Abstract
Background: Over the last decade, annual incidence rates for thyroid cancer have been among the highest of all cancers in the Western world. However, the genomic mechanisms impacting thyroid carcinogenesis remain elusive.
Methods: We employed an established mouse model of follicular thyroid cancer (FTC) with a homozygous proline to valine mutation (ThrbPV/PV) in the thyroid receptor β1 (TRβ1) and applied quantitative three-dimensional (3D) telomere analysis to determine 3D telomeric profiles in ThrbPV/PV, ThrbPV/+, and Thrb+/+ mouse thyrocytes before and after histological presentation of FTC.
Results: Using quantitative fluorescent in situ hybridization (Q-FISH) and TeloView™ image analysis, we found altered telomeric signatures specifically in mutant mouse thyrocytes. As early as 1 month of age, ThrbPV/PV mouse thyrocytes showed more telomeres than normal and heterozygous age-matched counterparts. Importantly, at the very early age of 1 month, 3D telomeric profiles of ThrbPV/PV thyrocyte nuclei reveal genetic heterogeneity with several nuclei populations exhibiting different telomere numbers, suggestive of various degrees of aneuploidy within the same animal. This was detected exclusively in ThrbPV/PV mice well before the presentation of histological signs of thyroid carcinoma.
Conclusions: We identified quantitative 3D telomere analysis as a novel tool for early detection and monitoring of thyrocyte chromosomal (in)stability. This technique has the potential to identify human patients at risk for developing thyroid carcinoma.
Introduction
Thyroid cancer is the most common carcinoma of endocrine glands and accounts for approximately 1% of all malignancies. For more than a decade now, thyroid cancer has seen one of the highest incidence rates annually of all cancers in the Western world, with 6.1 cases per 100,000 men and 18.2 cases per 100,000 women. These rates represent a sixfold increase since 1975 (1). A combination of more sensitive detection methods and unknown molecular/environmental factors likely contribute to this higher incidence of thyroid cancer (2). Among the three major groups of thyroid cancer—papillary (PTC), follicular (FTC), and undifferentiated (UTC)—PTC is the most common form, accounting for 80% of all thyroid carcinoma, followed by FTC and UTC at approximately 15% and 2% respectively (2). In 3–7% of cases, PTC and FTC occur as part of a hereditary syndrome (3,4), which involves mutations in the PTEN gene locus (10q22–23) and inactivating mutations in the gene encoding the regulatory subunit 1A of the cAMP-dependent protein kinase (PKAR1A; 2p16, 17q23) resulting in the constitutive activation of protein kinase A (5,6), which has been linked to nonthyroidal tumors in disease states such as Cowden's disease (5). Genetic instability with aneuploidy and allelic loss are common in FTC. Apart from a role for PTEN in FTC (4,7,8), both follicular adenoma and FTC contain mutations in all three RAS genes (H-RAS, N-RAS, K-RAS), with a higher prevalence in FTC. RAS mutations rarely coincide with oncogenic PAX8-PPARγ chromosomal 3p25 and 2q13 rearrangement found exclusively in FTC (9) and the inhibition of PPARγ tumor suppressor function contributes to FTC (10,11).
Thyroid hormone receptor β1 (TRβ1) is a nuclear thyroid hormone receptor and is regulated by triiodothyronine (T3). Mutations of TRβ1 have been found in human thyroid cancer (12). In human FTC and the TRβ1PV mutant mouse model (ThrbPV/PV) of FTC, RAS mutations, PAX8-PPARγ rearrangements, and mutations resulting in the activation of the AKT pathway are important contributors to the pathogenesis of FTC (11,13,14). Similar to human FTC, we have shown that homozygous ThrbPV/PV knock-in mice harbor a dominant negative TRβ1 mutant PV and spontaneously develop FTC and distant metastasis (15). These mice have a human-like thyroid hormone resistance syndrome resulting in the complete loss of T3-binding and transcriptional activation (16). TRβ1PV/PV homozygous mice have enlarged thyroid glands, are resistant to thyroid hormones, and display high serum thyrotropin (TSH; 400–500-fold increase over normal) and thyroid hormone (9–15-fold increase over normal) levels. ThrbPV/PV homozygotes develop highly invasive and metastasizing FTC in more than 80% of animals at 5–6 months of age, whereas no apparent abnormalities are observed in the wild-type Thrb+/+ and heterozygous ThrbPV/+ mice.
Telomeres are repetitive nucleotide sequences (TTAGGGn) at the ends of the chromosomes that protect chromosomes from fusions and illegitimate recombination events, as long as they are capped by a protein complex termed “shelterin” (17). The nuclear organization of telomeres in interphase nuclei is directly linked to chromosomal instability (CIN). We showed that deregulation of c-Myc causes telomere remodelling and promotes CIN and carcinogenesis (18,19). A shelterin complex composed of telomeric repeat binding factor (TRF) 1 and 2, and protection of telomeres (POT) 1 proteins directly interact with the TTAGGG repeat of the telomere sequence, and these proteins are interconnected by TPP1, TIN2, and Rap1 (17). Events leading to alterations in this shelterin complex can result in telomeres with impaired protective functions, which cause chromosomal fusions, continuous breakage–fusion–bridge (BFB) cycles, chromosomal imbalances, and gene amplifications. These events will ultimately lead to the generation of complex nonreciprocal translocations, a hallmark of solid tumours and genomic instability in general (20–23). We previously showed significant differences in telomeric profiles (i.e., in telomere signal intensity and number, the presence/absence of telomeric aggregates, and in the number of telomeres per nuclear volumes) in therapy-sensitive and therapy-resistant Hodgkin's lymphoma patients (24). These results demonstrated the prognostic value of three-dimensional (3D) telomere diagnostic imaging for glioblastoma patients (25), and suggested a 3D telomere dysfunction-based progression model leading to the progression of myelodysplastic syndromes to acute myeloid leukemias (26).
In the present study, we applied quantitative 3D telomere imaging techniques to monitor differences in telomere profiles during FTC in thyrocytes of homozygous ThrbPV/PV mice and compared these data to thyroid tissues from heterozygous ThrbPV/+ and wild type Thrb+/+ mice.
