Abstract
Despite the progress made thus far in the generation of small-diameter vascular grafts, cell sourcing still remains a problem. Human embryonic stem cells (hESCs) present an exciting new cell source for the regeneration applications due to their high proliferative and differentiation capabilities. In this study, the feasibility of creating small-diameter vascular constructs using smooth muscle cells (SMCs) differentiated from hESC-derived mesenchymal cells was evaluated. In vitro experiments confirmed the ability of these cells to differentiate into smooth muscle actin- and calponin-expressing SMCs in the presence of known inducers, such as transforming growth factor beta. Human vessel walls were constructed by culturing these cells in a bioreactor system under pulsatile conditions for 8 weeks. Histological analysis showed that vessel grafts had similarities to their native counterparts in terms of cellularity and SMC marker expression. However, markers of cartilage and bone tissue were also detected, thus raising questions about stable lineage commitment during differentiation and calling for more stringent analysis of differentiating cell populations.
Introduction
Cardiovascular disease is a leading cause of death in the United States, necessitating almost 500,000 bypass surgeries each year.1 In most cases, autologous grafts from saphenous veins, mammary arteries, or radial arteries are used. However, due to age, disease, or other complications, autologous veins or arteries are often not available to patients. For the past few decades, several different approaches for culturing arteries for bypass have emerged. Cells from various species have been seeded onto natural (e.g., fibrin)2 or synthetic (e.g., polymer)3 materials in various reactor configurations in the presence of soluble factors and/or mechanical conditioning to make tubular constructs with adequate mechanical properties. Varying degrees of success have been achieved so far. L'Heureux et al.4 have generated mechanically robust implantable vessels by fusing cell layers generated by culturing autologous human fibroblasts over the period of 6–9 months. In a different approach using fibrin gel and human neonatal fibroblasts, Syedain et al. generated collagen-rich vascular grafts with mechanical properties similar to native counterparts.2 A more recent milestone was achieved when Dahl et al. demonstrated the successful application of human cells to generate “off-the-shelf” tissue grafts.5 However, to date, the most successful approaches involving human cells still require long culture times and are particularly challenging when the donors are the elderly. Considering these issues, the search continues for a better cell source that is readily available and produces collagenous matrix more rapidly.
Stem cells present a novel alternative cell source due to their high proliferative and differentiation capabilities. Moreover, adult stem cells and progenitor cells, isolated from diverse sources, such as the bone marrow (BM),6 fat tissue,7 and hair follicles,8,9 have already been tested in engineering small-diameter vascular grafts. Mesenchymal stem cells (MSCs) are a type of adult stem cell that can self-renew and have the ability to differentiate into mesenchymal tissues in the adipogenic, chondrogenic, osteogenic, and myogenic lineages. Several studies have shown that human MSCs can differentiate into smooth muscle cells (SMCs). Various factors, such as growth factors, cell–cell contact, and mechanical stimulation as well as matrix substrate molecules, are known to influence the differentiation of MSCs into SMCs. In our laboratory, we had previously tested the ability of BM-derived mesenchymal cells to generate vascular grafts.10 Over 8 weeks, using a combination of growth factors, such as transforming growth factor beta 1 (TGFβ1), platelet-derived growth factor (PDGF), substrate coating such as fibronectin, and cyclic mechanical strain, human vessel grafts were generated, which contained 22% collagen by dry weight and had burst pressures over 200 mmHg. However, adult MSCs pose several challenges. They have a limited capacity to proliferate and often lose their ability to differentiate when expanded for long time periods ex vivo. Furthermore, aging and age-related disorders might also severely impair their survival and differentiation potential when used as autologous therapy. All these factors could lead to limited therapeutic efficiency.
Embryonic stem cells (ESCs) are cells that are derived from the preimplantation embryos at the blastocyst stage. Induced pluripotent stem cells (iPSCs) are derived by reprogramming mature adult cells into an embryonic-like state. The uniqueness of these cells lies in their higher proliferative capacity and ability to differentiate into many different lineages, thus making it possible to obtain large numbers of specialized cells types for tissue regeneration. Several groups have also focused on deriving MSCs from human ESCs (hESCs)11–14 and more recently from hiPSCs.15,16 Cells have been derived using various methods that include fluorescence-activated cell sorting (FACS) of CD73+ cells, coculture with OP9 mouse stromal cells, as well as from differentiating embryoid bodies. These MSCs have been shown to have higher proliferative capacity than BM-MSCs. Additionally, they were shown to be more effective in attenuating hindlimb ischemia in a mouse model.16 Additionally, hES-derived MSCs have been tested in vivo, as well as in certain tissue engineering applications, such as functional cartilage17 and tendon18 regeneration.
Due to their enhanced functionality over BM-MSCs, we test the potential of hES-derived MSCs as an alternative cell source for vascular engineering applications. We studied the in vitro SMC differentiation potential of hES-derived MSCs in the presence of growth factors, such as TGFβ1. In addition, we utilized SMCs differentiated from hES-MSCs to engineer vessel walls in a biomimetic system that involves long-term culture in a bioreactor under pulsatile strain. We examined the in vitro differentiated cells as well as the engineered vessel grafts for SMC marker expression and performed histological analysis on the vessel constructs. Overall, hES-derived MSCs proved to be a valuable cell source leading to the creation of collagen-rich vessel walls that contained SMC-like cells. However, our studies also revealed unexpected chondrogenic and osteogenic marker expression, raising questions about the stable lineage commitment of differentiating stem cells.
Materials and Methods
All cell culture reagents were obtained from Life Technologies, unless stated otherwise. Detailed information of all antibodies utilized in the study is provided in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/tea).
Isolation and cell culture of hES-MSCs
hES H1 cells were differentiated into mesenchymal cells by a previously published protocol.13,19 Briefly, hES H1 cells were regularly maintained on mouse embryonic fibroblast layers in a 5% O2 and 7.5% CO2 conditions. Spontaneously differentiating cells or “raclures” that appear at the edges of hES colonies were scraped, collected, and re-plated in a medium containing Dulbecco's modified Eagle's medium (DMEM), 10% fetal bovine serum (FBS), 1% penicillin–streptomycin (P/S), 1% nonessential amino acids, for several weeks until a thick cell layer was formed. While several cell isolates were derived by this method, one such isolate called P51R was utilized in this study.
hES-MSCs were routinely cultured in tissue-cultured treated flasks in the DMEM supplemented with 10% lot selected Hyclone FBS (Thermoscientific, Inc.) and 1% P/S (growth medium). The screening of FBS lot was based on the support of cell proliferation and low levels of TGFβ1 to attenuate differentiation and support the maintenance of undifferentiated state. Cells were also cultured in commercially available reduced serum medium MesenPro RS. Cultures were maintained at 37°C in a humidified environment containing 5% CO2. The medium was replaced twice a week, and the cultures were passaged when cells reached 80% confluency approximately once a week.
