Abstract
Chondrocytes have been generated in vitro from a range of progenitor cell types and by a number of strategies. However, achieving reconstitution of actual physiologically relevant, appropriately-laminated cartilage in situ that would be applicable to conditions, such as arthritis and cartilage degeneration remains elusive. This lack of success is multifactorial and includes limited cell source, decreased proliferation rate of mature chondrocytes, lack of maintenance of phenotype, reduced matrix synthesis, and poor integration with host tissue. We report an efficient approach for deriving mesenchymal chondroprogenitor cells from human embryonic stem cells. These cells generated tissue containing cartilage-specific matrix proteins that integrated in situ in a partial-thickness defect in ex vivo articular cartilage harvested from human arthritic joints. Given that stem cells provide a virtually inexhaustible supply of starting material and that our technique is easily scalable, cartilaginous tissue primed and grafted in this manner could be suitable for clinical translation.
Introduction
Arthritis and cartilage degeneration are leading causes of disability in both the aging population, as well as in younger demographics that engage in increasingly intense physical activity starting at an earlier age.1 The incidence of these conditions is growing significantly due to increasing life expectancy.2 As a tissue, cartilage is known for its metabolic inertness reflected by the absence of nerve and blood supply. Over time, mature adults remodel their cartilage matrix but cannot replenish the cells to synthesize matrix: in that sense cartilage is reminiscent of the heart and the brain. Furthermore, unlike more regenerative systems, such as skin and bone in which new tissues integrate with surrounding old tissue, grafted and newly regenerated cartilage does not bond well with preexisting mature cartilage.3–6
Surgical attempts to generate tissue resembling native cartilage have included microfracture, matrix scaffolds, and osteochondral grafting.7 Cell-replacement approaches, such as autologous chondrocyte transplantation are attractive for promoting repair and regeneration in lesions of cartilage. However, the regenerative capacity of such a cell source is restricted by the limited source of cells and the variable and often diminished capacity for proliferation and matrix synthesis of mature adult chondrocytes.6,8 Although generating chondrocytes from the ever broadening selection of stem cells would seem to be a logical solution to this problem, generating mature, physiologically relevant, layered, cartilage from such stem cell-derived chondrocytes has remained elusive.9
Developmentally, chondrocytes are derived from cells of mesenchymal origin.10 Chondrogenic differentiation was therefore, initially pursued extensively using mesenchymal stem cells (MSCs) isolated from bone marrow.11,12 Although chondrocytic differentiation was readily achieved, the generation of physiologically and clinically relevant cartilage was and continues to be an unexpected obstacle. Despite expression of promising genes, the generated matrix is of insufficient quality and is compounded by the propensity for MSCs, especially those derived from bone marrow, to undergo hypertrophy after chondrogenesis.13–15 The use of pluripotent cells, such as human embryonic stem cells (hESCs), allows one to start at an earlier developmental stage and is an attractive strategy to overcome this obstacle. Several methods have been employed to obtain mesenchymal progenitors from hESCs.16,17 These methods have usually included generating heterogeneous embryoid bodies,18–23 sorting hESC derivatives by selecting for mesenchymal cell surface markers, such as CD10524 or CD73,25 culturing cells on murine stromal cell lines, such as OP9,26 or by coculturing ESC with primary chondrocytes.27 Nevertheless, achieving reconstitution of actual physiologically relevant, appropriately laminated cartilage in situ that would be applicable to conditions such as arthritis and cartilage degeneration has not been successful.
We hypothesized that this impediment was due to lack of an efficient method to differentiate ESCs into functioning chondrocytes and due to an incomplete understanding of the developmental stage and the local microenvironment necessary for successful repair. We report an alternative approach that circumvents cumbersome sorting procedures based on a combination of markers and avoids animal feeder cells and substrates. The use of hESCs permits us to emulate the fundamental developmental forces of early organogenesis starting at the stage of primitive mesenchyme before the cells become mature chondrocytes.
Materials and Methods
Maintenance and differentiation of hESCs
The hESCs were obtained from NIH-line WA 09 and were supplied by WiCell (Madison, WI) as their H9 line. To differentiate the hESCs toward a mesenchymal lineage, the hESC colonies were mechanically dissected into small pieces under microscopic guidance, and then were transferred to tissue culture-treated six-well plates (Corning, Fisher Scientific, Waltham, MA). Culture media used was DMEM/F12 supplemented with nonessential amino acids and 10% fetal bovine serum (FBS; Invitrogen-Gibco, Grand Island, NY). The cells at this stage were considered passage 0 (P0). When the culture approached confluency in the six-well plates, cells were trypsinized and transferred to a new tissue culture flask at a nominal confluency of 25% using the same culture media. Culture media were changed every 3 days. As each culture approached confluency, the procedure was repeated in fresh tissue culture flasks up to nine passages. Excess cells were cryopreserved for replicate experiments.
Flow cytometry
Cell surface antigens on hESC-derived cells were analyzed by fluorescence-activated cell sorting. The cells were released from the tissue culture flask with Accutase, centrifuged, washed with phosphate-buffered saline (PBS), and blocked in 2% FBS for 0.5 h at room temperature. Cells (2×105) were then incubated with each of the following using a BD Stemflow™ Human MSC Analysis Kit (BD Biosciences, San Jose, CA): human MSC (hMSC) positive markers (CD73, CD90, CD105) and hMSC negative markers (CD11b, CD19, CD34, CD45, HLA-DR). After incubation, cells were washed and resuspended in PBS. Data were analyzed by collecting 20,000 events on a Cyan LX (Dako North America, Inc., Carpinteria, CA) instrument using WinMDI software. Nonspecific fluorescence was determined by incubation of similar cell aliquots with isotype-matched mouse monoclonal antibodies (PharMingen, San Diego, CA) or with secondary antibody alone.