Materials and Methods
Animal work
Animal study protocols were approved by the National Cancer Institute Animal Care and Use Committee. The generation of the ThrbPV/PV mice was described previously (27). Mice harboring the targeted PV mutation are designated as ThrbPV/PV and ThrbPV/+ for homozygous and heterozygous mutant mice respectively. Thyroid tissue sections of Thrb+/+, ThrbPV/+, and ThrbPV/PV animals were investigated for this study. The pathological progression of FTC in ThrbPV/PV mice was described previously (15). At the age of 12.9 months, only 50% of ThrbPV/PV mice were still alive, and 20% of ThrbPV/PV mice survived to the age of 14 months. Histopathologic findings were consistent with FTC progression. Information on animal numbers, sex, and age for each genotype are summarized in Table 1.
Table 1.
Summary of Thrb+/+, ThrbPV/+, and ThrbPV/PV Mice Used in Q-FISH Experiments
| Months | Thrb+/+(male/female) | ThrbPV/+(male/female) | ThrbPV/PV(male/female) |
|---|---|---|---|
| 1–4.9 | 4/2 | 5/4 | 8/8 |
| 5–12+ | 10/7 | 4/6 | 6/5 |
Quantitative fluorescent in situ hybridization
Q-FISH analysis was performed on 5 μm thick sections of mouse thyroid tissues. Tissue sections were deparaffinized in xylene for 15 min and placed for 1 min in 100% ethanol before a 20 min incubation at 80°C in 1 M sodium thiocyanate to remove RNA and tissue impurities causing autofluorescence before fixation (28). Tissues were postfixed with 10% formaldehyde in 1× phosphate buffer solution (PBS) for 20 min and washed twice for 5 min with PBS. Slides were soaked in 0.5% Triton X-100 for 10 min and transferred for 1 h to 20% glycerol. Thereafter, slides were briefly submerged in liquid nitrogen and allowed to thaw prior to submerging the slides in 20% glycerol. This process was repeated four times and then slides were washed in PBS three times for 5 min. The slides were incubated in 0.1 M HCl with pepsin at a final concentration of 50 μg/mL for 10 min at 37°C. Slides were washed in PBS and stored in 70% deionized formamide/2× standard saline citrate (SSC; pH 7.0), 4°C for at least 1 h. Peptide nucleic acid (PNA) telomere probe (Dako, Mississauga, Canada) was applied to the slides, denatured at 80°C for 3 min, and hybridized at 37°C for 2 h in a Hybrite™ hybridization chamber (Abbott, IL). Once the hybridization was completed, slides were washed twice in 70% formamide/10 mM Tris-HCl (pH 7.4) for 15 min each before being washed for 1 min in 1× PBS, then in 0.1× SSC for 5 min and twice for 5 min each in 2× SSC/0.05% Tween-20. Finally, 0.1 μg/mL DAPI was added to the slides before they were submerged in double-distilled H2O and dehydrated in a 70%, 90%, 100% ethanol series for 2 min each and coverslipped with Vectashield (Vector Labs Canada, Burlington, Canada).
Image analysis
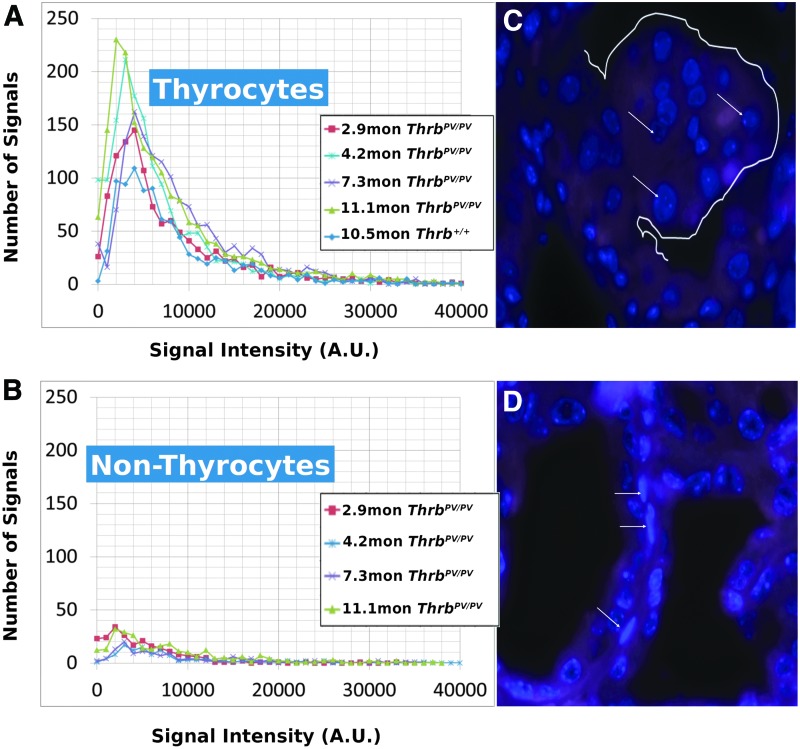
Using a ZeissAxioImager Z1 microscope with a Zeiss AxioCam HRm digital camera (Zeiss, Jena, Germany), 100 nuclear images per mouse sample were acquired. A rhodamine filter with an exposure time of 500 msec was used to image the cy3 labeled telomere probe with a DAPI chromatin counterstain. Images were taken at 100 focal planes (scale lateral: 0.107 μm/pixel; scale axial: 0.2 μm/slice) for each experiment. Images were deconvolved using a constrained iterative algorithm (29). Intensity analysis was performed with TeloView™ (30,31) with the rhodamine signal intensity providing the relative fluorescent intensities of telomere signals that are proportional to telomere size (32). Cells within the thyroid gland tissue that differed distinctly by their elongated cellular and nuclear shape from normal and carcinogenic thyrocytes were imaged as an internal control (Fig. 1B, white arrows) to confirm that changes in 3D telomeric architecture or signal intensity were not common to all cells in the thyroid tissue but specific to thyrocytes. A/C ratio analysis was performed with TeloView™ (30,31), and the rhodamine signal intensity provided relative fluorescent intensities of telomere signals proportional to telomere size (32). The TeloView™ software calculates the center of gravity and the integrated intensity of each telomere and the latter determines the length of the telomere. Telomere aggregates (TA) are larger than single telomeres, represent telomere clusters that cannot be further resolved by conventional microscopy, and show high-intensity hybridization signals. In all samples studied, we were unable to detect TA using TeloView™ software.