Confirmation of hES-MSC phenotype by FACS analysis
Cells were tested for their mesenchymal identity by flow cytometry for MSC markers. Fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD45, FITC-conjugated CD34, phycoerythrin-conjugated CD105, and allophycocyanine-conjugated CD90 were utilized in the study. Single-cell solutions were prepared as described previously (10). FACS was performed on the FACS Calibur cytometer (Becton Dickinson), and the data were analyzed using FlowJo software.
Multilineage potential of hES-MSCs
Mesenchymal cells are commonly defined by their ability to differentiate into multiple lineages giving rise to osteocytes, adipocytes, and chondrocytes. Cells were seeded in well plates and induced to osteogenic and adipogenic lineages using commercial media supplements as described previously.10
Induction of hES-MSCs into SMCs
Cells were initially maintained in the MesenPro medium until the point of differentiation. To determine the conditions for optimal differentiation, cells were seeded at 5.6×103 cells/cm2 in six-well plates in a basal medium (DMEM supplemented with 2% FBS and 1% P/S). The following day, SMC differentiation was initiated by increasing the amount of FBS in the DMEM from 2% to 5% or 10% or by adding recombinant human TGFβ1 (R&D systems) (0, 0.01, or 1 ng/mL) in the DMEM with 2% FBS. Serum utilized for differentiation experiments was lot selected for high TGFβ1 levels to promote SMC growth. Cells were cultured for 14 days under various conditions, and the medium was replaced twice a week. TGFβ1, if applicable, was added at each media change. As a control, cells were grown in parallel in the basal DMEM with 2% FBS and 1% P/S (no growth factors added).
TGFβ1/ALK5 signaling
hES-MSCs were plated in six-well plates at 0.5×106 cells/well in the regular growth medium. Twenty-four hours before the start of the experiment, cells were serum-starved (DMEM/1% P/S without addition of any serum). In some wells, the ALK5-specific inhibitor SB525334 (Tocris, Inc.) was added to block the ALK5 pathway at this time. At t=0, the starvation medium was replaced by the medium containing 1% FBS, and this was maintained for the duration of the experiment. Additionally, TGFβ1 was added to the cells at a concentration of 1 ng/mL. At desired time points of 24 or 72 h, RNA was collected from the cells, whereas some cells were fixed and stained for SMC markers.
Collagen I gel contraction assay
Rat tail collagen I (Life Technologies) was prepared as recommended by the manufacturer to a concentration of 4 mg/mL. A single-cell suspension of hES-MSCs was prepared in the collagen gel solution at a cell density of 1.5–2×105 cells/mL. The pH of the final gel solution was measured to ensure that it remained at 7 for optimal polymerization. A 250 μL volume was spotted onto plastic Petri dishes and allowed to polymerize for 1 h at 37°C. After polymerization had occurred, the spot was gently lifted using a cell scraper and transferred to a six-well plate. The medium was added to each well (3 mL per well) and the constructs were cultured for 5 days, with media change every other day. “Differentiation medium” refers to a solution of the DMEM supplemented with 10% lot selected FBS and 1% P/S. Gross images were acquired at days 2 and 5. The contraction of the gel was assessed first by calibrating the image to the scale bar present in each image. Then, the average of the construct length in two perpendicular directions was taken using ImageJ program and the circular area was calculated. The average area of the conditions was normalized to the control (gel without cells).
Enzyme-linked immunosorbent assays
The media concentrations of TGFβ1 were measured using a Quantikine Human TGFβ1 ELISA kit (R&D Systems). Different media conditions (differentiation media, growth media; MesenPro) were run in triplicate. After assay was completed, the optical density of each well was measured using a Synergy HT multimode microplate reader (Biotek, Inc.). Readings of 570 nm were subtracted from 450 nm readings to obtain the average corrected OD, as described by the manufacturer.
Immunofluorescence studies
Staining for alpha smooth muscle actin (αSMA) and calponin was performed on day 7 of SMC induction for cells exposed to 1 ng/mL TGFβ1 in the DMEM supplemented with 2% FBS and 1% P/S. Control cells were grown in the MesenPro RS.
Cells were fixed for 20 min in 4% paraformaldehyde at room temperature and then stained and visualized under a fluorescent microscope as previously described.10 Primary antibodies were utilized at 1:200 for αSMA and 1:100 for calponin.
Small-diameter vessel culture
Basic engineering protocol
Human blood vessel grafts were engineered using techniques similar to those described previously.3,5,20
The blood vessel bioreactors and the polyglycolic acid (PGA) polymer mesh scaffolds were prepared as previously described.10,21 Treated PGA sheets (length ∼8.5–9 cm) were sutured around 1-mm silicone tubing, and each PGA scaffold was seeded with 5×106 differentiated SMCs. The reactors were filled with high-glucose DMEM supplemented with 20% FBS, CuSO4 (3 ng/mL), proline (50 μg/mL), alanine (20 μg/mL), glycine (50 μg/mL), and basic fibroblast growth factor (bFGF) (5 ng/mL). Half of the medium volume was replaced every 5 days with freshly prepared medium. Ascorbic acid (50 μg/mL) was added to the reactor three times a week. A week after seeding the cells in the reactor, a pulsatile perfusion system was applied to introduce ∼5% cyclic strain. The vessel culture was continued for 8 weeks, at which time, the vessels were harvested from the reactors for analysis.
Analysis of the engineered vessel walls
Each engineered vessel was cut into three pieces. One piece was fixed in 10% neutral buffered formalin and embedded in paraffin. Sections were cut, deparaffinized and stained with hematoxylin and eosin (H&E) and Masson's trichrome for collagen. Immunostaining was performed for proliferating cell nuclear antigen (PCNA), SMC markers (αSMA and calponin), adipogenesis marker (fatty acid binding protein, FABP4), chondrogenesis marker (aggrecan), and osteogenesis marker (osteocalcin). Total protein was extracted from the last piece using RIPA buffer containing protease inhibitor for western blot analysis. For polymerase chain reaction (PCR) analysis, RNA was extracted from formalin-fixed paraffin-embedded tissue sections.
