Abstract
Background: Takotsubo or stress-induced cardiomyopathy is a form of reversible cardiomyopathy commonly associated with emotional or physical stress. Thyrotoxicosis has been identified as a rare cause of Takotsubo cardiomyopathy, with only 12 cases reported in the literature. Here, we report a case of thyroid storm presenting with Takotsubo cardiomyopathy in the setting of Graves' disease.
Patient Findings: A 71-year-old woman presented with abdominal pain, vomiting, confusion, and history of weight loss. She was initially diagnosed and treated for diabetic ketoacidosis at another hospital and was transferred to our hospital one day after initial presentation because of concern for acute coronary syndrome. A diagnosis of Takotsubo cardiomyopathy was made on the basis of cardiac catheterization. At that time, she was diagnosed and treated for thyroid storm. Follow-up 7 weeks later revealed improvement of her cardiac function and near-normalization of thyroid hormone levels.
Summary: In this patient, who presented with symptoms of heart failure, acute coronary syndrome was initially considered, but the diagnosis of Takotsubo cardiomyopathy associated with thyroid storm was ultimately made based on cardiac catheterization and laboratory investigation.
Conclusions: Thyrotoxicosis is associated with adverse disturbances in the cardiovascular system. Takotsubo cardiomyopathy could be a presenting manifestation of thyroid storm, perhaps related to excess catecholamine levels or sensitivity.
Introduction
Historically, the effects of thyroid hormone on the cardiovascular system were first described by Robert Graves in 1835 (1). It was not until later in 1924 when Levine and Sturgis (2) introduced the concept of cardiac disease provoked by hyperthyroidism on the basis of their clinical observations of patients who presented with atrial fibrillation, tachyarrhythmias, and heart failure, masked by underlying hyperthyroidism.
Thyroid hormone has both direct and indirect actions on the cardiovascular system. Biologically active thyroid hormone, triiodothyronine (T3), increases cardiac contractility both directly by acting on cardiac myocytes and indirectly by causing peripheral vasodilatation that leads to a decrease in systemic vascular resistance and eventually to an increase in cardiac output and blood volume (3). However, in cases of thyrotoxicosis the hypervolemic burden can lead to reduction in myocardial contractile reserve and finally to heart failure.
Stress-induced cardiomyopathy, also known as Takotsubo cardiomyopathy, is a form of reversible, nonischemic cardiomyopathy reported to be triggered by stressful events. It was first described in Japan in the early 1990s (4) and has been increasingly recognized since then. It is characterized by normal coronary arteries and a distinctive left ventricular contraction pattern of apical or midventricular dyskinesis or akinesis, which explains its description as “apical ballooning syndrome” (5). The precipitants of Takotsubo cardiomyopathy are either emotional or physical stress, including acute medical illness (6). We report a case of Takotsubo cardiomyopathy in a patient with thyrotoxicosis associated with Graves' disease.
Patient
A 71-year-old woman was admitted to our institution because of abdominal pain, vomiting, and altered mental status. The patient had been well until 2 days prior to presentation when she started complaining of abdominal pain associated with nausea and vomiting; she was also noted by her family to be more confused with slurred speech. According to her son, she had been complaining of dizziness, dry mouth, sweating, palpitations, diarrhea, and a weight loss of 25 pounds (11.34 kg) over a period of 6 weeks before presentation. Her medical history was significant for type-2 diabetes of 15 years, as well as a remote history of colon cancer. She worked as a waitress, was separated from her husband, and lived alone. Her home medications included glargine-insulin 12 units daily, aspart-insulin 10 units with meals, and metformin 500 mg twice daily. One day prior to admission to this hospital, she presented to the emergency department of another hospital where she was found to have diabetic ketoacidosis and was treated with insulin infusion. A computed tomography of the head without contrast at that time was unremarkable. In addition, an electrocardiogram revealed ST and T wave changes consistent with anterolateral ischemia, and the cardiac markers were found to be elevated (creatine kinase MB 26.3 ng/mL, reference range 0.5–3.6; troponin 5.67 ng/mL, reference range 0.0–0.045). The patient was transferred to our institution the same day for further evaluation and management because of concern for acute coronary syndrome.