FIG. 1.
(A) Intensity measurements of ThrbPV/PV thyrocytes at various ages compared with normal and an example area of a polyp (white outline) in a ThrbPV/PV mouse thyroid section (B) containing thyrocytes (white arrows). (C) Intensity measurements of ThrbPV/PV fibroblasts and an example image in a ThrbPV/PV thyroid (D; white arrows) used as an internal control for telomere intensity and number.
Statistics
p-Values for comparing means were calculated using a two-tailed Student's t-test assuming equal variance. Given that we obtained more samples from one genotype than another for most of our age groups, we elected to express the total number of cells with a given number of signals as a percentage of cells within that genotype, as well as the number of signals with a given intensity per 100 cells in order to prevent favoring larger sample sizes.
Results
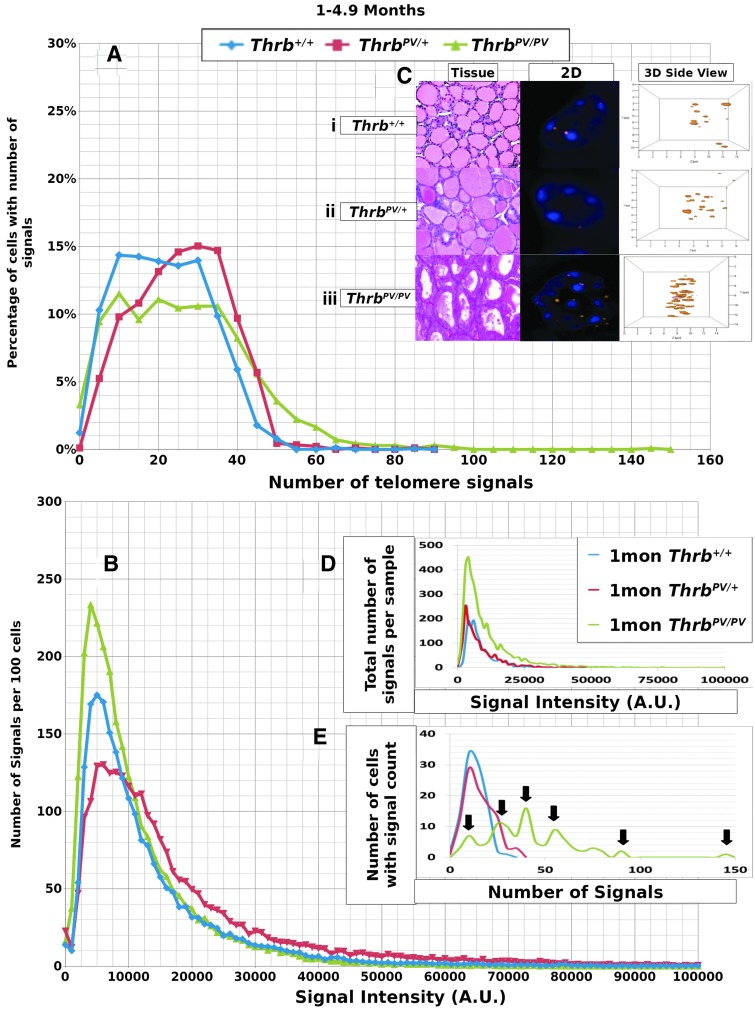
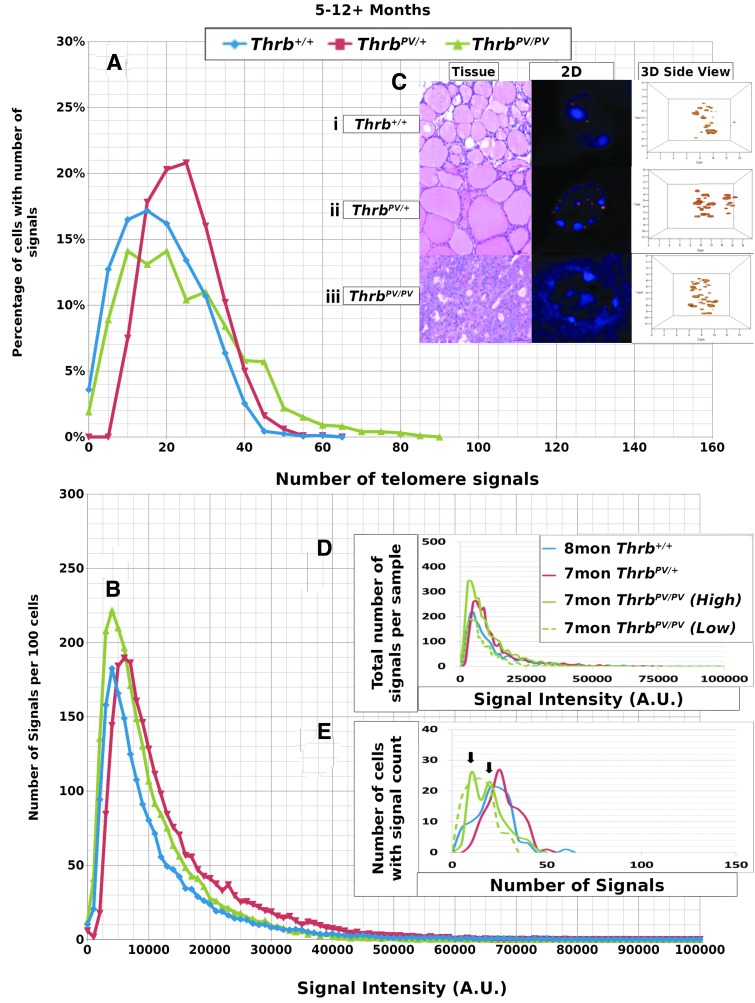
We performed Q-FISH for the detection of telomeres in interphase nuclei on thyroid tissues of ThrbPV/PV, ThrbPV/+, and Thrb+/+ mice between 1 and 13 months of age (Table 1) followed by quantitative 3D image analysis. Independent of the sex of the animals, the Q-FISH telomere analysis revealed changes in mean telomere signal numbers (Table 2) that were specific to thyrocytes (Fig. 1A, B) but were not observed in other nonfollicular cell populations in the thyroid gland as determined by their interfollicular location and nuclear shape (Fig. 1C, D). Of the expected 160 telomeres of mouse chromosomes (four signals per chromosome), 3D telomere FISH and subsequent quantitative analysis using TeloView detects on average 40 separated telomeres in whole interphase nuclei of cultured cells with complete hybridization efficiency as shown in 2D FISH metaphase spreads (30,31). This number is equivalent to previously published findings (33–35). For our analysis, we did not investigate cultured cells but 5 μm tissue sections. We measured a range for thyrocyte nuclear diameters between 4.5 μm and 10.2 μm in hematoxylin and eosin (H&E) sections, indicating that nuclei in tissue sections are not always complete but represent segments of different nuclear volumes. Our analysis confirmed this situation, as we detected on average 21 telomere signals in thyrocyte nuclei of Thrb+/+ controls (Figs. 2A, 3A; Table 2). Thus, we considered nuclear segments in a 5 μm thick section of thyroid tissue having more than 30 telomeric signals as potentially being aneuploid and nuclear segments having more than 40 signals as potentially being near tetraploid or tetraploid. As early as 1 month of age (Fig. 2A–E), thyrocytes in the ThrbPV/PV and ThrbPV/+ group showed larger number of telomere signals compared to Thrb+/+ thyrocytes (Fig. 2C–E; Table 2). Thyrocytes of the ThrbPV/+ and ThrbPV/PV genotypes continued to show a significantly higher average number of telomere signals compared to the Thrb+/+ controls for all age groups studied (Table 2). In both mutants, we observed a higher percentage of nuclei showing higher telomere signals than corresponding controls (Figs. 2A, 3A). Intensity measurements of these signals showed that thyrocyte nuclei of homozygous mutants amassed a larger number of low intensity signals, indicative of shorter telomeres (32), than their normal counterparts in the 1–4.9 months age group (Fig. 2B). However, only thyrocyte nuclei of ThrbPV/PV showed a peak in the number of critically short telomeres in the 1–4.9 months age group (Fig. 2A) and continued to have an increased number of short telomeres in the 5–12+months age group (Fig. 3A). Compared to the controls, the ThrbPV/+ heterozygous mice showed an increased number of telomere signals with higher intensities in the 1–4.9 months (Fig. 2A) and the 5–12+ months age group (Fig. 3A).