Western blot analysis
Protein lysates were quantified by the Bradford assay, using the Quick Start™ Bradford Dye Reagents according to the manufacturer's protocol. Samples containing 15 μg of protein were separated on a 4–15% Tris–HCl SDS-PAGE precast gel, transferred to polyvinylidene difluoride membranes, and probed as previously described.10 Anti-human primary antibodies were utilized as follows: αSMA=1:800; SM22α=1:10,000; calponin=1:200; GAPDH=1:10,000.
Real-time PCR
RNA was either isolated from cells or from formalin-fixed paraffin-embedded tissue samples using the appropriate RNA extraction kit (Qiagen, Inc.) according to the manufacturer's instructions. RNA concentration and purity was evaluated using the NanoDrop spectrophotometer. cDNA was generated using the iScript cDNA synthesis kit (BioRad, Inc.) according to the manufacturer's instructions. Comparative real-time PCR was performed for αSMA, SM22α, CNN1, OCN, PPARγ, and COL2a using the iQ™ SYBR Green SuperMix (BioRad, Inc.). GAPDH was used as a reference control to normalize equal loading of template cDNA. For positive controls, RNA from human tissue was purchased from OriGene, Inc.
Statistics
Student's t-test was performed where required to show significant differences between conditions. Significant differences between samples (with p<0.05) are marked with an asterisk in each figure. Data are presented as mean±SD.
Results
Generation and characterization of cells
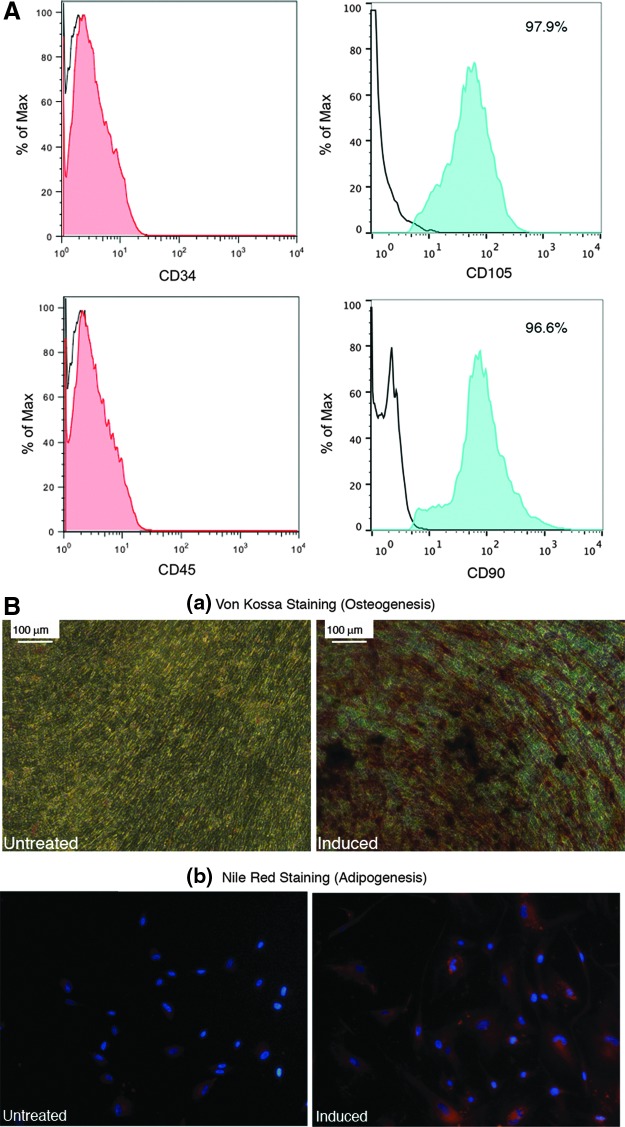
hES H1 cells were differentiated into mesenchymal cells by a previously published protocol19 as described in the Materials and Methods section. In our hands, hES-MSCs displayed a fibroblast-like morphology, were contact inhibited, and formed a cellular monolayer when cultured in tissue culture plastic flasks. We verified the mesenchymal identity of these cells by analyzing their surface antigen expression and found that they express known markers CD90 and CD105, as well as CD73 and CD71 (data not shown) while they remained negative for markers CD34 and CD45 (Fig. 1A). Additionally, we characterized the developmental potential of hES-MSCs by differentiating them into other mesenchymal tissues (Fig. 1B). Bone nodules were formed when the hES-MSCs were differentiated in the commercially available osteogenic medium. In contrast, cells that were not treated with the osteogenic medium did not show any positive staining. Furthermore, when cells were exposed to the adipogenic inducing medium for several weeks, they differentiated into adipocytes that stained positive with Nile Red, a marker for lipid molecules. These tests confirm the mesenchymal identity of hES-MSCs.
FIG. 1.
Characterization of hES-derived MSCs. (A) Flow cytometry analysis of hES-MSCs. Black lines indicate unstained cells. In each plot, the Y-axis represents the number of cells, whereas the X-axis represents the fluorescent intensity. (B) Differentiation potential of hES-MSCs. (a) Von Kossa staining (red-brown color) for osteogenesis. (b) Nile Red lipid staining for adipogenesis. hES, human embryonic stem cell; MSC, mesenchymal stem cells.