On physical examination the patient was markedly confused and disoriented, tachycardic (heart rate 132 bpm), and tachypneic (respiratory rate 28/min), with a blood pressure of 170/119 mmHg in supine position and a body temperature of 37°C. No pallor, jaundice, or proptosis was noted. Neck examination revealed a palpable thyroid gland weighing approximately 40 g, with no discrete masses or nodules, and no bruit. Chest examination revealed bibasilar rales; tachycardia with normal S1 and S2 and with no murmurs, rubs, or gallops; and displacement of apical impulse leftward. She had hyperactive bowel sounds and a soft abdomen without abdominal tenderness or distention. Her skin was warm and had no edema in her extremities.
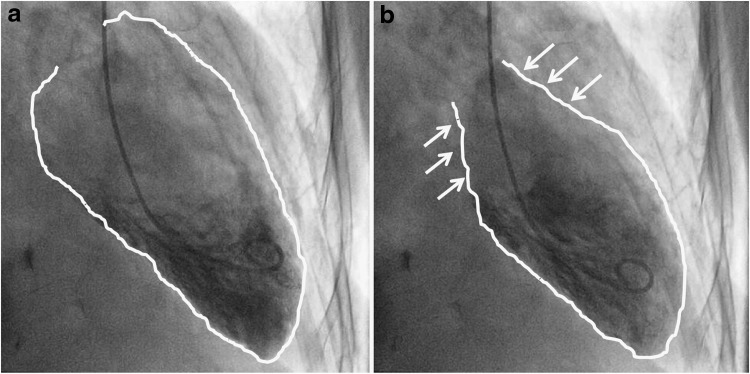
Within 30 minutes after presentation to our hospital, she underwent cardiac catheterization, which revealed normal coronary arteries and severe left ventricular dysfunction (ejection fraction 30%) associated with apical and midcavity hypokinesis (Fig. 1), features consistent with Takotsubo cardiomyopathy (5). Laboratory work-up done within the first 1 hour after presentation included a serum chemistry panel showing elevated sodium (Na 153 mmol/L) and creatinine (Cr 1.40 mg/dL) levels, a normal anion gap, and mildly elevated transaminases (alanine aminotransferase 155 U/L, aspartate aminotransferase 183 U/L) (Table 1). Thyroid function tests were also determined and were found to be abnormal with an undetectable thyrotropin (<0.05 mU/L, reference range 0.4–4.0) and an elevated free thyroxine (T4; 5.3 ng/dL, reference range 0.76–1.46) and total T3 (232 ng/dL, reference range 60–180). Antithyroperoxidase antibodies were negative, while the thyroid-stimulating immunoglobulin level was 3.4 (normal <1.3).
FIG. 1.
Left ventriculogram with wall motion abnormalities in the distal segments and normal wall motion in basal segments (arrows) comparable to stress-induced cardiomyopathy. (a) Diastolic phase. (b) Systolic phase.
Table 1.
Laboratory Data
| Variable | Reference value, adults | On admission | 2nd day | 3rd day | 4th day | 5th day | 6th day | 11th day | 16th day | 40th day (3 weeks postdischarge) | 70th day (7 weeks postdischarge) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TSH (μIU/mL) | 0.400–4.000 | UD | UD | UD | UD | UD | UD | UD | UD | 0.008 | 1.40 |
| T4 free (ng/dL) | 0.76–1.46 | 4.91 | 5.30 | 3.87 | 3.12 | 3.01 | 1.77 | 1.63 | 0.79 | 0.63 | |
| T3 total (ng/dL) | 60.0–180.0 | 232 | 127 | 97.2 | 125 | ||||||
| TSI (index) | ≤1.3 | 3.4 | |||||||||
| Antithyroperoxidase Ab (IU/mL) | 0.0–35.0 | <10.0 | |||||||||
| Antithyroglobulin Ab (IU/mL) | 0.0–40.0 | <20.0 | |||||||||
| Creatine kinase MB (U/L) | 0.5–3.6 | 22.8 | 7.5 | ||||||||
| Troponin (ng/mL) | 0.00–0.045 | 9.080 | 4.050 | ||||||||
| Hemoglobin (g/dL) | 11–14.5 | 10.4 | 10.0 | 10.0 | 10.5 | 12.2 | 12.1 | 11.1 | 10.1 | 11.5 | |
| Hematocrit (%) | 34.5–44.0 | 31.0 | 30.6 | 31.1 | 32.6 | 37.8 | 37.9 | 35.9 | 32.2 | 35.9 | |
| White cell count (per mm3) | 4.0–10.8 | 16.8 | 15.6 | 13.8 | 12.2 | 13.3 | 17.3 | 16.9 | 9.4 | 6.9 | |
| Platelet count (K/μL) | 145–400 | 222 | 218 | 165 | 136 | 126 | 75 | 172 | 388 | 389 | |
| Aspartate aminotransferase (U/L) | 3–34 | 183 | 55 | 52 | 39 | 33 | 28 | ||||
| Alanine aminotransferase (U/L) | 15–41 | 155 | 137 | 121 | 94 | 59 | 22 | ||||
| Alkaline phosphatase (U/L) | 38–126 | 109 | 134 | 177 | 165 | 107 | 83 |
Ab, antibodies; T3, triiodothyronine; T4, thyroxine; TSH, thyrotropin; TSI, thyroid-stimulating immunoglobulin; UD, undetected.