Table 2.
Summary of Mean Number of Signals and Statistical Analysis for All Age Groups
| Thrb+/+ | ThrbPV/+ | ThrbPV/PV | |
|---|---|---|---|
| 1–4.9 months | |||
| Mean number of signals | 23.5539 | 28.0067 | 28.3045 |
| SD | 11.2705 | 11.5752 | 16.6082 |
| vs. ThrbPV/+ | p=2.09E–021 | ||
| vs. ThrbPV/PV | p=1.37E–021 | p=0.639 | |
| 5–12+months | |||
| Mean number of signals | 20.4151 | 26.1780 | 26.2810 |
| SD | 10.2038 | 8.6749 | 14.9949 |
| vs. ThrbPV/+ | p=5.90E–48 | ||
| vs. ThrbPV/PV | p=7.71E–32 | p=0.851 | |
SD, standard deviation.
FIG. 2.
Data for 1–4.9 month old mouse thyrocytes. (A) y-Axis indicates the percentage of cells in the given genotypes that have the number of signals shown on the x-axis. This percentage was determined by dividing the number of cells with a specific number of signals by the total number of cells observed for each particular genotype. A shift to the right was observed in the ThrbPV/+ and especially the ThrbPV/PV genotypes and indicates cells with higher numbers of signals. (B) y-Axis indicates the number of signals per 100 cells observed at an intensity shown on the x-axis in arbitrary units (AU). This number was determined by dividing the number of signals observed by the total number of thyroid samples observed for each genotype. A shift upward indicates more signals of lesser intensity for ThrbPV/+ and ThrbPV/PV genotypes, and a shift to the right indicates more signals of higher intensity for ThrbPV/PV. (C) Representative thyroid areas (H&E stained) and individual thyrocyte nuclei selected from each genotype, DAPI nuclear stain with rhodamine-labelled telomere signals, (i) homozygous normal Thrb+/+, (ii) heterozygous ThrbPV/+ mutant, (iii) homozygous ThrbPV/PV mutant, shown as both 2D and 3D side nuclear images of telomere signals. Nuclei with more telomere signals are seen in nuclei of ThrbPV/PV thyrocytes. (D) Data from representative thyroid samples from mice of each genotype displaying signal intensity data. (E) Data from representative thyroid samples of mice for each genotype showing the number of signals per thyrocyte. Arrows indicate separate populations of cells with differing numbers of telomeres in the multipeaked ThrbPV/PV example.
FIG. 3.
Data for thyrocytes of mice sacrificed at 5–12+ months of age. (A) y-axis indicates the percentage of cells in the given genotypes that have the number of signals shown on the x-axis. (B) y-Axis indicates the number of signals per 100 thyrocytes observed at an intensity shown on the x-axis. In nuclei of ThrbPV/PV thyrocytes, a higher numbers of less intense telomere signals were observed, whereas in nuclei of the ThrbPV/+ thyrocytes higher intensity signals were detected than in the nuclei of age-matched controls. (C) Representative H&E stained thyroid tissue sections and individual thyrocyte nuclei taken from each genotype, DAPI nuclear stain with rhodamine-labeled telomere signals, (i) homozygous normal Thrb+/+, (ii) heterozygous ThrbPV/+ mutant, (iii) homozygous ThrbPV/PV mutant, shown as both 2D and 3D side nuclear images of telomere signals. (D) Data from representative mouse thyroid samples of each genotype showing signal intensity versus number of signals. (E) Data from representative mouse thyroid samples of each genotype showing the number of signals per thyrocyte. Arrows highlight multiple ThrbPV/PV thyrocyte populations with specific signal counts.