TGFβ1 induces differentiation of hES-MSCs into smooth muscle phenotype
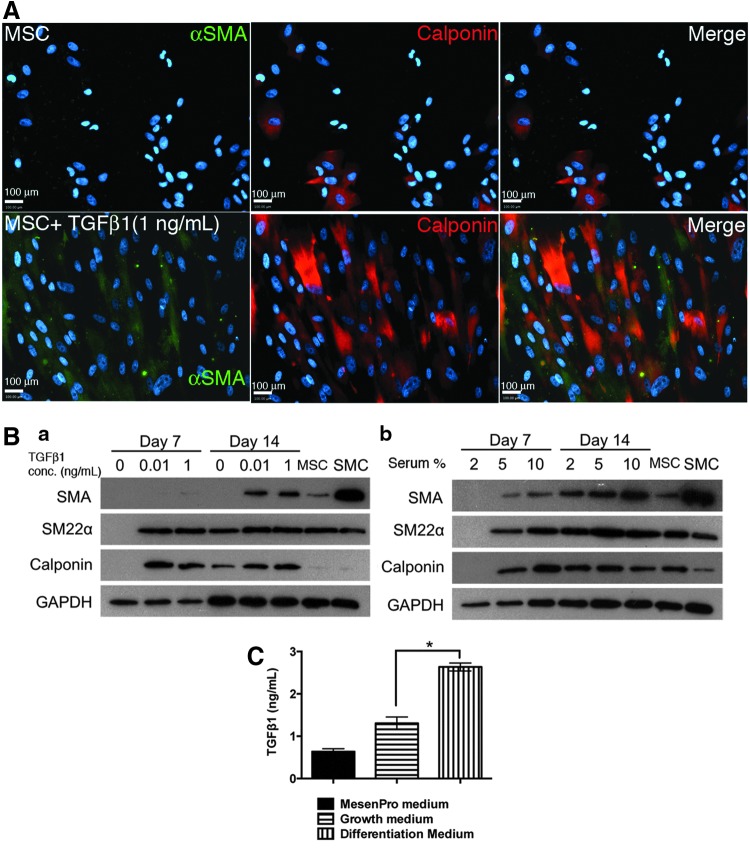
Our previous works with adult BM-derived mesenchymal cells, as well as several other studies, have shown that TGFβ1 is a strong inducer of smooth muscle differentiation. As seen in Figure 2A, we found that TGFβ1 had the potential to differentiate hESC-derived mesenchymal cells toward a smooth muscle lineage. hES-MSCs maintained in the growth medium reveal low levels of αSMA expression. When cells are exposed to TGFβ1 (1 ng/mL) for 7 days, they stained positive for early marker (αSMA) and mid-marker (calponin). Immunostaining clearly indicates that the differentiated cells display prominent contractile filaments, a notable feature of SMCs. To access the extent of SMC differentiation, we performed western blot analysis for contractile protein expression. hES-MSCs expressed SMC proteins in a TGFβ1 dose-dependent manner over a period of 7–14 days [Fig. 2B(a)]. Even at the lowest TGFβ1 concentration of 10 pg/mL, cells express mid-marker calponin as early as day 7. By day 14, cells express all SMC markers, such as αSMA, SM22α, and calponin. Since serum that is commonly utilized for the maintenance of cell cultures also contains varying levels of TGFβ1, we also tested the effects of various serum amounts on the differentiation of hES-MSCs [Fig. 2B(b)]. At the lowest 2% serum concentration, cells did not express any SMC markers at day 7. However, by day 14, we observed the expression of several SMC markers, such as αSMA, SM22α, and calponin. Higher serum concentrations enhanced the rate of SMC differentiation. Exposure to a 5% serum medium led to the expression of all SMC markers as early as day 7. Our data thus suggest that serum is a strong inducer of smooth muscle differentiation. In fact, a 10% serum medium induces SMC marker expression at an earlier time frame, compared to a 1 ng/mL TGFβ1 exposure.
FIG. 2.
TGFβ1 induces the differentiation of hES-MSCs into calponin-positive smooth muscle-like cells. (A) Immunofluorescent staining for SMC markers, such as (1) αSMA and (2) calponin. (B) Western blot analysis for protein detection. Cells treated with various concentrations of (a) TGFβ1 or (b) serum over 7 to 14 days. (C) Enzyme-linked immunosorbent assay (ELISA) for TGFβ1 in various media. *p<0.05. αSMA, alpha smooth muscle actin; SMC, smooth muscle cells; TGFβ1, transforming growth factor beta 1.
To understand the enhanced differentiation observed in the presence of FBS, we performed an enzyme-linked immunosorbent assay (ELISA) for activated TGFβ1 in various media utilized in our studies. The results from the ELISA (Fig. 2C) reveal very high levels (>2.5 ng/mL) of TGFβ1 in the differentiation medium compared to the growth medium. These TGFβ1 levels were higher than the highest TGFβ1 concentration (1 ng/mL) used for the in vitro differentiation studies. The commercially available MesenPro growth medium had the least amounts of TGFβ1 (almost fivefold lower than the differentiation medium). Overall, these results hint at the potent role of TGFβ1 in SMC differentiation, thus providing some explanation for why the differentiation medium was effective in inducing differentiation of MSCs into SMCs.
Functional characterization of hES-MSCs
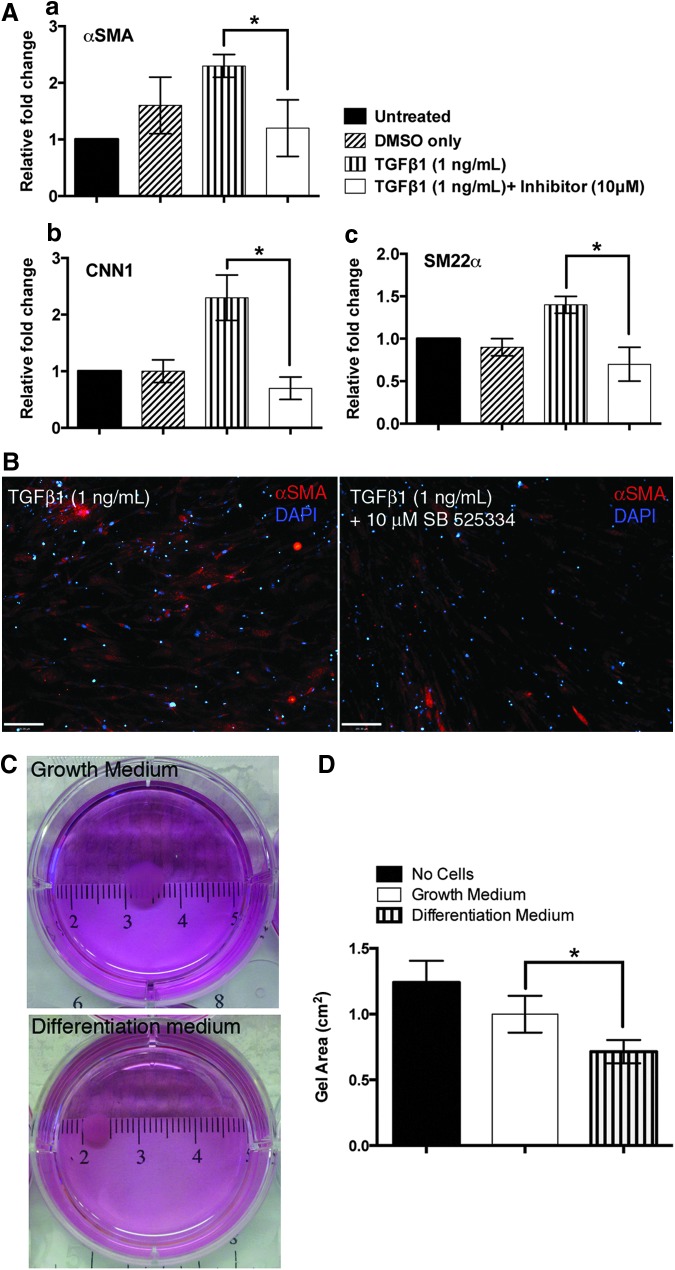
In BM stromal cells22 and SMCs,23 TGFβ1 regulates SMC differentiation by the activation of ALK5 (TGFβ type II receptor) that leads to Smad phosphorylation and further SMC marker expression. To test whether our in vitro differentiated cells possessed the same signaling machinery, we treated hES-MSCs with an ALK5-specific inhibitor,24 SB525334. When cells are exposed to TGFβ1 (1 ng/mL), there is upregulation of several SMC markers, such as αSMA, SM22α, and mid-marker calponin. This indicates that TGFβ1 induces a smooth muscle-like phenotype in hES-MSCs. However, when cells were exposed to the inhibitor SB525334 (10 μM) for 24 h, before exposure to TGFβ1, the expression of SMC markers is significantly decreased, as seen both by PCR (Fig. 3A) and by immunofluorescence (Fig. 3B). We observed these results for αSMA, SM22α, as well as calponin. Overall, these results provide evidence that hES-MSCs possess the TGFβ signaling mechanism that has been documented previously to be involved in SMC differentiation.14,25
FIG. 3.