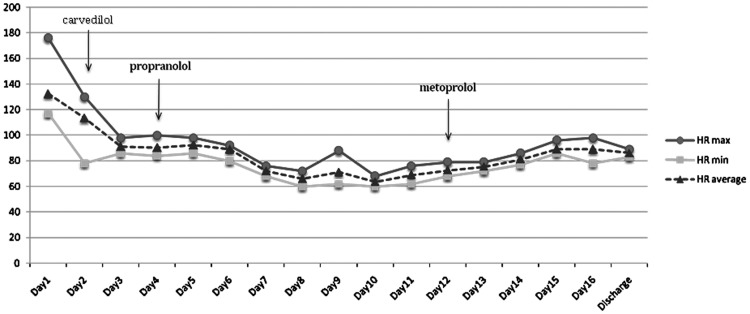
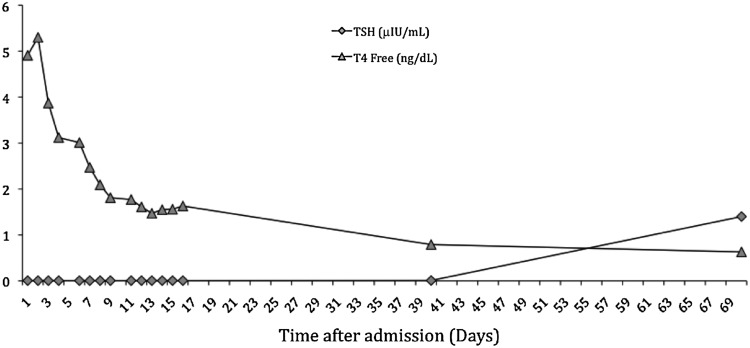
According to the Burch–Wartofsky criteria for thyrotoxicosis (7), the patient had a score of >45 (delirium=20; nausea, vomiting=10; tachycardia=20; moderate pulmonary edema=10), therefore making the diagnosis of thyroid storm highly likely. On the basis of this evidence, the patient was started on high-dose dexamethasone at 4 mg (intravenously) every 8 hours, methimazole at 20 mg every 8 hours, propranolol 40 mg every 8 hours, and captopril 12.5 mg twice daily. This successfully controlled her heart rate (down to 80s; Fig. 2) and lowered free T4 levels (Fig. 3); however, her mental status failed to significantly improve. Because of these persistent symptoms, a surgical evaluation was sought and further imaging of the neck with computed tomography was pursued; this showed a diffuse goiter (right thyroid lobe, 3.2 cm×3.2 cm×5.4 cm; left thyroid lobe, 3.4 cm×2.7 cm×4.6 cm; isthmus thickened at 16 mm) without nodules or cervical lymphadenopathy. At the same time, the patient was started on Lugol's solution at 0.25 mL/day for 5 days. On hospital day 6, methimazole was switched to propylthiouracil (300 mg three times daily) due to thrombocytopenia of unknown cause, which resolved shortly after switching to propylthiouracil and stopping proton-pump-inhibitor therapy simultaneously. Over the next 10 days, the patient's symptoms gradually improved and her free T4 reached near-normal levels (Fig. 3). Surgical intervention was deferred. A repeat echocardiogram performed 10 days after initial presentation showed improved left ventricular function with an ejection fraction of 45–50%. At a follow-up visit 7 weeks after discharge from hospital, she was clinically euthyroid with improved mental status and a detectable thyrotropin (1.40 mU/L), along with a near-normal free T4 (0.63 ng/dL) and a normal total T3 level (125 ng/dL).