Chondrogenic differentiation
The hESCs (2.5×105 cells) were collected in 15-mL conical tubes and centrifuged at 150 g for 5 min after which they were transferred to serum-free chondrogenic media (Lonza, Basel, Switzerland) in the presence or absence of transforming growth factor β3 (TGFβ3, 10 ng/mL; PeproTech, Rocky Hill, NJ). The media were changed twice weekly. At the end of 3 weeks, some cell pellets were fixed with zinc-buffered formalin (Z-Fix, Anatech, Battle Creek, MI), paraffin-embedded, sectioned, and assessed for their chondrogenic differentiation status using histochemical stains, immunocytochemical markers, and mRNA levels as described below. Experiments with hESC were run in triplicate. For comparison, adult human articular chondrocytes (n=5 donors) and adult bone marrow-derived MSCs (BM-MSC, n=3) were cultured under similar chondrogenic conditions.
Histochemical and immunocytochemical characterization
To test for immunocytochemical markers of the undifferentiated pluripotent state (e.g., Oct 3/4) vs. markers of early differentiation (e.g., vimentin), hESCs or their derivatives were cultured in 24-well plates, fixed in cold methanol for 1 min, blocked with 10% FBS in PBS, and incubated with the appropriate primary antibody diluted in 1.5% bovine serum albumin/PBS at room temperature for 1 h. Mouse anti-Oct4 and rabbit anti-vimentin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). After washing, the cells were incubated for 1 h with a species-appropriate fluorochrome-conjugated secondary antibody: FITC-goat anti-mouse (BD Biosciences) or Alexa Fluor® 568 goat anti-rabbit (Invitrogen, Carlsbad, CA). Normal mouse or rabbit IgG were used as negative controls. For histology, cartilage explants, and cell pellets were fixed in Z-Fix (Anatech) for 24 h and were embedded in paraffin. Paraffin-embedded samples were sectioned at 4 μm, deparaffinized in a xylene substitute, Pro-Par Clearant (Anatech), passed through an ethanol series, and finally placed in water for rehydration. Sections were stained with Safranin-O/fast green. For immunohistochemical staining, sections were washed with PBS, and blocked with 0.1% Tween 20 with 3% normal goat serum for 30 min at room temperature. Primary antibodies anti-lubricin (Goat IgG at 1 μg/mL; Santa Cruz), anti-type II collagen (II-II6B3; Hybridoma Bank, University of Iowa at 2 μg/mL), and negative controls (normal goat IgG and normal mouse IgG and; 1 μg/mL) were applied and incubated for 1 h at room temperature. After washing with PBS, sections were incubated with biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA) for 30 min. Sections were then incubated with horseradish peroxidase (Zymed Laboratories, San Francisco, CA) for 10 min at room temperature and were stained with 3,3-diaminobenzidine (Vector Laboratories) substrate for 3–10 min.
Gene expression
Total RNA was extracted from cell pellets with RNeasy® kit (Invitrogen) and was reverse transcribed to cDNA with SuperScript® (Invitrogen). Real-time RT-PCR of collagen 2A1 and aggrecan was performed using Taqman® Gene expression assays as per manufacturer's instructions (Applied Biosystems®, Foster City, CA).
Repairing cartilage defects in adult human arthritic joints using hESC-derived cells
Osteochondral explants were obtained from tissue surgically resected from the joints of adult arthritic human patients (n=6) undergoing total knee arthroplasty. Two osteochondral explants ∼6 mm in diameter were obtained from each donor: one assigned to the cell treatment group and the other to the control group. A surgical curette was used to make partial-thickness defects ∼2 mm in size in the articular surface of each explant. In the cell treatment group, the defects were filled with hESC-derived chondrogenic precursors that had been aggregated under the following conditions: 5×105 cells centrifuged in 15-mL conical tubes at 150 g for 5 min in DMEM/F12 supplemented with 10% FBS and incubated overnight in the presence or absence of TGFβ3. In the control group, the defect was left untreated. Explants embedded with cells and control explants without cell treatment were cultured in DMEM/F12 supplemented with nonessential amino acids and 1% FBS (Invitrogen-Gibco). Culture media were changed every 3 days. After 4 weeks, explants were air-dried and digital photographs were obtained with a digital camera (Canon EOS Rebel X) using a macro lens. Specimens were then fixed, paraffin-embedded, sectioned, and stained with Safranin-O for histologic analysis or for immunohistochemical analysis for collagen II and lubricin detection and distribution (as described above).
Research ethics approval
All experiments were approved by the Scripps Health Institutional Review Board and Stem Cell Oversight Committee at the University of California, San Diego.