Functional characterization of hES-MSCs. (A, B) TGFβ1/ALK5 pathway is active in hES-MSCs. (A) Real-time PCR data (normalized to GAPDH) for various SMC genes: (a) αSMA, (b) CNN1, (c) SM22α. (B) Downregulation of αSMA in the presence of ALK5 inhibitor by immunostaining. (C, D) hES-MSCs display a contractile phenotype. (C) Gross images of seeded collagen gels. (D) Quantitation of gel surface area. *p<0.05. PCR, polymerase chain reaction.
Additionally, one of the characteristics of mesenchymal cells is their ability to exert contractile forces on the surrounding collagenous connective tissue. This property is generally evaluated experimentally by the contraction of collagen gels.26,27 When hES-MSCs were seeded in collagen I gels, shrinkage was observed depending on the culture conditions as seen in the gross images of the seeded collagen gels (Fig. 3C). Particularly, when seeded gels were cultured under normal growth conditions (that maintain the mesenchymal phenotype), gels contracted by ∼20% after 4 days, compared to control gels that had no cells seeded in them (Fig. 3D). In contrast, when cells were seeded in the gels and maintained under differentiation conditions (that induce the contractile phenotype), the gels contracted even more, by ∼50% compared to the unseeded control gels. Collectively, these results show that hES-MSCs possess the ability to exert different contractile forces depending on the media conditions.
Creating a vascular construct using hES-MSCs
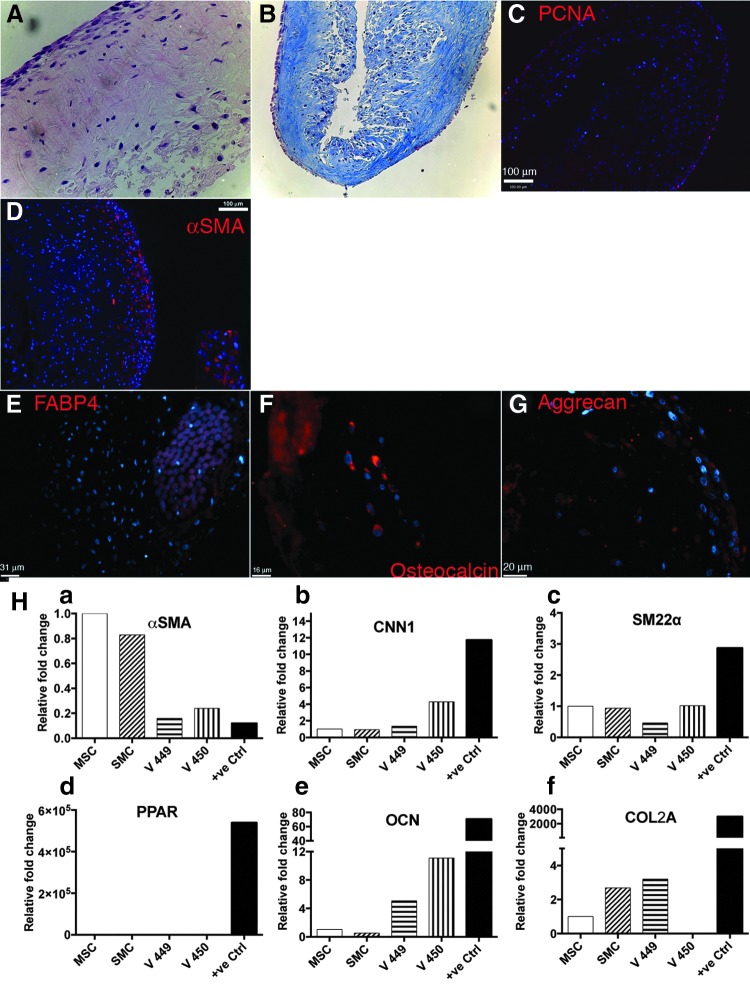
We cultured engineered vascular tissue using a previously published protocol in a bioreactor system under pulsatile conditions. Four vessels were generated in two separate bioreactors (two vessels per reactor). The cells were grown in a static condition for the first 2 weeks, after which pulsatile strain was introduced. After a total of 8 weeks of culture in the bioreactor system, the histological appearance (H&E staining) showed cellularity throughout the thickness of the vessel (Fig. 4A). More cells were detected closer to the surface of the vessel than the lumen. Masson's trichrome staining also revealed the production of collagen (Fig. 4B). Small remnants of the PGA were seen closer to the lumen indicating that the polymer had not completed degraded. Verhoeff Van-Gieson stain did not reveal any deposition of elastin (data not shown). Additionally, PCNA staining revealed that the cells were mostly actively dividing and proliferating (Fig. 4C). All vessels expressed αSMA by immunostaining (Fig. 4D). Most of the positive αSMA staining was found closer to the outer surface of the vessel. No calponin staining was detected (data not shown). Taken together, these results indicate that the cells that were seeded onto the PGA mesh and cultured in the bioreactor were able to proliferate and differentiate into smooth muscle phenotype while also laying down collagenous matrix.