Data from representative thyroid samples from each genotype for every age group are shown as signal intensity per 100 nuclei (Figs. 2D, 3D) and as the number of nuclei with defined number of telomere signals per 100 thyrocytes (Figs. 2E, 3E). As expected, the graphs in D resembled the curves for the average number of signals analyzed in all samples per age group (Figs. 2B, 3B). When we separated the number of nuclei with defined signal numbers for each animal, ThrbPV/PV mutants revealed a spiked pattern (arrows in Figs. 2E, 3E) resembling several cell populations with different telomere numbers indicative of various degrees of aneuploidy per sample. We observed a small number of cells with more than 80 telomere signals exclusively in ThrbPV/PV thyrocytes at the 1–4.9 months age group (Fig. 2E) and thyrocyte populations showing 50 or more telomere signals per nucleus indicative of the presence of tetraploid thyrocytes. ThrbPV/VP mice at 5–12+ months of age revealed fewer cell populations and reduced number of telomere signals when compared to thyrocytes of ThrbPV/+ or controls (Fig. 3E) and the few ThrbPV/PV mice investigated at the age of 12 months and above appeared to have only two populations. None of the ThrbPV/+ heterozygous mice or the controls presented a spiked pattern in any of the age groups investigated.
We have previously shown that the spherical nature of telomere distribution (the a/c ratio) is associated with the cell cycle phase, with higher ratios indicating more cells at the G2/M boundary and thus a higher overall level of cell proliferation: a/c ratios of 1.4±0.1, 1.5±0.2 and 14±2 refer to nuclei in Go/G1, S, and G2 respectively (30,36). Although we determined a higher a/c ratio in the ThrbPV/PV and the ThrbPV/+, mutants in the 1–4.9 month age group (Supplementary Table S1; Supplementary Data are available online at www.liebert.com/thy), an a/c ratio of just over 2.0 fails to indicate the presence of a telomeric disc at G2/M or a higher rate of cell division. However, the analysis of nuclear segments in tissues rather than complete nuclei (as achievable in cultured cells) may limit the informative value of the a/c parameter. Since the segmental nuclei we analyzed did not reveal an a/c ratio indicative of G2 phase (30), we concluded that more than 40 telomere signals per nucleus reflected near tetraploid or tetraploid thyrocytes.
Discussion
Like other interphase nuclear FISH techniques (37), 3D telomere Q-FISH analysis of interphase nuclei is a highly sensitive method and was used in this study to quantify the length, number of telomeres, presence of TA, and the overall distribution of telomeres (a/c ratio) in individual thyroid epithelial cell nuclei in the ThrbPV/PV mouse model of FTC. In the thyroid gland of ThrbPV/PV mice, alterations in 3D nuclear telomere architecture were exclusively observed in thyrocytes and absent in other cell types within the thyroid gland. The latter finding exemplifies a strength of 3D telomere FISH over Southern blot-based terminal restriction fragment assays (TRF), which also measures contamination of surrounding stromal, vascular, or interstitial cells. Similarly, 2D FISH only assesses telomere signals in a single optical plane and requires metaphases reflective of the proliferative cell population only. By contrast, 3D Q-FISH analysis of interphase nuclei assesses telomere signals in every single cell of the population analyzed.
Our 3D telomere FISH analysis revealed, for the first time, changes in telomere number and length as early as 1 month of age in thyrocytes of ThrbPV/PV mice. Similarly, in 2.4 month old ThrbPV/PV mice, the combined use of histopathology and quantitative 3D Q-FISH revealed the presence of an altered telomere architecture and an increased number of critically short telomeres in thyrocyte aggregates protruding into the thyroid follicular lumen (Fig. 1A, B). All ThrbPV/PV mice investigated revealed an increased average number of total telomeres as early as 1–4.9 months of age (Table 2; Fig. 2A) and showed a higher percentage of cells with short telomeres (Fig. 2B). These results suggest early focal appearance of thyrocytes with abnormal telomere phenotypes which may contribute to FTC in the ThrbPV/PV mouse model. Precancerous stages and cancer can both present with telomere shortening (38,39), and FISH-based detection of shorter telomeres in histological specimens was shown to be an early event in human prostate, pancreas, bladder, large intestine, esophagus, oral cavity, and uterine cervix cancer development (39–41). However, shorter telomeres are not always associated with cancer but have also been described in breast ductal carcinoma in situ and in normal breast secretory cells (38).
Telomere length is an essential parameter of telomere protective function and maintained in various cell types either by telomerase-dependent or alternative telomere lengthening mechanisms (ALT) (42,43). Telomere length characteristics are species-specific, differ considerably between human and mouse (44,45), between mouse strains (44), and in different tissues of the same mouse strain with age. While brain and spleen mouse tissues develop significant changes in telomere length with age, terminal restriction fragment (TRF) failed to detect changes in telomere length in liver, testis, or kidney (44). Our 3D telomere FISH analysis revealed that, up to 12 months of age, the average telomere length in thyrocytes of wild type Thrb+/+ mice with C57BL/J6 genetic background remained relatively stable (see B graphs of all figures). This allowed us to compare telomere profiles across different age groups and genotypes and demonstrate that homozygous ThrbPV/PV mice displayed the highest level of genomic instability followed by the ThrbPV/+ genotype. Although we observed a higher number of shorter telomeres at early age in both ThrbPV/PV and the ThrbPV/+ mutants, the ThrbPV/PV mice exclusively presented with various cell populations with different number of telomere signals (Fig. 2E) within the same animal. This suggests an early phase of telomere instability creating cells with various levels of genomic instability, including tetraploidy and aneuploidy. These alterations in 3D telomere profiles were apparent at very early ages and long before histological signs of FTC (13,15) were detectable in ThrbPV/PV mice. We previously described numerical (ranging from near diploid to near tetraploid) and structural chromosome aberration in thyroid cancer cell lines established from ThrbPV/PV mice (46). The presence of ThrbPV/PV thyrocyte populations with 40 or more telomere signals in several age groups is consistent with the notion that both aneuploid and/or tetraploid cells have tumorigenic properties (47–49). Aneuploidy is common in many human cancers (50,51) and serves as independent prognostic factor for clinical outcome of numerous cancer types, including differentiated and undifferentiated/anaplastic thyroid cancer (52–54).