Results
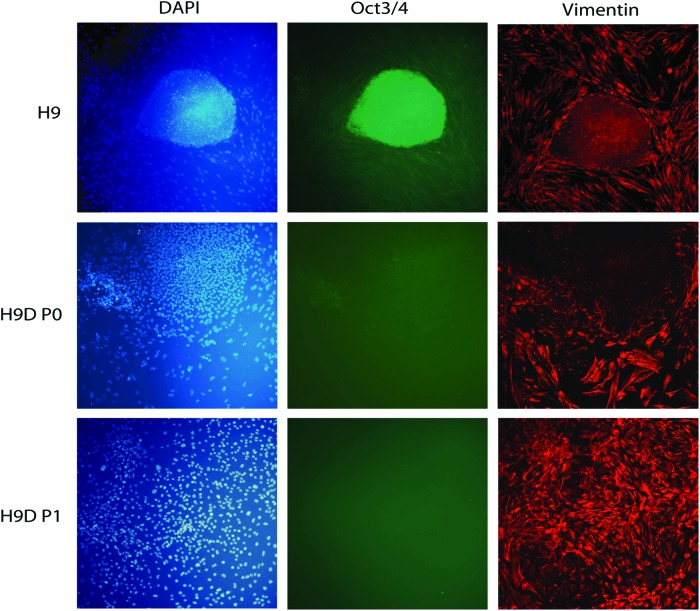
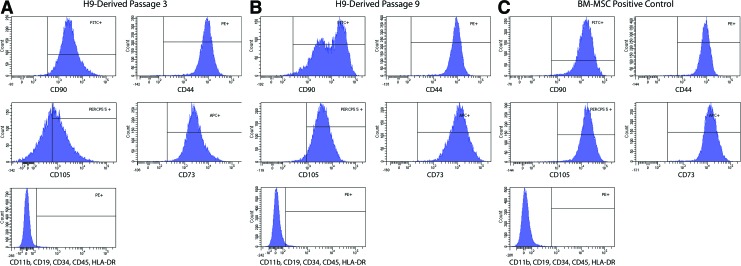
To derive chondroprogenitors from hESC, small clumps of hESCs were transferred to tissue-culture treated polystyrene wells containing 10% FBS. After plating, a heterogeneous population of cells emerged and migrated out from the edge of the small clumps, which were distinct from the typical compact colonies of undifferentiated hESCs. By five passages, almost all the cells were spindle-shaped, resembling adult human BM-MSCs (Fig. 1). In contrast to undifferentiated hESCs within the densely packed colony, which expressed markers of the undifferentiated pluripotent state, such as Oct3/4, the cells that migrated beyond the periphery of the colony ceased expressing Oct3/4 and began instead to express vimentin, an intermediate filament associated with and often used as a marker of epithelial–mesenchymal transition.28 Vimentin immunoreactivity was absent within the hESC colony but was not initially uniform in the migrating cells (Fig. 2, top and middle rows). However, when these migrating cells were isolated, trypsinized, and passaged after 2 to 4 weeks in vitro to confluence, the surviving cell population appeared homogenous in morphology and entirely vimentin immunopositive (Fig. 2, bottom row). Cytometric analysis of these cells indicated that they expressed surface markers traditionally associated with BM-MSCs and in a profile similar to that cell type (Fig. 3). After the first passage, very few of the hESC-derived cells were positive for CD73, CD90, CD44, and CD105. After 3 passages in standard tissue culture polystyrene plates with no further manipulation, 55.4% of cells expressed all four markers, and by passage 9, 95.7% of cells were positive. Only 1.1% of cells remained positive for negative markers of MSC (CD11b, CD19, CD34, CD45, HLA-DR) suggesting that our relatively simple protocol had either permitted hESC-derived MSC-like cells to acquire a survival advantage and to dominate the culture or had allowed a spontaneous differentiation process to unfold, which directed hESCs preferentially toward a mesenchymal progenitor cell-like lineage.
FIG. 1.
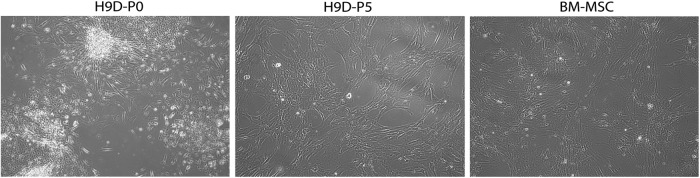
Derivation of chondrogenic precursors from human embryonic stem cells (hESCs) starting first with differentiation toward mesenchymal progenitor cells. Small clumps of cells were scraped from colonies of hESCs and cultured in basal medium supplemented with 10% fetal bovine serum. When confluent (“hESC-differentiated [-D] Passage 0 [P0]”), these hESC-derived cells were trypsinized and passaged up to five times. After five passages (“hESC-D-P5”), these hESC-derived cells resembled mesenchymal stem cells (MSCs) isolated directly from bone marrow (“BM-MSC”) by morphology (phase contrast microscope).
FIG. 2.
Immunofluorescent staining for the marker of the undifferentiated pluripotent state of hESCs, Oct-3/4 (green); for the marker of early differentiating migrating hESC-derived cells, vimentin (red); and for all cell nuclei (DAPI, blue). The differentiating hESCs lose Oct-3/4 expression and become increasingly vimentin immunopositive by as early as the first passage (“hESC-D-P1”). Color images available online at www.liebertpub.com/tea
FIG. 3.
At passage 3, over 90% of hESC-derived cells express three of the four mesenchymal markers CD90, CD44, CD 105, and CD73. (A) By passage 9, over 95% of hESC-derived cells express all four markers (B), to an extent similar to BM-MSC (C) More than 98% of H9-derived cells at passage 3 or 9 were negative for CD11b, CD19, CD34, CD45, HLA-DR. Color images available online at www.liebertpub.com/tea
We then assessed chondrogenic potential by growing them as high density 3-dimensional aggregates for 3 weeks, while exposing them to TGFβ3 in culture medium that also contained dexamethasone and high glucose concentration.29,30 As negative controls, some cultures were grown in the absence of TGFβ3. Pellet cultures of adult human articular chondrocytes (n=5 donors) and adult BM-MSC (n=3) were cultured under similar chondrogenic conditions as positive controls.