FIG. 2.
Diagram of patient's heart rate (HR) in relation to treatment with beta-blockers.
FIG. 3.
Thyroid function tests during hospital course and outpatient follow-up visits. TSH, thyrotropin; T4, thyroxine.
Discussion
Takotsubo cardiomyopathy is characterized by transient left ventricular dysfunction with chest symptoms, elevated cardiac enzymes, and electrocardiogram changes that resemble myocardial infarction. It is estimated that approximately 2% of patients with suspected acute ST-elevation myocardial infarction have the syndrome (5). The prevalence is higher among postmenopausal women, and attacks are usually preceded by emotional or physical stress (5). The mortality rate is, however, higher in males than in females and may be related to underlying critical illness (8). Patients have no discrete lesions in their coronary arteries, usually documented by normal coronary angiography. On ventricular angiography, the left ventricle has a characteristic morphologic appearance described as “apical ballooning” that resembles the shape of Japanese octopus fishing pots (i.e., tako tsubo; tako=octopus, tsubo=pot).
Several pathophysiological mechanisms have been proposed to explain the unusual features of this syndrome, including multivessel epicardial spasm, coronary microvascular impairment, and microvascular spasm (9). However, evidence supporting any of these possible mechanisms is limited and with some conflicting results. For example, multivessel epicardial spasm is only demonstrated in a small minority of these patients (10,11). Currently, the most popular theory focuses on the role of endogenous catecholamines in inducing myocardial dysfunction and stunning. In fact, considerable literature supports this theory based on the findings of abnormal cardiac sympathetic innervation with sympathetic hyperactivity at the cardiac apex in patients presenting with the syndrome (12,13) and the distinct marked elevation of plasma catecholamine and neuropeptide levels in these patients compared with patients with myocardial infarction (14). Case reports of Takotsubo cardiomyopathy caused by catecholamine-secreting tumors and exogenous administration of catecholamines further support the notion of catecholamine-induced myocardial stunning (15,16). The precise mechanism of catecholamine-mediated myocardial stunning in Takotsubo cardiomyopathy remains unclear. A direct toxic effect on cardiac myocytes has been suggested (17). Catecholamines decrease myocyte viability through calcium overload mediated by cyclic adenosine monophosphate, resulting in contraction band necrosis, a histological pattern of myocytes injury that has been observed in Takotsubo cardiomyopathy (14) and other hyperadrenergic states (18). Data from animal models also support the hypothesis of catecholamine-mediated myocardial injury. In a rat model of Takotsubo cardiomyopathy, myocardial injury was prevented by pretreatment with combined α- and β-adrenoreceptor blockade (19). Similarly in monkeys, Takotsubo cardiomyopathy was induced by the infusion of intravenous epinephrine, resulting in increased myocytolysis in the apical portion of the ventricle. Administration of the beta-blocker metoprolol decreased the observed epinephrine-mediated myocytolysis and resulted in improvement in left ventricular ejection fraction (20).
Inflammation might also play a role, as this has been shown in endomyocardial biopsy specimens that demonstrated focal areas with mononuclear inflammatory infiltration and fibrosis (21). In fact, a recent study suggested a significant contribution of oxidative stress to the pathogenesis of Takotsubo cardiomyopathy (22), which further supports the notion that regional hypokinesis of myocardium may be a sign of inflammation related to stress. Moreover, there is evidence to suggest that the release of brain natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP) in these patients might be a response to inflammation; therefore, BNP/NT-proBNP may provide the basis for improved early diagnosis (23). Specifically, Nguyen et al. (24) showed that the extent of BNP and pro-BNP elevation was directly correlated with the severity of Takotsubo cardiomyopathy, as measured by the extent of normetanephrine elevation and of left ventricular systolic dysfunction, but it did not vary with pulmonary capillary wedge pressure. The authors therefore concluded that in the absence of acute pulmonary edema, a different physiologic stimulus, such as inflammation, might be the basis for this BNP/NT-proBNP release.
Finally, the cause of the strong female predisposition for Takotsubo cardiomyopathy is unknown but may be related to sex differences in myocardial sensitivity to catecholamines (25) and the potentially important role of estrogen in the pathogenesis of this syndrome (26).