In mammalian cells, the 3D nuclear position of telomeres is dependent on the cell cycle and cell type. In normal cells, telomeres are found in microterritories (35) and usually do not overlap or create aggregates (31). A telomeric disks is formed during G2 phase of the cell cycle (31) as determined by a substantially increase in the a/c ratio (30). Despite the thyroid hyperplasia in ThrbPV/PV mice (27), we failed to detect differences in the a/c ratio in thyroid epithelial cells of any mouse genotype studied. These results may in part be explained by the fact that, contrary to nuclei of cultured cells which are scanned entirely, it is difficult to assess the entire a/c ratio of telomere distribution in nuclei of tissue samples, since only the nuclear segment as presented within a particular tissue section can be analyzed. We have previously identified TA as a specific signature of tumor cells (19,31) which is independent of telomerase activity and telomere length (18). TA are telomere clusters with stronger intensity hybridization signals that cannot be further resolved as separate signals by conventional microscopy and likely represent end-to-end telomere fusions which cause breakage–fusion–bridge cycles (18,55,56), thus facilitating chromosomal rearrangements.
Telomere shortening was observed in human PTC (57). In our FTC mouse model, we detected the presence of shorter telomeres at a very early stage, suggesting that in FTC, as in other cancer types, alterations to the organization of the 3D nucleus promotes tumorigenesis as a result of chromosomal instability (58). Short and especially critically shortened telomeres represent telomere dysfunction and may form aggregates that likely are not classified as TA by the software, as their cumulative intensity falls below the threshold level for the fluorescence intensity of normal telomeres. In ThrbPV/PV mice, telomere instability is likely to be responsible for the appearance of several cell populations with deletions, gene amplifications, and/or nonreciprocal translocations we described earlier in cell lines derived from ThrbPV/PV tumors (46). Telomere-driven genomic instability in thyroid lesions was also detected by interphase FISH for cytogenetic subtyping of human thyroid lesions, revealing the presence of specific breakpoint chromosomal aberrations (37). Close proximity of telomere territories combined with altered telomere architecture can increase the risk of recombination especially in subtelomeric regions fuelling genomic instability (59). Our findings implicate telomere architectural changes to trigger early onset, continual, and competitive clonal selection of thyrocyte populations throughout follicular carcinogenesis.
Intriguingly, the 3D telomere profile of ThrbPV/+ thyrocytes was different from wild type controls throughout all age groups. The majority of segmental nuclei of ThrbPV/+ heterozygous thyrocytes showed increased average telomere numbers (Table 2), a higher percentage of nuclei with increased telomere signals (Figs. 2A, 3A), and longer telomeres (Figs. 2B, 3B). ThrbPV/+ nuclei lacked the presence of multiple cell populations with different degrees of aneuploidy. Instead, they showed a homogeneous population of aneuploid nuclei with increased numbers of telomere signals (Figs. 2E, 3E). We interpret these observations as an underlying genomic instability in ThrbPV/+ thyrocytes that requires additional stimuli for carcinoma initiation. Indeed, we demonstrated that elevated thyroid hormone levels (60), the lack of a wild type thyroid hormone receptor beta (TRβ) (13), and TSH levels that are elevated beyond a critical threshold level (61) can promote the initiation of FTC in ThrbPV/+ thyrocytes. The exact mechanisms by which thyroid hormones and/or TSH can affect telomere architecture in ThrbPV/+ thyrocytes remains to be elucidated.
In conclusion, 3D telomere FISH analysis was able to detect thyrocyte-specific alterations in telomere architecture starting as early as 1 month of age prior to histological signs of FTC in the ThrbPV/PV mouse model. In male and female ThrbPV/PV mice alike, FTC involved the dynamic selection of thyrocytes with shorter telomeres and aneuploid and tetraploid thyrocyte populations. These telomere changes were absent in heterozygous ThrbPV/+ or control mice. Our findings confirm previous reports identifying 3D telomere FISH as an effective diagnostic tool for early detection of individual cancer cells, tumor subtypes, and suitable prognostic indicator of disease progression (24,25,31,62). Recently, we developed an automated 3D telomere scanning procedure capable of rapid imaging of telomere profiles from interphase nuclei, and specific TeloScan software was shown to identify individual tumor cells in large samples (63). This novel 3D telomere technique will make it feasible to test large numbers of human thyroid tissues in clinical screening rapidly.
Supplementary Material
Acknowledgments
S.H.K. and T.K. are both grateful for financial support from the Natural Sciences and Engineering Council of Canada (NSERC), the Manitoba Institute of Child Health (MICH), and the Department of Surgery Research Fund at the Faculty of Medicine, University of Manitoba. L.W. is the recipient of a Manitoba Health Research Council (MHRC) fellowship and S.M. is supported by the Canadian Institutes of Health Research (CIHR). The authors thank Ms. Mary Cheang for statistical analysis of the data.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.National Cancer Institute 2012 SEER stat fact sheets: thyroid cancer Available online at http://seer.cancer.gov/statfacts/html/thyro.html
- 2.Bhaijee F, Nikiforov YE.2011Molecular analysis of thyroid tumors. Endocr Pathol 22:126–133 [DOI] [PubMed] [Google Scholar]
- 3.Nishisho I, Nakamura Y, Miyoshi Y.1991Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 253:665–669 [DOI] [PubMed] [Google Scholar]
- 4.Liaw D, Marsh D, Li J, Dahia P, Wang S.1997Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nature 16:64–67 [DOI] [PubMed] [Google Scholar]