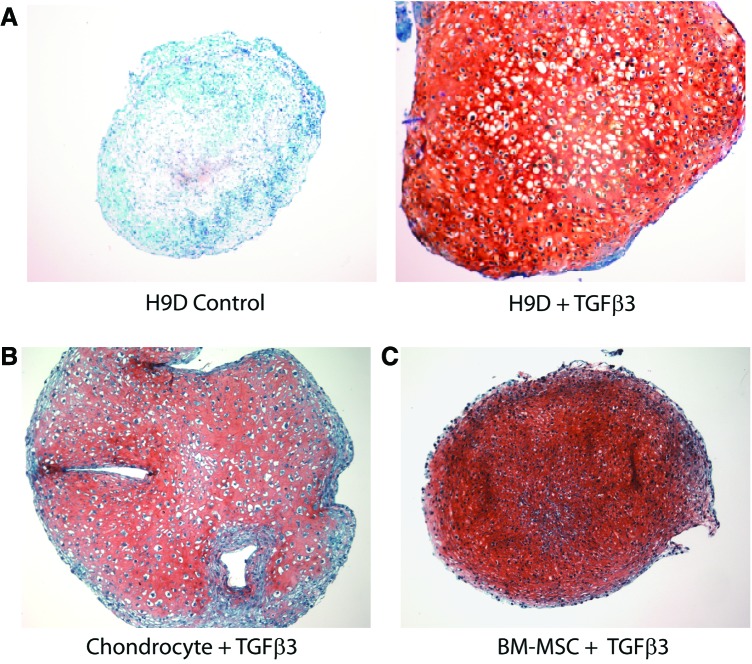
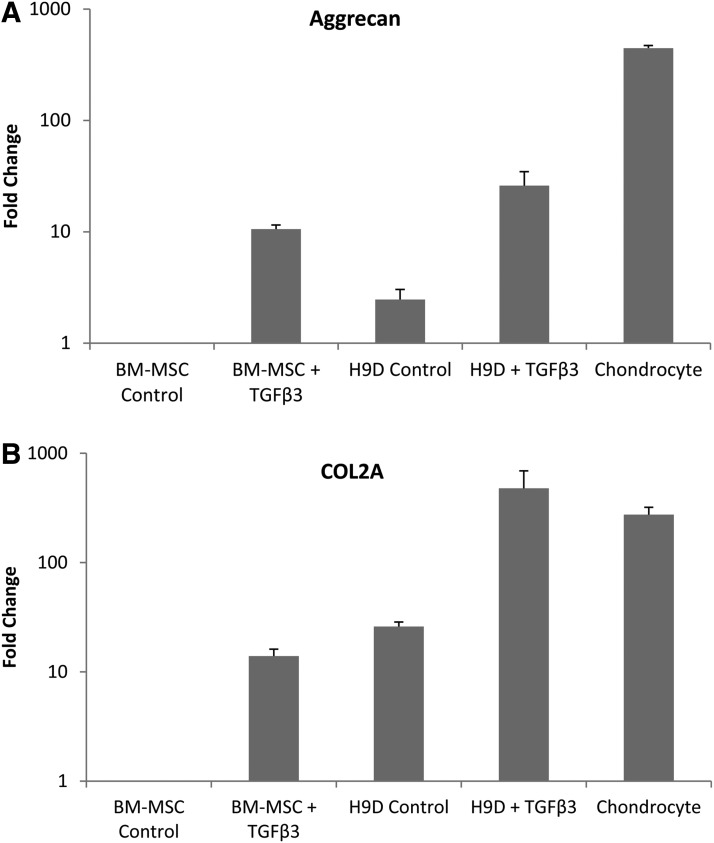
With TGFβ3 treatment, aggregates increased in size along with a significant accumulation of proteoglycans as indicated by Safranin-O histochemical staining (Fig. 4). Cells embedded in the matrix exhibited the typically rounded morphology of chondrocytes. TGFβ3 also induced a several-fold higher expression of chondrocyte-specific genes: aggrecan (Fig. 5A) and collagen type IIA1 (Fig. 5B). The combined histochemical, cell morphologic, and gene expression pattern indicated that the hESC derivatives had now acquired chondrogenic potential.
FIG. 4.
hESC-derived mesenchymal progenitors underwent chondrogenic differentiation in pellet culture. (A) Safranin-O (red) staining of tight cell aggregates shows proteoglycan expression when cultured in the presence of TGFβ3 (A: right), but not in its absence (A: left). Pellet cultures of adult human chondrocytes (B) and BM-MSC (C) are shown for comparison. Color images available online at www.liebertpub.com/tea
FIG. 5.
Gene expression (RT-PCR) of chondrocytic markers aggrecan (A) and COL2A (B) increased substantially with TGFβ3 treatment of hESC-derived mesenchymal progenitors (H9D) in pellet culture. Gene expression was normalized to that of BM-MSC without TGFβ3 stimulation.