Clinically, hyperthyroidism mimics a state of adrenergic excess. The thyroid and adrenergic axes are closely interrelated, and pathologically high levels of thyroid hormones cause exaggerated chronotropic and inotropic responses to catecholamines (27). This is partially mediated by the upregulation of β-adrenergic receptors by thyroid hormones in many tissues, including the heart (28,29). This indirect effect of thyroid hormones on adrenergic response is validated by the observation of normal or low levels of catecholamines in patients with thyrotoxicosis (30,31). Consequently, potentiation of catecholamine action by an excess of thyroid hormone has been invoked as an explanation (32).
However, the increase in β-adrenergic receptors is not always accompanied by a corresponding increase in cardiovascular sensitivity to catecholamines (33,34). Experimental animal data have also shown that cardiovascular responses to hyperthyroidism are preserved in mice lacking all three β-adrenergic receptors (β1, β2, β3) compared with wild-type mice (35). Thus, an additional direct action of thyroid hormone at the intracellular level has been suggested based on the finding of thyroid hormone receptor expression on cardiac myocytes (36).
There have been several case reports of Takotsubo cardiomyopathy associated with hyperthyroidism. We identified a total of 12 cases in the literature (Table 2), most of which were associated with Graves' disease (37–42). Other causes included exogenous levothyroxine intake (43–45), Hashimoto's thyroiditis (46), and toxic multinodular goiter (47). In all cases, the patients had a complete recovery of the cardiomyopathy after treatment for thyrotoxicosis.
Table 2.
Case Reports of Takotsubo Cardiomyopathy Associated with Hyperthyroidism
| Author (year) | Age | Sex | Chief complaint at presentation | Chest pain (Y/N) | Elevated cardiac enzymes (Y/N) | Etiology of thyroid storm | Initial LVEF (%) | FU LVEF (%) | Resolution of LV dysfunction (days) | Outcome | Maximum FU period |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Miyazaki et al. (2004) (37) | 79 | F | Palpitations | N | N | Graves' | 45 | NL | 9 | Good | 3 months |
| Sakaki et al. (2004) (46) | 74 | F | Chest pain | Y | Y | Hashimoto's | nr | nr | nr | Good | 6 months |
| Rossor et al. (2007) (38) | 61 | F | Dyspnea | N | Y | Graves' | nr | nr | 1 | Good | 1 month |
| Radhakrishnan and Granato (2009) (39) | 65 | F | Dyspnea, diarrhea | N | Y | Graves' | 25 | 60 | 4 | Good | nr |
| Kwon et al. (2010) (43) | 55 | F | Chest pain | Y | Y | Exogenous levothyroxine | nr | nr | nr | Good | 3 months |
| Sarullo et al. (2009) (41) | 55 | F | Dyspnea | N | Y | Graves' | 28 | nr | 18 | Good | 6 months |
| van de Donk et al. (2009) (47) | 73 | M | Dyspnea | N | Y | Toxic multinodular goiter+131I thyroiditis | 25 | 57 | 4 | Good | 7 weeks |
| Alidjan et al. (2010) (40) | 66 | F | Palpitations | N | Y | Graves' | 40 | NL | 30 | Good | 1 month |
| Tsao et al. (2010) (44) | 31 | F | Chest pain | Y | nr | Exogenous levothyroxine | nr | NL | 14 | Good | 2 weeks |
| Hutchings et al. (2011) (45) | 79 | F | Dyspnea | Y | nr | Nr | nr | NL | 5 | Good | nr |
| Hutchings et al. (2011) (45) | 55 | F | Dyspnea | Y | Y | Exogenous levothyroxine | nr | nr | nr | Good | 4 months |
| Gundara et al. (2012) (42) | 40 | F | Anxiety, dyspnea | Y | Y | Thyroidectomy in Graves' disease patient | nr | nr | nr | nr | nr |
EF, ejection fraction; F, female; FU, follow-up; LV, left ventricle; M, male; N, no; NL, normal; nr, not reported; Y, yes.
In our case, the patient received supportive care with beta-blockers and angiotensin-converting enzyme inhibitors as indicated for treatment of Takotsubo cardiomyopathy in addition to antithyroid drugs and steroids for treatment of thyrotoxicosis, resulting in full recovery and improvement of cardiac function. Whereas the ejection fraction did not restore to normal levels, it should be noted that complete recovery takes at least several months (23) and despite our efforts to obtain a repeat echocardiogram after the patient became euthyroid, the patient could not be re-evaluated.