FIG. 4.
Blood vessels engineered from differentiated hES-MSCs in a pulsatile bioreactor for 8 weeks. (A) Hematoxylin and eosin; (B) Masson's trichrome staining; (C) PCNA staining; (D–G) immunofluorescent staining of engineered tissue. (H) PCR profiles of engineered tissue (MSCs and SMCs cultured in vitro before bioreactor culture, V449- and V450-engineered tissue from separate experiments) for genes (a) αSMA, (b) CNN1, (c) SM22α, (d) PPAR, (e) OCN, and (f) COL2A. PCNA, proliferating cell nuclear antigen.
Since not all cells in the tissue construct stained positive for SMC markers, we were interested in understanding the phenotype of these cells that did not have a SMC phenotype. To account for possible dedifferentiation of hES-MSCs, we stained for additional mesenchymal tissue lineages, such as adipocytes, chondrocytes, and osteocytes. While the adipogenic marker FABP4 was not detected (Fig. 4E), we detected some positive staining for osteocalcin, a marker for osteoblasts (Fig. 4F), and aggrecan, a marker for chondrocytes (Fig. 4G). The staining distribution was detected closer to the lumen in the cellular population that mostly did not stain positive for αSMA. To further confirm the nature of the cells in the engineered vessel, we created gene expression profiles of cells in the engineered tissue and compared them to those of the starting cellular populations before bioreactor culture, namely hES-MSCs and SMCs (14 day in vitro differentiation of hES-MSCs). PCR analysis (Fig. 4H) showed that indeed our engineered vessels had upregulated expression of early SMC markers, such as αSMA and SM22α as well as mid-marker calponin, thus confirming the presence of smooth muscle-like phenotype. However, similar to our observations by immunostaining, we also detected upregulation of OCN (osteogenic marker) and COL2a (chondrogenic marker). There was no positive upregulation of adipogenic marker PPARγ. Collectively, these results show that our engineered vessel construct mainly composed of smooth muscle-like cells. However, some other mesenchymal lineages, such as osteoblasts and chondrocytes, were also detected.
Discussion
In this study, we have evaluated the potential of hES-derived MSCs as a novel cell source for the generation of vascular tissue constructs. First, we confirmed the mesenchymal identity of hES-MSCs by common surface marker expression, as well as their ability to differentiate into mesenchymal lineages, such as adipocytes and osteocytes. Furthermore, we utilized TGFβ1, a known strong inducer of smooth muscle phenotype, to differentiate hES-MSCs into αSMA- and calponin-positive smooth muscle-like cells in vitro. An analysis of the major signaling pathway involved in SMC differentiation revealed that the TGFβ1/ALK5 pathway is indeed active in the hES-MSCs. These cells also demonstrate the ability to contract collagen gels, thus providing evidence for the ability to generate a contractile cellular phenotype. Overall, these studies confirmed the mesenchymal identity of the human embryonic-derived MSCs and provided evidence for the ability of these cells to contribute to a contractile myogenic phenotype.
We then proceeded to test the potential of these cells to engineer tissue grafts in a bioreactor system. After 8 weeks of culture, our experiments resulted in tissue-like constructs that displayed good cellularity, stained positive for SMC markers such as αSMA, and also revealed the deposition of extracellular matrix (ECM) molecules such as collagen. These results show that hES-MSCs were capable to divide, proliferate, and lay down collagenous matrix over long-term culture periods, leading to generation of connective tissue-like constructs.
Studies to date have shown that it is possible to differentiate adult mesenchymal as well human embryonic-derived and human pluripotent cells into functional SMCs.10,15,28,29 Some of the cues that have proven to be significant in the differentiation of MSCs into smooth muscle phenotype include growth factors, such as TGFβ1, PDGFBB, and ECM proteins as well as the application mechanical strain. Of all these factors, TGFβ1 is a well-known strong inducer of smooth muscle differentiation. The role of TGFβ1 in the regulation, proliferation, and differentiation of SMCs is well documented. It has been shown that TGFβ1 promotes vessel maturation by ECM production and by inducing differentiation of mural cells in mammalian development. Our own previous work with BM-MSCs showed that TGFβ1 enabled differentiation into αSMA- and calponin-postitive SMCs.10 Others have also utilized TGFβ1 in conjunction with other molecules, such as bone morphogenetic protein 4 (BMP4)7 or PDGFBB,29 to enhance SMC differentiation. Additionally, we also found serum to be a strong inducer of SMC differentiation. Some reports14 suggest no influence of serum on cellular differentiation, although TGFβ1 strongly induced the expression of αSMA, calponin, and SM22α in these studies. This could possibly be attributed to serum lot selection. In our own work, we used different lots of serum for the growth and differentiation of MSCs. When probed further using ELISA, we found that the serum that induced SMC differentiation to the greatest extent contained very high levels of TGFβ1 (2.6 ng/mL). This sheds some light on the responsiveness of hES-MSCs to serum. There is evidence in the literature that TGFβ1 induces SMA expression in cell types other than SMCs, such as endothelial cells and myofibroblasts. αSMA is a less specific marker of SMCs as it is expressed in many other cell types, including transiently in cardiac and skeletal myocytes as well as in myofibroblasts.30,31 However, calponin is mainly restricted to vascular smooth muscle phenotype.32,33 Overall, our cells behave similarly to BM-derived mesenchymal cells and differentiate into a SMC phenotype when induced with TGFβ1.
A significant challenge in tissue engineering with stem cells is the maintenance of stable cell lineage commitment. In fact, studies have shown that in vitro differentiation may not guarantee stable lineage commitment and differentiation in vivo. For example, chondrogenic-like MSC pellets when implanted into SCID mice were found to undergo ossification rather than adopting a stable chondrocyte phenotype.34 In a complementary study,35 researchers found that the failure of the maintenance of chondrogenic phenotype in vivo was due to incomplete preimplantation differentiation of the chondrocytes in vitro. In these studies, cells that were differentiated for less than 8 weeks in vitro did not maintain a stable phenotype and underwent partial ossification when implanted in nude rats.