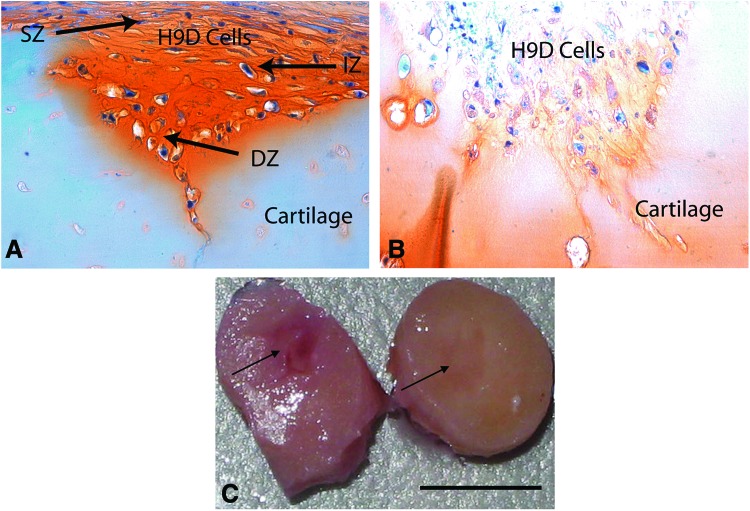
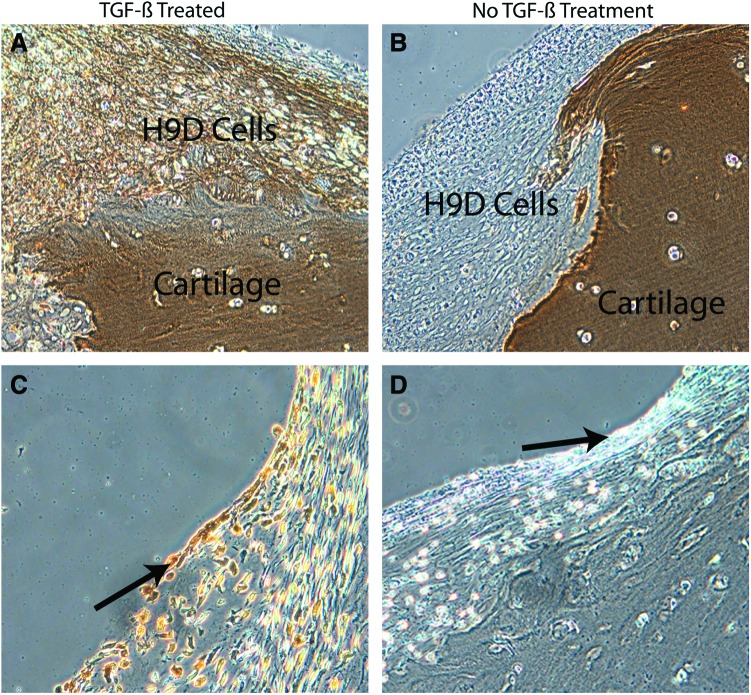
Chondrogenesis during embryologic development has been recapitulated in vitro in an elegant series of experiments involving hESC.31 However, forming cartilaginous tissue in vitro is still too far from physiologically relevant tissue capable of performing in vivo, which has been the principal stumbling block to repair strategies to date. We hypothesized that the entire profile of developmental signals needed for cartilage development was likely not necessary and simplified our protocol to include the minimum biochemical stimuli. We further assumed that cells and matrix in diseased tissue would provide cues that trigger and enhance differentiation and generation of repair tissue. As proof of principle, a partial-thickness defect was created in human adult articular cartilage explanted from the arthritic joints of patients undergoing total knee arthroplasty. Such defects do not heal spontaneously in vivo or in vitro. Furthermore, arthritic cartilage is characterized by a surface that is fibrillated, has lost its superficial zone, is depleted in matrix content, and possesses a lower density of viable cells. After 4 weeks, the defects in the arthritic joint tissue became filled with Safranin-O staining regenerated tissue that integrated with the surrounding host cartilage and restored the cartilage surface (Fig. 6). Defects without cells persisted unchanged (Fig. 6C). This seamlessly integrated repair tissue, which was also strongly positive for the hyaline articular cartilage-specific marker collagen II (Fig. 7A), contained cells typical of rounded chondrocytes in lacunae. Normal articular cartilage has a unique and highly ordered structure with characteristic zones (superficial, intermediate, and deep). Most significantly, the embedded hESC-derived cells exhibited this zonal/laminated distribution. The superficial cells were compacted, spindle-shaped, transversely elongated, and aligned tangential and parallel to the articular surface (covering the surface of the repair and replacing the superficial layer typically lost in arthritic joints), while the deeper cells were more rounded (with large cytoplasmic areas and small nuclei) within lacunae surrounded by pericellular matrix. Superficial zone protein, also known as lubricin, is a functional lubricant found primarily in the superficial zone of normal articular cartilage.32 As a marker of zonal organization in repair tissue, sections of cartilage seeded with hESC-derived progenitor cells were stained with anti-lubricin antibody. The superficial layer was stained positive for lubricin (Fig. 7C), which further supported our premise that hESC-derived cells are attractive candidates for cartilage repair. The cells closest to the host cartilage consistently accumulated the most proteoglycans as indicated by the darker staining, suggesting that direct contact with components of extracellular cartilage matrix likely helps induce, facilitate, and maintain chondrogenesis.33,34 That the recipient human articular surface was from an arthritic joint was reinforced by noting that the matrix staining (Fig. 6, Safranin-O red stain) was more intense in the embedded cells than in the surrounding host cells. The margins of chronic defects in mature cartilage are essentially devitalized with no living cells to generate repair tissue to fill the arthritic defect. In our protocol, immature precursors were permitted to mature under physiologic conditions to generate this seamless integration. While the hESC-derived progenitors were chondrogenic, whether or not additional exogenous TGFβ3 was provided, TGFβ3 supplementation significantly enhanced matrix formation (i.e., both proteoglycan and collagen II content).
FIG. 6.
hESC-derived chondrogenic precursors were embedded in partial thickness defects in human adult articular cartilage from an arthritic joint. After 4 weeks of culture in the presence of TGF-β, the partial-thickness defects was filled completely with donor cell-derived intensely Safranin-O staining material (indicative of proteoglycan), which seamlessly integrated with the surrounding host cartilage and restored the surface of the cartilage (A) Such defects never heal spontaneously. Note the poor Safranin-O staining of the host tissue due to arthritis. Also note that the repaired tissue has the highly-ordered unique laminated structure of normal articular cartilage with the three distinct characteristic zones indicated: superficial, intermediate, and deep. Cells in the superficial zone (SZ) were compact, spindle-shaped, and aligned tangential and parallel to the articular surface covering the surface of the repair, while the deeper cells (DZ) were rounded with large cytoplasms and small nuclei within lacunae surrounded by pericellular matrix. (Arthritic cartilage is usually devoid of a superficial layer.) While elimination of transforming growth factor β3 (TGFβ3) did generate some neotissue, the matrix content was suboptimal: note the diminished Safranin-O staining (B). (C) Digital photograph of the gross appearance of repaired site at 4 weeks shows complete healing with H9D (right) and no healing without H9D (left). Arrows point to the location of the defect. H9D, human embryonic stem cell-derived progenitors; cartilage, host human arthritic cartilage. Scale bar=5 mm. Color images available online at www.liebertpub.com/tea
FIG. 7.