In regard to the patient's neuropsychiatric symptoms of mental retardation and impaired cognitive function, these are well known to occur in the setting of acute thyrotoxicosis (48). Typically, there is an improvement in cognitive and behavioral impairment when patients become euthyroid, but the response can be delayed, as it was observed in our patient. In fact, one study reported that there was persistent cognitive impairment for many years after successful treatment for thyrotoxicosis (49). The explanation is not entirely clear. We know that restoration of thyroid function tests to normal may coincide with or precede amelioration of the tissue effects of hyperthyroidism. Several biomarkers, such as sex hormone-binding globulin, ferritin, and angiotensin-converting enzyme levels, have been used to indirectly assess thyroid hormone peripheral tissue sensitivity or action in hyperthyroid patients, and such measurements may have been helpful in the patient described (50,51).
In conclusion, we present a case of Takotsubo cardiomyopathy in the setting of thyrotoxicosis secondary to Graves' disease. Physicians should be aware of the clinical manifestations of thyrotoxicosis and its effects on the cardiovascular system so that a reversible cause of heart failure is not missed.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Graves R.1835Newly observed affection of the thyroid gland in females. Lond Med Surg J 7(Part 2):516–517 [Google Scholar]
- 2.Levine SA, Sturgis CC.1924Hyperthyroidism masked as heart disease. Boston Med Surg J 190:233–237 [Google Scholar]
- 3.Klein I, Ojamaa K.2001Thyroid hormone and the cardiovascular system. N Engl J Med 344:501–509 [DOI] [PubMed] [Google Scholar]
- 4.Dote K, Sato H, Tateishi H, Uchida T, Ishihara M.1991[Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases]. J Cardiol 21:203–214 [PubMed] [Google Scholar]
- 5.Pilgrim TM, Wyss TR.2008Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: a systematic review. Int J Cardiol 124:283–292 [DOI] [PubMed] [Google Scholar]
- 6.Bybee KA, Prasad A.2008Stress-related cardiomyopathy syndromes. Circulation 118:397–409 [DOI] [PubMed] [Google Scholar]
- 7.Burch HB, Wartofsky L.1993Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab Clin North Am 22:263–277 [PubMed] [Google Scholar]
- 8.Brinjikji W, El-Sayed AM, Salka S.2012In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J 164:215–221 [DOI] [PubMed] [Google Scholar]
- 9.Nef HM, Mollmann H, Akashi YJ, Hamm CW.2010Mechanisms of stress (Takotsubo) cardiomyopathy. Nat Rev Cardiol 7:187–193 [DOI] [PubMed] [Google Scholar]
- 10.Kurisu S, Sato H, Kawagoe T, et al. 2002Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J 143:448–455 [DOI] [PubMed] [Google Scholar]
- 11.Abe Y, Kondo M, Matsuoka R, Araki M, Dohyama K, Tanio H.2003Assessment of clinical features in transient left ventricular apical ballooning. J Am Coll Cardiol 41:737–742 [DOI] [PubMed] [Google Scholar]
- 12.Akashi YJ, Nakazawa K, Sakakibara M, Miyake F, Musha H, Sasaka K.2004123I-MIBG myocardial scintigraphy in patients with “Takotsubo” cardiomyopathy. J Nucl Med 45:1121–1127 [PubMed] [Google Scholar]
- 13.Burgdorf C, von Hof K, Schunkert H, Kurowski V.2008Regional alterations in myocardial sympathetic innervation in patients with transient left-ventricular apical ballooning (Tako-tsubo cardiomyopathy). J Nucl Cardiol 15:65–72 [DOI] [PubMed] [Google Scholar]
- 14.Wittstein IS, Thiemann DR, Lima JA, et al. 2005Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 352:539–548 [DOI] [PubMed] [Google Scholar]