In our experiments, despite the detection of collagen in our engineered constructs, we also detected some chondrogenic and osteogenic marker expression using gene expression analysis and immunostaining, thus raising caution about the lineage commitment of differentiated cells. Although hES-MSCs were differentiated in vitro for 2 weeks and expressed SMC markers before bioreactor culture, it is possible that it would take longer time for the cells to develop all the cellular machinery of mature SMCs The appearance of a somewhat distinct “inner layer” that is positive for chondrogenic markers is indeed very intriguing. It remains unclear whether the SMC transdifferentiated into an osteo-chondrocytic lineage or the microenvironment in the bioreactor caused the SMCs to dedifferentiate into a mesenchymal phenotype. Clearly, the inner layers of cells experience a different differentiating microenvironment than the cell layers on the outside. Several possible explanations remain viable. Enhanced serum phosphate levels have been shown to induce transdifferentiation of mature SMC into osteo-chondrocytic lineages in calcifying arteries.36,37 However, there are no obvious reasons for enhanced phosphate levels in our bioreactor culture. We suspect that mass transfer limitations might play a role. Possibly, the cells on the outer layers experience a greater nutrient-rich environment than the inner layers closer to the lumen. The presence of collagen could have additionally skewed the cells to differentiate toward a chondrogenic lineage. Additionally, PGA hydrolysis has been shown to cause dedifferentiation of SMC in the areas close to any PGA remnants.38 Previous work by Gong and Niklason10 showed that by dividing the culture period into proliferation and differentiation stages, they were able to improve the quality of the vessel grafts significantly. In our reactor system, we utilized a lot selected serum as the main inducer of differentiation and maintenance of the reactor cultures. This serum lot contained high levels of TGFβ1 (as determined by ELISA) favoring SMC differentiation. We utilized increased serum concentrations (20% serum) to maintain the SMCs in a synthetic phenotype.39
Other factors that can further influence the fate of the bioreactor cultures are the growth factor cocktails that the cells are exposed to over the long culture periods. Our reactor culture medium also contained bFGF at a 5 ng/mL concentration. bFGF has been known to enhance the proliferation of SMCs and is thus a favorable growth factor additive for culturing SMC-based vessels.40 However, studies involving MSCs reveal that bFGF antagonizes SMC marker expression10 Some investigators have shown that bFGF in the presence of TGFβ1 reduces αSMA expression in MSCs that are differentiated toward a chondrogenic lineage.41,42 All these factors could have possibly resulted in the dedifferentiation of SMCs cultured in the bioreactor and further resulted in the expression of the “off-target” markers that we observed in some of our cultures. These results highlight the importance of engineering the microenvironment of differentiated stem cells to maintain a stable lineage commitment. Overall, further optimization of bioreactor conditions to control and maintain the desired smooth muscle phenotype is warranted.
In summary, vascular constructs have been engineered using hESC-derived mesenchymal cells. This is the first report of the utilization of hES-derived cells for vascular construct generation. While the engineered tissue displayed some similarities to native tissue in terms of cellular markers and ECM components, several issues remain before PSC-based grafts can be tested in an in vivo setting. First, it will be necessary to optimize the culture conditions to create mechanically strong vascular constructs with a uniform SMC phenotype. Altering the growth factor scheduling, as well as mechanical stimulation, will be necessary to improve the specificity of cellular differentiation under long-term culture conditions. Our studies have raised important questions regarding the “rogue differentiation” of cells into undesirable phenotypes. The elimination of bFGF as well as utilization of BMP inhibitors (to avoid osteogenic–chondrogenic differentiation) might aid the maintenance of SMC phenotype. It also remains unclear whether in vitro differentiation into SMC phenotype provides any particular advantages in tissue graft generation over the direct utilization of MSCs. Longer culture times have the potential to aid in improving the cellular density, production of ECM, and ultimately, the mechanical properties of the vessel constructs. Finally, creating a functional endothelium will be required to counter thrombogenesis, especially in small-diameter in vivo applications. Strategies such as seeding HUVECs or endothelial progenitor cells21 in a perfusion-based system for the development of a confluent and functional endothelium can be utilized to circumvent problems of immune rejection.
Engineering of tissue constructs from ESC populations poses unique challenges. While hESC and hiPSC remain an exciting cell source due to their strong proliferative and differentiation capabilities, careful consideration of their pitfalls is necessary. Controlling differentiation toward specific lineages as well as ensuring the nonexistence of undesired cell populations will be extremely important in the utilization of these cells for tissue regeneration applications.
Conclusion
hESC-derived mesenchymal cells can be utilized as an alternative cell source for vascular tissue engineering. However, careful optimization of growth conditions in the bioreactor will be required to control the differentiation toward required smooth muscle phenotype. Additionally, it will be necessary to ensure nonexistence of undesired cell populations when utilizing stem cells for tissue engineering applications.
Supplementary Material
Acknowledgments
This work was supported by NIH R01 HL083895-06A1 (L.N.); NIH 1P01HL107205-01A1 (Simons); and Connecticut Innovations CT Stem Cell 08-SCB-YSME-025 (L.N.). This material is based on the work supported by the State of Connecticut under the Connecticut Stem Cell Research Grants Program. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the State of Connecticut, the Department of Public Health of the State of Connecticut or Connecticut Innovations, Inc.
Authors' Contributions
S.S.: conception and design, collection and assembly of data, data analysis and interpretation, writing of the article.
A.E.: collection and assembly of data, data analysis and interpretation.
A.S.: collection and assembly of data, data analysis and interpretation.
C.Q.: provision of materials, final approval of the article.
L.N.: conception and design, data analysis and interpretation, final approval of the article, financial support.