Collagen II (A, B) and lubricin (C, D) (indicated by brown immunoreactivity) are present in the transplanted hESC-derived cells in cartilage. Treatment with TGFβ (A) generated neotissue positive for collagen II (indicated by brown immunoreactivity) in contrast to absence of TGFβ treatment (B). Lubricin (C) (indicated by brown immunoreactivity) was present in the superficial zone (arrow) of the neotissue (D, isotype control). H9D, human embryonic stem cell-derived progenitors; cartilage, host human arthritic cartilage. Color images available online at www.liebertpub.com/tea
Discussion
We developed an efficient and convenient method of assembling the requisite components for cartilage formation: (1) proper developmental stage (prechrondrocytic mesenchyme), (2) proper trophic factor support, and (3) initial cell-cell contact. The use of hESCs was most amenable to achieving each of these steps. For pellet culture, primary human adult articular chondrocytes were used in parallel with hESC-derived chondrogenic progenitors as positive controls and for validation.
Our data indicated that generating chondroprogenitors from human ESC does not require embryonic body (EB) formation, a step previously used in hESC chondrogenesis.19,20,23 In addition, we were able to induce effective chondrogenic differentiation and to generate chondral tissue without the need of recapitulating the entire sequence of events that occurs during embryologic development.31 We and others have noted that adult MSCs can be isolated from bone marrow by mere adhesion to standard tissue culture-treated polystyrene.11,35,36 By extending this simple observation, we cultured undifferentiated hESCs directly on tissue culture-treated polystyrene and induced differentiation into MSC-like progenitor cells. While more than 95% of cells expressed MSC-like cell surface markers, we did not test for differentiation into adipocyte or osteoblast lineages. Our translational objective was to generate progenitors with optimal chondrogenic potential rather than proving that the cells were mesenchymal in nature.
Hwang et al. derived chondrocytes from hESC with20 or without27 EB formation. When EB was not involved; however, hESC were cocultured with primary chondrocytes isolated from bovine cartilage. Nakagawa et al. reported generating chondroprogenitors from hESC without EB formation by directly plating single dissociated hESC onto polystyrene culture dishes.37 Their protocol involved digestion of hESC with collagenase and trypsin before plating cells on tissue culture plates, while no enzyme digestion of hESC was used in our generation of chondroprogenitors. Oldershaw et al. developed a protocol that directed hESC through developmental stages of primitive streak and mesoderm before differentiation into chondrocytes.31 Our protocol was simpler and directly differentiated hESC into cells of MSC-like phenotype with high chondrogenic potential.
The mechanical properties of the underlying substrate directly modulate differentiation of pluripotent and multipotent cells.38 This factor was most likely responsible for spontaneous differentiation of ESC into MSC-like phenotype when cultured on polystyrene. A gradient in the substrate stiffness is also likely to induce changes in cell response.39 MSC tend to migrate to areas of higher substrate stiffness. We observed histologic evidence of this effect as we often found cells and neo-tissue permeating cracks and fissures in host arthritic tissue. In addition, temporal changes in matrix stiffness also drive differentiation and maturation signals.40 The repair process develops from an initial highly cellular construct of low stiffness to progressively increasing stiffness with generation and deposition of extracellular matrix components. This process also likely contributed to maturation of the cells.
While TGF-β enhanced the quality of repair tissue, even without TGF-β we noted substantial repair tissue. Components of the extracellular matrix in general modulate cell attachment, proliferation, differentiation, and the differentiated functions of cells.41–44 More specifically, collagen II and Perlecan domain 1 can enhance chondrogenic differentiation.45–49 Other extracellular matrix substrates also affect the differentiation capacity of human chondrocytes toward chondrogenic and osteogenic lineages.50 Therefore, the extracellular matrix in the host tissue likely generated powerful differentiation and repair signals. Cellular interactions are at least as important as matrix components in directing differentiation. Coculture with chondrocytes enhances chondrogenic differentiation of MSC and reduces the need for exogenous growth factors.51,52 The lack of efficacy of media conditioned with chondrocyte culture indicates that bidirectional communication between mature and differentiating cells. Of direct relevance to our arthritic tissue repair model, is osteoarthritic chondrocytes in culture generated a spectrum of extracellular matrix components and growth factors. When cocultured with adult hMSC, primary osteoarthritic chondrocytes induced chondrogenesis even in the absence of exogenous growth factors.51 However, this chondrogenic effect of primary osteoarthritic chondrocytes was lost when the cells were passaged for expansion. Implanting cells at a more primitive progenitor status, in which capacity for cell proliferation, as well as further differentiation is still high, may be an attractive strategy for cartilage tissue healing.
Three major challenges facing repair of articular cartilage are generation of hyaline tissue, integration with host tissue, and reproduction of the superficial zone. The most common surgical repair procedures are microfracture, autologous osteochondral grafting, and autologous chondrocyte implantation.7 Microfracture generates a blood clot in the cartilage defect, which contains MSC from the subchondral bone marrow. This clot matures into a fibrocartilage that typically has positive outcomes only in the short term. Fibrocartilage differs from hyaline cartilage since it is low in glycosaminoglycans and contains primarily collagen I (rather than type II). Autologous osteochondral grafting fills the joint defect directly with mature bone and hyaline cartilage. However, integration of the graft with the host tissue is poor to nonexistent, primarily due to the poor intrinsic healing of articular cartilage. Autologous chondrocyte implantation relies on a cell suspension that is injected into the defect to generate repair tissue. While this procedure has been shown to generate hyaline tissue, residual problems are integration with host tissue and reproduction of the superficial zone of articular cartilage. An approach using differentiated ESC that addresses all three major hurdles is therefore, very compelling.