- 5.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA.2000Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet 26:89–92 [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal GM, Dennis PA.2008PTEN hamartoma tumor syndromes. Eur J Hum Genet 16:1289–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondo T, Ezzat S, Asa SL.2006Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer 6:292–306 [DOI] [PubMed] [Google Scholar]
- 8.Yeager N, Klein-Szanto A, Kimura S, Di Cristofano A.2007Pten loss in the mouse thyroid causes goiter and follicular adenomas: insights into thyroid function and Cowden disease pathogenesis. Cancer Res 67:959–966 [DOI] [PubMed] [Google Scholar]
- 9.Nikiforova MN.2003RAS point mutations and PAX8-PPARgamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab 88:2318–2326 [DOI] [PubMed] [Google Scholar]
- 10.Kroll TG.2000PAX8-PPARgamma 1 fusion in oncogene human thyroid carcinoma. Science 289:1357–1360 [DOI] [PubMed] [Google Scholar]
- 11.Kato Y, Ying H, Zhao L, Furuya F, Araki O, Willingham MC, Cheng SY.2006PPARgamma insufficiency promotes follicular thyroid carcinogenesis via activation of the nuclear factor-kappaB signaling pathway. Oncogene 25:2736–2747 [DOI] [PubMed] [Google Scholar]
- 12.Puzianowska-Kuznicka M, Krystyniak A, Madej A, Cheng S-Y, Nauman J.2002Functionally impaired TR mutants are present in thyroid papillary cancer. J Clin Endocrinol Metab 87:1120–1128 [DOI] [PubMed] [Google Scholar]
- 13.Kato Y, Ying H, Willingham MC, Cheng S-Y.2004A tumor suppressor role for thyroid hormone beta receptor in a mouse model of thyroid carcinogenesis. Endocrinology 145:4430–4438 [DOI] [PubMed] [Google Scholar]
- 14.Araki O, Ying H, Furuya F.2005Thyroid hormone receptor beta mutants: dominant negative regulators of peroxisome proliferator-activated receptor gamma action. Proc Natl Acad Sci USA 102:16251–16256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki H, Willingham M, Cheng S.2002Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid 12:963–969 [DOI] [PubMed] [Google Scholar]
- 16.Ferrara AM, Onigata K, Ercan O, Woodhead H, Weiss RE, Refetoff S.2012Homozygous thyroid hormone receptor β-gene mutations in resistance to thyroid hormone: three new cases and review of the literature. J Clin Endocrinol Metab 97:1328–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Lange T.2005Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 19:2100–2110 [DOI] [PubMed] [Google Scholar]
- 18.Louis SF, Vermolen BJ, Garini Y, Young IT, Guffei A, Lichtensztejn Z, Kuttler F, Chuang TC, Moshir S, Mougey V, Chuang AY, Kerr PD, Fest T, Boukamp P, Mai S.2005c-Myc induces chromosomal rearrangements through telomere and chromosome remodeling in the interphase nucleus. Proc Natl Acad Sci USA 102:9613–9618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mai S, Garini Y.2006The significance of telomeric aggregates in the interphase nuclei of tumor cells. J Cell Biochem 97:904–915 [DOI] [PubMed] [Google Scholar]
- 20.Desmaze C, Soria J-C, Freulet-Marrière M-A, Mathieu N, Sabatier L.2003Telomere-driven genomic instability in cancer cells. Cancer Lett 194:173–182 [DOI] [PubMed] [Google Scholar]
- 21.Gollin SM.2008Mechanisms leading to nonrandom, nonhomologous chromosomal translocations in leukemia. Semin Cancer Biol 17:74–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuttler F, Mai S.2007Formation of non-random extrachromosomal elements during development, differentiation and oncogenesis. Semin Cancer Biol 17:56–64 [DOI] [PubMed] [Google Scholar]
- 23.Mai S.2010Initiation of telomere-mediated chromosomal rearrangements in cancer. J Cell Biochem 109:1095–1102 [DOI] [PubMed] [Google Scholar]
- 24.Knecht H, Kongruttanachok N, Sawan B, Turcotte E, Lichtensztejn Z, Lichtensztejn D, Mai S.2012Three-dimensional telomere signatures of Hodgkin- and Reed-Sternberg cells at diagnosis identify patients with poor response to conventional chemotherapy. Transl Oncol 5:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gadji M, Fortin D, Tsanaclis A.2010Three-dimensional nuclear telomere architecture is associated with differential time to progression and overall survival in glioblastoma patients. Neoplasia 12:183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gadji M, Adebayo Awe J, Rodrigues P, Kumar R, Houston DS, Klewes L, Dièye TN, Rego EM, Passetto RF, de Oliveira FM, Mai S.2012Profiling three-dimensional nuclear telomeric architecture of myelodysplastic syndromes and acute myeloid leukemia defines patient subgroups. Clin Cancer Res 18:3293–3304 [DOI] [PubMed] [Google Scholar]
- 27.Kaneshige M, Kaneshige K, Zhu X, Dace a, Garrett L, Carter TA, Kazlauskaite R, Pankratz DG, Wynshaw-Boris A, Refetoff S, Weintraub B, Willingham MC, Barlow C, Cheng S.2000Mice with a targeted mutation in the thyroid hormone beta receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci USA 97:13209–13214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopman AH, Van Hooren E, Van De Kaa CA, Vooijs PG, Ramaekers FC.1991Detection of numerical chromosome aberrations using in situ hybridization in paraffin sections of routinely processed bladder cancers. Mod Pathol 4:503–513 [PubMed] [Google Scholar]
- 29.Schaefer LH, Schuster D, Herz H.2001Generalized approach for accelerated maximum likelihood based image restoration applied to three-dimensional fluorescence microscopy. J Microscopy 204:99–107 [DOI] [PubMed] [Google Scholar]
- 30.Vermolen BJ, Garini Y, Mai S, Mougey V, Fest T, Chuang TC, Wark L, Young IT.2005Characterizing the three-dimensional organization of telomeres. Cytometry 6:144–150 [DOI] [PubMed] [Google Scholar]
- 31.Chuang T, Moshir S, Garini Y, Ya- A, Chuang C, Young IT, Vermolen B, van den Doel R, Mougey V, Perrin M, Braun M, Kerr PD, Fest T, Boukamp P, Mai S.2004The three-dimensional organization of telomeres in the nucleus of mammalian cells. BMC Biol 2:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poon SS, Martens UM, Ward RK, Lansdorp PM.1999Telomere length measurements using digital fluorescence microscopy. Cytometry 36:267–278 [DOI] [PubMed] [Google Scholar]
- 33.Nagele RG, Velasco a Q, Anderson WJ, McMahon DJ, Thomson Z, Fazekas J, Wind K, Lee H.2001Telomere associations in interphase nuclei: possible role in maintenance of interphase chromosome topology. J Cell Sci 114:377–388 [DOI] [PubMed] [Google Scholar]
- 34.Weierich C, Brero A, Stein S, Von Hase J, Cremer C, Cremer T, Solovei I.2003Three-dimensional arrangements of centromeres and telomeres in nuclei of human and murine lymphocytes. Chromosome Res 11:485–502 [DOI] [PubMed] [Google Scholar]