Disclosure Statement
L.N. has a financial interest in Humacyte, Inc., a regenerative medicine company. Humacyte did not fund this study, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
References
- 1.Roger V.L., Go A.S., Lloyd-Jones D.M., Benjamin E.J., Berry J.D., Borden W.B., et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 125,e2, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Syedain Z.H., Meier L.A., Bjork J.W., Lee A., and Tranquillo R.T.Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials 32,714, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niklason L.E., Gao J., Abbott W.M., Hirschi K.K., Houser S., Marini R., et al. Functional arteries grown in vitro. Science 284,489, 1999 [DOI] [PubMed] [Google Scholar]
- 4.L'Heureux N., Mcallister T.N., and la Fuente de L.M.Tissue-engineered blood vessel for adult arterial revascularization. N Engl J Med 357,1451, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Dahl S.L.M., Kypson A.P., Lawson J.H., Blum J.L., Strader J.T., Li Y., et al. Readily available tissue-engineered vascular grafts. Sci Transl Med 3,68ra9, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Gong Z., and Niklason L.E.Use of human mesenchymal stem cells as alternative source of smooth muscle cells in vessel engineering. Methods Mol Biol 698,279, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C., Cen L., Yin S., Liu Q., Liu W., Cao Y., et al. A small diameter elastic blood vessel wall prepared under pulsatile conditions from polyglycolic acid mesh and smooth muscle cells differentiated from adipose-derived stem cells. Biomaterials 31,621, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Peng H.F., Liu J.Y., Andreadis S.T., and Swartz D.D.Hair follicle-derived smooth muscle cells and small intestinal submucosa for engineering mechanically robust and vasoreactive vascular media. Tissue Eng Part A 17,981, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J.Y., Peng H.F., and Andreadis S.T.Contractile smooth muscle cells derived from hair-follicle stem cells. Cardiovasc Res 79,24, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Gong Z., and Niklason L.E.Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J 22,1635, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barberi T., Willis L.M., Socci N.D., and Studer L.Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med 2,e161, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vodyanik M.A., Yu J., Zhang X., Tian S., Stewart R., Thomson J.A., et al. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell 7,718, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivier E.N., Rybicki A.C., and Bouhassira E.E.Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells 24,1914, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Boyd N.L., Nunes S.S., Jokinen J.D., Krishnan L.N., Chen Y., Smith K.H., et al. Microvascular mural cell functionality of human embryonic stem cell derived mesenchymal cells. Tissue Eng Part A 17,1537, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajpai V.K., Mistriotis P., Loh Y.-H., Daley G.Q., and Andreadis S.T.Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates. Cardiovasc Res 96,391, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lian Q., Zhang Y., Zhang J., Zhang H.K., Wu X., Zhang Y., et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation 121,1113, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Hwang N.S., Varghese S., Lee H.J., Zhang Z., Ye Z., Bae J., et al. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci U S A 105,20641, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J.L., Yin Z., Shen W.L., Chen X., Heng B.C., Zou X.H., et al. Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials 31,9438, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Olivier E.N., and Bouhassira E.E.Differentiation of human embryonic stem cells into mesenchymal stem cells by the “raclure” method. Methods Mol Biol 690,183, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Niklason L., Abbott W., and Gao J.Morphologic and mechanical characteristics of engineered bovine arteries. J Vasc Surg 33,628, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Quint C., Kondo Y., Manson R.J., Lawson J.H., Dardik A., and Niklason L.E.Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A 108,9214, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurpinski K., Lam H., Chu J., Wang A., Kim A., Tsay E., et al. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells 28,734, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Tang Y., Yang X., Friesel R.E., Vary C.P.H., and Liaw L.Mechanisms of TGF-β-induced differentiation in human vascular smooth muscle cells. J Vasc Res 48,485, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grygielko E.T., Martin W.M., Tweed C., Thornton P., Harling J., Brooks D.P., et al. Inhibition of gene markers of fibrosis with a novel inhibitor of transforming growth factor-beta type I receptor kinase in puromycin-induced nephritis. J Pharmacol Exp Ther 313,943, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Sinha S., Hoofnagle M.H., Kingston P.A., McCanna M.E., and Owens G.K.Transforming growth factor-beta1 signaling contributes to development of smooth muscle cells from embryonic stem cells. Am J Physiol Cell Physiol 287,C1560, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Kinner B., Zaleskas J.M., and Spector M.Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp Cell Res 278,72, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Boyd N.L., Robbins K.R., Dhara S.K., West F.D., and Stice S.L.Human embryonic stem cell-derived mesoderm-like epithelium transitions to mesenchymal progenitor cells. Tissue Eng Part A 15,1897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang A., Tang Z., Li X., Jiang Y., Tsou D.A., and Li S.Derivation of smooth muscle cells with neural crest origin from human induced pluripotent stem cells. Cells Tissues Organs (Print) 195,5, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung C., Bernardo A.S., Trotter M.W.B., Pedersen R.A., and Sinha S.Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol 30,165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darby I., Skalli O., and Gabbiani G.Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 63,21, 1990 [PubMed] [Google Scholar]
- 31.Sawtell N.M., and Lessard J.L.Cellular distribution of smooth muscle actins during mammalian embryogenesis: expression of the alpha-vascular but not the gamma-enteric isoform in differentiating striated myocytes. J Cell Biol 109(6 Pt 1), 2929, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida T., and Owens G.K.Molecular determinants of vascular smooth muscle cell diversity. Circ Res 96,280, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Gan Q., Yoshida T., Li J., and Owens G.K.Smooth muscle cells and myofibroblasts use distinct transcriptional mechanisms for smooth muscle alpha-actin expression. Circ Res 101,883, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Pelttari K., Winter A., Steck E., Goetzke K., Hennig T., Ochs B.G., et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum 54,3254, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Liu K., Zhou G.D., Liu W., Zhang W.J., Cui L., Liu X., et al. The dependence of in vivo stable ectopic chondrogenesis by human mesenchymal stem cells on chondrogenic differentiation in vitro. Biomaterials 29,2183, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Speer M.Y., Yang H.-Y., Brabb T., Leaf E., Look A., Lin W.-L., et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res 104,733, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau W.L., Pai A., Moe S.M., and Giachelli C.M.Direct effects of phosphate on vascular cell function. Adv Chronic Kidney Dis 18,105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins S.P., Solan A.K., and Niklason LE.Effects of polyglycolic acid on porcine smooth muscle cell growth and differentiation. J Biomed Mater Res A 67,295, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Wanjare M., Kuo F., and Gerecht S.Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells. Cardiovasc Res 97,321, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee M., Wu B.M., Stelzner M., Reichardt H.M., and Dunn J.C.Y.Intestinal smooth muscle cell maintenance by basic fibroblast growth factor. Tissue Eng Part A 14,1395, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Khan I.M., Evans S.L., Young R.D., Blain E.J., Quantock A.J., Avery N., et al. Fibroblast growth factor 2 and transforming growth factor β1 induce precocious maturation of articular cartilage. Arthritis Rheum 63,3417, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Li Q., Liu T., Zhang L., Liu Y., Zhang W., Liu W., et al. The role of bFGF in down-regulating α-SMA expression of chondrogenically induced BMSCs and preventing the shrinkage of BMSC engineered cartilage. Biomaterials 32,4773, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.