Articular cartilage is immunoprivileged, supporting the clinical feasibility of allogeneic cell therapy for partial-thickness cartilage lesions. Potential sources of chondroprogenitor cells include bone marrow, synovial and adipose tissue, cord blood, amniotic fluid, and Wharton's jelly.11,53–56 However, limitations in isolating and expanding these cells exist. Therefore, hESCs are a very attractive source of allogeneic cells due to the nearly unlimited number of cells available at an immature, more plastic developmental stage. A quick, efficient, convenient, inexpensive, and safe method of deriving chondroprogenitor cells from hESCs capable of differentiating homogenously into chondrocytes and generating zonally structured cartilaginous tissue that integrates seamlessly into arthritic (or injured) human articular cartilage is an extremely useful alternative.
Particularly for arthritis, cells that differentiate into chondrocytes that secrete healthy matrix and reconstruct the superficial zone could provide new therapeutic options. Most importantly and instructive to practitioners of all of the above-mentioned potentially chondrogenic cell types, we report an approach that invokes fundamental developmental principles pertinent to this unique tissue. As noted above, leveraging developmental forces depends on recreating elements of early organogenesis and starting with cells at a primitive mesenchyme stage before their differentiation into chondrocytes as the use of hESCs permits. There are enough unrecognized similarities between cartilage and other organs that make insights into generating structurally appropriate cartilage perhaps instructive for other tissues as well.
Acknowledgments
This work was supported by California Institute for Regenerative Medicine (CIRM) grants (TR1-01216, TR1-01276) and NIH grants (UL1 RR025774, P01 AG007996). The collagen type II antibody used in this study was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The Univetsity of Iowa, Department of Biology, Iowa city, IA 52242.
Disclosure Statement
No competing financial interests exist.
References
- 1.Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2003–2005. MMWR Morb Mortal Wkly Rep 55,1089, 2006 [PubMed] [Google Scholar]
- 2.Fontaine K.R., Haaz S., and Heo M.Projected prevalence of US adults with self-reported doctor-diagnosed arthritis, 2005 to 2050. Clin Rheumatol 26,772, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Tam H.K., Srivastava A., Colwell C.W., Jr., and D'Lima D.D.In vitro model of full-thickness cartilage defect healing. J Orthop Res 25,1136, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Enders J.T., Otto T.J., Peters H.C., Wu J., Hardouin S., Moed B.R., et al. A model for studying human articular cartilage integration in vitro. J Biomed Mater Res 94,509, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Ahmed T.A., and Hincke M.T.Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev 16,305, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Horas U., Pelinkovic D., Herr G., Aigner T., and Schnettler R.Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am 85-A,185, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Hunziker E.B.Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage 10,432, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Grogan S.P., Barbero A., Diaz-Romero J., Cleton-Jansen A.M., Soeder S., Whiteside R., et al. Identification of markers to characterize and sort human articular chondrocytes with enhanced in vitro chondrogenic capacity. Arthritis Rheum 56,586, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Jukes J.M., Moroni L., van Blitterswijk C.A., and de Boer J.Critical steps toward a tissue-engineered cartilage implant using embryonic stem cells. Tissue Eng Part A 14,135, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Hall B.K., and Miyake T.All for one and one for all: condensations and the initiation of skeletal development. Bioessays 22,138, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science 284,143, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Noth U., Steinert A.F., and Tuan R.S.Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol 4,371, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Murphy J.M., Fink D.J., Hunziker E.B., and Barry F.P.Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 48,3464, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Mueller M.B., Fischer M., Zellner J., Berner A., Dienstknecht T., Prantl L., et al. Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-beta isoforms and chondrogenic conditioning. Cells Tissues Organs 192,158, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller M.B., and Tuan R.S.Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum 58,1377, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toh W.S., Lee E.H., and Cao T.Potential of human embryonic stem cells in cartilage tissue engineering and regenerative medicine. Stem Cell Rev 7,544, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Hematti P.Human embryonic stem cell-derived mesenchymal progenitors: an overview. Methods Mol Biol 690,163, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Hwang N.S., Varghese S., Zhang Z., and Elisseeff J.Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng 12,2695, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Lee E.J., Lee H.N., Kang H.J., Kim K.H., Hur J., Cho H.J., et al. Novel embryoid body-based method to derive mesenchymal stem cells from human embryonic stem cells. Tissue Eng Part A 16,705, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Hwang N.S., Varghese S., Lee H.J., Zhang Z., Ye Z., Bae J., et al.In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci U S A 105,20641, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai H.Y., Chen G.A., Mao G.H., Song T.R., and Wang Y.X.Three step derivation of cartilage like tissue from human embryonic stem cells by 2D-3D sequential culture in vitro and further implantation in vivo on alginate/PLGA scaffolds. J Biomed Mater Res 94,539, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Gong G., Ferrari D., Dealy C.N., and Kosher R.A.Direct and progressive differentiation of human embryonic stem cells into the chondrogenic lineage. J Cell Physiol 224,664, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Toh W.S., Lee E.H., Richards M., and Cao T.In vitro derivation of chondrogenic cells from human embryonic stem cells. Methods Mol Biol 584,317, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Lian Q., Lye E., Suan Yeo K., Khia Way Tan E., Salto-Tellez M., Liu T.M., et al. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells 25,425, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Barberi T., Willis L.M., Socci N.D., and Studer L.Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med 2,e161, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trivedi P., and Hematti P.Simultaneous generation of CD34+ primitive hematopoietic cells and CD73+ mesenchymal stem cells from human embryonic stem cells cocultured with murine OP9 stromal cells. Exp Hematol 35,146, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Hwang N.S., Varghese S., and Elisseeff J.Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tissue regeneration. PLoS One 3,e2498, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ullmann U., In't Veld P., Gilles C., Sermon K., De Rycke M., Van de Velde H., et al. Epithelial-mesenchymal transition process in human embryonic stem cells cultured in feeder-free conditions. Mol Hum Reprod 13,21, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Mackay A.M., Beck S.C., Murphy J.M., Barry F.P., Chichester C.O., and Pittenger M.F.Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 4,415, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Johnstone B., Hering T.M., Caplan A.I., Goldberg V.M., and Yoo J.U.In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238,265, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Oldershaw R.A., Baxter M.A., Lowe E.T., Bates N., Grady L.M., Soncin F., et al. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol 28,1187, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Lee S.Y., Niikura T., and Reddi A.H.Superficial zone protein (lubricin) in the different tissue compartments of the knee joint: modulation by transforming growth factor beta 1 and interleukin-1 beta. Tissue Eng Part A 14,1799, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Grassel S., and Ahmed N.Influence of cellular microenvironment and paracrine signals on chondrogenic differentiation. Front Biosci 12,4946, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Vats A., Bielby R.C., Tolley N., Dickinson S.C., Boccaccini A.R., Hollander A.P., et al. Chondrogenic differentiation of human embryonic stem cells: the effect of the micro-environment. Tissue Eng 12,1687, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Friedenstein A.J., Chailakhjan R.K., and Lalykina K.S.The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 3,393, 1970 [DOI] [PubMed] [Google Scholar]
- 36.Colter D.C., Class R., DiGirolamo C.M., and Prockop D.J.Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A 97,3213, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakagawa T., Lee S.Y., and Reddi A.H.Induction of chondrogenesis from human embryonic stem cells without embryoid body formation by bone morphogenetic protein 7 and transforming growth factor beta1. Arthritis Rheum 60,3686, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Engler A.J., Sen S., Sweeney H.L., and Discher D.E.Matrix elasticity directs stem cell lineage specification. Cell 126,677, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Tse J.R., and Engler A.J.Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS One 6,e15978, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young J.L., and Engler A.J.Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 32,1002, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang K.G., Saris D.B., Geuze R.E., Helm Y.J., Rijen M.H., Verbout A.J., et al. Impact of expansion and redifferentiation conditions on chondrogenic capacity of cultured chondrocytes. Tissue Eng 12,2435, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Yang W., Gomes R.R., Brown A.J., Burdett A.R., Alicknavitch M., Farach-Carson M.C., et al. Chondrogenic differentiation on perlecan domain I, collagen II, and bone morphogenetic protein-2-based matrices. Tissue Eng 12,2009, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veilleux N.H., Yannas I.V., and Spector M.Effect of passage number and collagen type on the proliferative, biosynthetic, and contractile activity of adult canine articular chondrocytes in type I and II collagen-glycosaminoglycan matrices in vitro. Tissue Eng 10,119, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Shin H., Jo S., and Mikos A.G.Biomimetic materials for tissue engineering. Biomaterials 24,4353, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Yang W.D., Gomes R.R., Jr., Alicknavitch M., Farach-Carson M.C., and Carson D.D.Perlecan domain I promotes fibroblast growth factor 2 delivery in collagen I fibril scaffolds. Tissue Eng 11,76, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gustafsson E., Aszodi A., Ortega N., Hunziker E.B., Denker H.W., Werb Z., et al. Role of collagen type II and perlecan in skeletal development. Ann N Y Acad Sci 995,140, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Poole A.R., Kojima T., Yasuda T., Mwale F., Kobayashi M., and Laverty S.Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Related Res 391 Suppl,S26, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Aszodi A., Bateman J.F., Gustafsson E., Boot-Handford R., and Fassler R.Mammalian skeletogenesis and extracellular matrix: what can we learn from knockout mice? Cell Struct Funct 25,73, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Zhu Y., Oganesian A., Keene D.R., and Sandell L.J.Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-beta1 and BMP-2. J Cell Biol 144,1069, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barbero A., Grogan S.P., Mainil-Varlet P., and Martin I.Expansion on specific substrates regulates the phenotype and differentiation capacity of human articular chondrocytes. J Cell Biochem 98,1140, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Aung A., Gupta G., Majid G., and Varghese S.Osteoarthritic chondrocyte-secreted morphogens induce chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheum 63,148, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hwang N.S., Varghese S., Puleo C., Zhang Z., and Elisseeff J.Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J Cell Physiol 212,281, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Estes B.T., Diekman B.O., Gimble J.M., and Guilak F.Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc 5,1294, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fong C.Y., Subramanian A., Gauthaman K., Venugopal J., Biswas A., Ramakrishna S., et al. Human umbilical cord Wharton's jelly stem cells undergo enhanced chondrogenic differentiation when grown on nanofibrous scaffolds and in a sequential two-stage culture medium environment. Stem Cell Rev 8,195, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Kolambkar Y.M., Peister A., Soker S., Atala A., and Guldberg R.E.Chondrogenic differentiation of amniotic fluid-derived stem cells. J Mol Histol 38,405, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Wang J.F., Wang L.J., Wu Y.F., Xiang Y., Xie C.G., Jia B.B., et al. Mesenchymal stem/progenitor cells in human umbilical cord blood as support for ex vivo expansion of CD34(+) hematopoietic stem cells and for chondrogenic differentiation. Haematologica 89,837, 2004 [PubMed] [Google Scholar]