- 15.Scott IU, Gutterman DD.1995Pheochromocytoma with reversible focal cardiac dysfunction. Am Heart J 130:909–911 [DOI] [PubMed] [Google Scholar]
- 16.Abraham J, Mudd JO, Kapur NK, Klein K, Champion HC, Wittstein IS.2009Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists. J Am Coll Cardiol 53:1320–1325 [DOI] [PubMed] [Google Scholar]
- 17.Mann DL, Kent RL, Parsons B, Cooper GT.1992Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation 85:790–804 [DOI] [PubMed] [Google Scholar]
- 18.Drislane FW, Samuels MA, Kozakewich H, Schoen FJ, Strunk RC.1987Myocardial contraction band lesions in patients with fatal asthma: possible neurocardiologic mechanisms. Am Rev Respir Dis 135:498–501 [DOI] [PubMed] [Google Scholar]
- 19.Ueyama T, Kasamatsu K, Hano T, Yamamoto K, Tsuruo Y, Nishio I.2002Emotional stress induces transient left ventricular hypocontraction in the rat via activation of cardiac adrenoceptors: a possible animal model of “tako-tsubo” cardiomyopathy. Circ J 66:712–713 [DOI] [PubMed] [Google Scholar]
- 20.Izumi Y, Okatani H, Shiota M, et al. 2009Effects of metoprolol on epinephrine-induced takotsubo-like left ventricular dysfunction in non-human primates. Hypertens Res 32:339–346 [DOI] [PubMed] [Google Scholar]
- 21.Nef HM, Mollmann H, Kostin S, et al. 2007Tako-Tsubo cardiomyopathy: intraindividual structural analysis in the acute phase and after functional recovery. Eur Heart J 28:2456–2464 [DOI] [PubMed] [Google Scholar]
- 22.Nef HM, Mollmann H, Troidl C, et al. 2008Expression profiling of cardiac genes in Tako-Tsubo cardiomyopathy: insight into a new cardiac entity. J Mol Cell Cardiol 44:395–404 [DOI] [PubMed] [Google Scholar]
- 23.Neil CJ, Nguyen TH, Sverdlov AL, et al. 2012Can we make sense of takotsubo cardiomyopathy? An update on pathogenesis, diagnosis and natural history. Expert Rev Cardiovasc Ther 10:215–221 [DOI] [PubMed] [Google Scholar]
- 24.Nguyen TH, Neil CJ, Sverdlov AL, et al. 2011N-terminal pro-brain natriuretic protein levels in takotsubo cardiomyopathy. Am J Cardiol 108:1316–1321 [DOI] [PubMed] [Google Scholar]
- 25.Mori H, Ishikawa S, Kojima S, et al. 1993Increased responsiveness of left ventricular apical myocardium to adrenergic stimuli. Cardiovasc Res 27:192–198 [DOI] [PubMed] [Google Scholar]
- 26.Ueyama T, Kasamatsu K, Hano T, Tsuruo Y, Ishikura F.2008Catecholamines and estrogen are involved in the pathogenesis of emotional stress-induced acute heart attack. Ann NY Acad Sci 1148:479–485 [DOI] [PubMed] [Google Scholar]
- 27.Silva JE, Bianco SD.2008Thyroid-adrenergic interactions: physiological and clinical implications. Thyroid 18:157–165 [DOI] [PubMed] [Google Scholar]
- 28.Bilezikian JP, Loeb JN.1983The influence of hyperthyroidism and hypothyroidism on alpha- and beta-adrenergic receptor systems and adrenergic responsiveness. Endocr Rev 4:378–388 [DOI] [PubMed] [Google Scholar]
- 29.Bahouth SW.1991Thyroid hormones transcriptionally regulate the beta 1-adrenergic receptor gene in cultured ventricular myocytes. J Biol Chem 266:15863–15869 [PubMed] [Google Scholar]
- 30.Bayliss RI, Edwards OM.1971Urinary excretion of free catecholamines in Graves' disease. J Endocrinol 49:167–173 [DOI] [PubMed] [Google Scholar]
- 31.Coulombe P, Dussault JH, Walker P.1976Plasma catecholamine concentrations in hyperthyroidism and hypothyroidism. Metabolism 25:973–979 [DOI] [PubMed] [Google Scholar]
- 32.Levey GS, Klein I.1990Catecholamine-thyroid hormone interactions and the cardiovascular manifestations of hyperthyroidism. Am J Med 88:642–646 [DOI] [PubMed] [Google Scholar]