- 35.De Vos WH, Hoebe R a, Joss GH, Haffmans W, Baatout S, Van Oostveldt P, Manders EM.2009Controlled light exposure microscopy reveals dynamic telomere microterritories throughout the cell cycle. Cytometry A 75:428–439 [DOI] [PubMed] [Google Scholar]
- 36.Caporali A, Wark L, Vermolen BJ, Garini Y, Mai S.2007Telomeric aggregates and end-to-end chromosomal fusions require myc box II. Oncogene 26:1398–1406 [DOI] [PubMed] [Google Scholar]
- 37.Drieschner N, Rippe V, Laabs A, Dittberner L, Nimzyk R, Junker K, Rommel B, Kiefer Y, Belge G, Bullerdiek J, Sendt W.2011Interphase fluorescence in situ hybridization analysis detects a much higher rate of thyroid tumors with clonal cytogenetic deviations of the main cytogenetic subgroups than conventional cytogenetics. Cancer Genet 204:366–374 [DOI] [PubMed] [Google Scholar]
- 38.Meeker AK, Hicks JL, Iacobuzio-Donahue CA, Montgomery EA, Westra WH, Chan TY, Ronnett BM, De Marzo AM.2004Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin Cancer Res 10:3317–3326 [DOI] [PubMed] [Google Scholar]
- 39.Meeker AK, Hicks JL, Gabrielson E, Strauss WM, De Marzo AM, Argani P.2004Telomere shortening occurs in subsets of normal breast epithelium as well as in situ and invasive carcinoma. Am J Pathol 164:925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meeker AK, Hicks JL, Platz EA, March GE, Bennett CJ, Delannoy MJ, De Marzo AM.2002Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res 62:6405–6409 [PubMed] [Google Scholar]
- 41.Van Heek NT, Meeker AK, Kern SE, Yeo CJ, Lillemoe KD, Cameron JL, Offerhaus GJ, Hicks JL, Wilentz RE, Goggins MG, De Marzo AM, Hruban RH, Maitra A.Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol 161:1541–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cesare AJ, Reddel RR.2010Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet 11:319–330 [DOI] [PubMed] [Google Scholar]
- 43.Shay JW, Wright WE.2011Role of telomeres and telomerase in cancer. Semin Cancer Biol 21:349–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coviello-McLaughlin GM, Prowse KR.1997Telomere length regulation during postnatal development and ageing in Mus spretus. Nucleic Acids Res 25:3051–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA.1999Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96:701–712 [DOI] [PubMed] [Google Scholar]
- 46.Zimonjic DB, Kato Y, Ying H, Popescu NC, Cheng S-Y.2005Chromosomal aberrations in cell lines derived from thyroid tumors spontaneously developed in TRbetaPV/PV mice. Cancer Genet Cytogenet 161:104–109 [DOI] [PubMed] [Google Scholar]
- 47.Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D.2005Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 437:1043–1047 [DOI] [PubMed] [Google Scholar]
- 48.Ganem NJ, Storchova Z, Pellman D.2007Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev 17:157–162 [DOI] [PubMed] [Google Scholar]
- 49.Davoli T, De Lange T.2012Telomere-driven tetraploidization occurs in human cells undergoing crisis and promotes transformation of mouse cells. Cancer Cell 21:765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordon DJ, Resio B, Pellman D.2012Causes and consequences of aneuploidy in cancer. Nat Rev Genet 13:189–203 [DOI] [PubMed] [Google Scholar]
- 51.Jonsdottir AB, Stefansson OA, Bjornsson J, Jonasson JG, Ogmundsdottir HM, Eyfjord JE.2012Tetraploidy in BRCA2 breast tumours. Eur J Cancer 48:305–310 [DOI] [PubMed] [Google Scholar]
- 52.Sturgis CD, Caraway NP, Johnston DA, Sherman SI, Kidd L, Katz RL.1999Image analysis of papillary thyroid carcinoma fine-needle aspirates: significant association between aneuploidy and death from disease. Cancer 87:155–160 [DOI] [PubMed] [Google Scholar]
- 53.Miura D, Wada N, Chin K, Magrane GG, Wong M, Duh Q-Y, Clark OH.2003Anaplastic thyroid cancer: cytogenetic patterns by comparative genomic hybridization. Thyroid 13:283–290 [DOI] [PubMed] [Google Scholar]
- 54.Pinto AE, Silva G, Banito ANA, Leite V, Soares J.2008Aneuploidy and high S-phase as biomarkers of poor clinical outcome in poorly differentiated and anaplastic thyroid carcinoma. Oncol Rep 20:913–919 [PubMed] [Google Scholar]
- 55.McClintock B.1941The stability of broken ends of chromosomes in Zea Mays. Genetics 26:234–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mueller J.1938A synthetic medium for the cultivation of C. diphtheriae. J Baceteriol 36:499–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Capezzone M, Cantara S, Marchisotta S, Busonero G, Formichi C, Benigni M, Capuano S, Toti P, Pazaitou-Panayiotou K, Caruso G, Carli AF, Palummo N, Pacini F.2011Telomere length in neoplastic and nonneoplastic tissues of patients with familial and sporadic papillary thyroid cancer. J Clin Endocrinol Metab 96:1852–1856 [DOI] [PubMed] [Google Scholar]
- 58.Maicher A, Kastner L, Dees M, Luke B.2012Deregulated telomere transcription causes replication-dependent telomere shortening and promotes cellular senescence. Nucleic Acid Res 40:6649–6659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Linardopoulou E, Williams E, Fan Y.2005Human subtelomeres are hot spots of interchromosomal recombination and segmental duplication. Nature 437:94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu C, Zhu X, Willingham MC, Cheng S-Y.2012Activation of tumor cell proliferation by thyroid hormone in a mouse model of follicular thyroid carcinoma. Oncogene 31:2007–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao L, Zhu X, Won Park J, Fozzatti L, Willingham M, Cheng S.2012Role of TSH in the spontaneous development of asymmetrical thyroid carcinoma in mice with a targeted mutation in a single allele of the thyroid hormone-β receptor. Endocrinology 153:5090–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuzyk A, Mai S.2012Selected telomere length changes and aberrant three-dimensional nuclear telomere organization during fast-onset mouse plasmacytomas. Neoplasia 14:344–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klewes L, Höbsch C, Katzir N, Rourke D, Garini Y, Mai S.2011Novel automated three-dimensional genome scanning based on the nuclear architecture of telomeres. Cytometry A 79:159–166 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.