.Scintigraphic evaluation of osteoblastic activity in extraction sockets treated with platelet-rich fibrin. J Oral Maxillofac Surg 68,980, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Tajima N., Sotome S., Marukawa E., and Omura K.A three-dimensional cell-loading system using autologous plasma loaded into a porous β-tricalcium-phosphate block promotes bone formation at extraskeletal sites in rats. Mater Sci Eng 27,625, 2007 [Google Scholar]
- 37.Werner S., and Grose R.Regulation of wound healing by growth factors and cytokines. Physiol Rev 83,835, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Marden L.J., Fan R.S., Pierce G.F., Reddi A.H., and Hollinger J.O.Platelet-derived growth factor inhibits bone regeneration induced by osteogenin, a bone morphogenetic protein, in rat craniotomy defects. J Clin Invest 92,2897, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsieh S.C., and Graves D.T.Pulse application of platelet-derived growth factor enhances formation of a mineralizing matrix while continuous application is inhibitory. J Cell Biochem 69,169, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Harris S.E., Bonewald L.F., Harris M.A., Sabatini M., Dallas S., Feng J.Q., Ghosh-Choudhury N., Wozney J., and Mundy G.R.Effects of transforming growth factor beta on bone nodule formation and expression of bone morphogenetic protein 2, osteocalcin, osteopontin, alkaline phosphatase, and type I collagen mRNA in long-term cultures of fetal rat calvarial osteoblasts. J Bone Miner Res 9,855, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Kasperk C.H., Wergedal J.E., Mohan S., Long D.L., Lau K.H., and Baylink D.J.Interactions of growth factors present in bone matrix with bone cells: effects on DNA synthesis and alkaline phosphatase. Growth Factors 3,147, 1990 [DOI] [PubMed] [Google Scholar]
- 42.Lu L., Yaszemski M.J., and Mikos A.G.TGF-beta1 release from biodegradable polymer microparticles: its effects on marrow stromal osteoblast function. J Bone Joint Surg Am 83-A,S82, 2001 [PubMed] [Google Scholar]
- 43.Lieb E., Vogel T., Milz S., Dauner M., and Schulz M.B.Effects of transforming growth factor beta1 on bonelike tissue formation in three-dimensional cell culture. II: osteoblastic differentiation. Tissue Eng 10,1414, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Cassiede P., Dennis J.E., Ma F., and Caplan A.I.Osteochondrogenic potential of marrow mesenchymal progenitor cells exposed to TGF-beta 1 or PDGF-BB as assayed in vivo and in vitro. J Bone Miner Res 11,1264, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Kawase T., Okuda K., Wolff L.F., and Yoshie H.Platelet-rich plasma-derived fibrin clot formation stimulates collagen synthesis in periodontal ligament and osteoblastic cells in vitro. J Periodontol 74,858, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Kawase T., Okuda K., Saito Y., and Yoshie H.In vitro evidence that the biological effects of platelet-rich plasma on periodontal ligament cells is not mediated solely by constituent transforming-growth factor-beta or platelet-derived growth factor. J Periodontol 76,760, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Yeo A.B., and Ong M.M.Principles and implications of site preservation for alveolar ridge development. Singapore Dent J 26,15, 2004 [PubMed] [Google Scholar]
- 48.Schettino A.M., Franco D., Franco T., Filho J.M., and Vendramin F.S.Use of autologous fibrin glue (platelet-poor plasma) in abdominal dermolipectomies. Aesthetic Plast Surg 36,1296, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Franco D., Franco T., Schettino A.M., Filho J.M., and Vendramin F.S.Protocol for obtaining platelet-rich plasma (PRP), platelet-poor plasma (PPP), and thrombin for autologous use. Aesthetic Plast Surg 36,1254, 2012 [DOI] [PubMed] [Google Scholar]