- 33.Liggett SB, Shah SD, Cryer PE.1989Increased fat and skeletal muscle beta-adrenergic receptors but unaltered metabolic and hemodynamic sensitivity to epinephrine in vivo in experimental human thyrotoxicosis. J Clin Invest 83:803–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crozatier B, Su JB, Corsin A, Bouanani N-H.1991Species differences in myocardial beta-adrenergic receptor regulation in response to hyperthyroidism. Circ Res 69:1234–1243 [DOI] [PubMed] [Google Scholar]
- 35.Bachman ES, Hampton TG, Dhillon H, et al. 2004The metabolic and cardiovascular effects of hyperthyroidism are largely independent of beta-adrenergic stimulation. Endocrinology 145:2767–2774 [DOI] [PubMed] [Google Scholar]
- 36.Dillmann WH.2002Cellular action of thyroid hormone on the heart. Thyroid 12:447–452 [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki S, Kamiishi T, Hosokawa N, et al. 2004Reversible left ventricular dysfunction “takotsubo” cardiomyopathy associated with hyperthyroidism. Jpn Heart J 45:889–894 [DOI] [PubMed] [Google Scholar]
- 38.Rossor AM, Pearce SH, Adams PC.2007Left ventricular apical ballooning (takotsubo cardiomyopathy) in thyrotoxicosis. Thyroid 17:181–182 [DOI] [PubMed] [Google Scholar]
- 39.Radhakrishnan A, Granato JE.2009An association between Takotsubo cardiomyopathy and thyroid storm. Postgrad Med 121:126–130 [DOI] [PubMed] [Google Scholar]
- 40.Alidjan F, Ezzhati M, Bruggeling W, van Guldener C.2010Takotsubo cardiomyopathy precipitated by thyrotoxicosis. Thyroid 20:1427–1428 [DOI] [PubMed] [Google Scholar]
- 41.Sarullo FM, Americo L, Accardo S, et al. 2009Tako-tsubo cardiomyopathy observed in a patient with sepsis and transient hyperthyroidism. Monaldi Arch Chest Dis 72:33–36 [DOI] [PubMed] [Google Scholar]
- 42.Gundara JS, Lee JC, Ip J, Sidhu S.2012Takotsubo cardiomyopathy complicating thyroidectomy for Graves' disease. Thyroid 22:975–976 [DOI] [PubMed] [Google Scholar]
- 43.Kwon SA, Yang JH, Kim MK, et al. 2010A case of Takotsubo cardiomyopathy in a patient with iatrogenic thyrotoxicosis. Int J Cardiol 145:e111–e113 [DOI] [PubMed] [Google Scholar]
- 44.Tsao YT, Lin CS, Lin SH.2010Thyrotoxicosis factitia induces Takotsubo cardiomyopathy in end-stage renal disease: a pathogenetic hypothesis. Kidney Int 77:468. [DOI] [PubMed] [Google Scholar]
- 45.Hutchings DC, Adlam D, Ferreira V, Karamitsos TD, Channon KM.2011Takotsubo cardiomyopathy in association with endogenous and exogenous thyrotoxicosis. QJM. 104:433–435 [DOI] [PubMed] [Google Scholar]
- 46.Sakaki T, Fujioka Y, Akagami T, et al. 2004Cardiac wall motion abnormalities observed in a patient with transient hyperthyroidism. Jpn Heart J 45:1071–1077 [DOI] [PubMed] [Google Scholar]
- 47.van de Donk NW, America YG, Zelissen PM, Hamer BJ.2009Takotsubo cardiomyopathy following radioiodine therapy for toxic multinodular goitre. Neth J Med 67:350–352 [PubMed] [Google Scholar]
- 48.Bunevicius R, Prange AJ., Jr2006Psychiatric manifestations of Graves' hyperthyroidism: pathophysiology and treatment options. CNS Drugs 20:897–909 [DOI] [PubMed] [Google Scholar]
- 49.Perrild H, Hansen JM, Arnung K, Olsen PZ, Danielsen U.1986Intellectual impairment after hyperthyroidism. Acta Endocrinol (Copenh) 112:185–191 [DOI] [PubMed] [Google Scholar]
- 50.Smallridge RC, Chernow B, Snyder R, Zaloga GP, Burman KD.1985Angiotensin-converting enzyme activity. A potential marker of tissue hypothyroidism in critical illness. Arch Intern Med 145:1829–1832 [DOI] [PubMed] [Google Scholar]
- 51.Smallridge RC, Rogers J, Verma PS.1983Serum angiotensin-converting enzyme. Alterations in hyperthyroidism, hypothyroidism, and subacute thyroiditis. JAMA 250:2489–2493 [DOI] [PubMed] [Google Scholar]