Abstract
A prerequisite for the realization of human pluripotent stem cell (hPSC) therapies is the development of bioprocesses for generating clinically relevant quantities of undifferentiated hPSCs and their derivatives under xeno-free conditions. Microcarrier stirred-suspension bioreactors are an appealing modality for the scalable expansion and directed differentiation of hPSCs. Comparative analyses of commercially available microcarriers clearly show the need for developing synthetic substrates supporting the adhesion and growth of hPSCs in three-dimensional cultures under agitation-induced shear. Moreover, the low seeding efficiencies during microcarrier loading with hPSC clusters poses a significant process bottleneck. To that end, a novel protocol was developed increasing hPSC seeding efficiency from 30% to over 80% and substantially shortening the duration of microcarrier loading. Importantly, this method was combined with the engineering of polystyrene microcarriers by surface conjugation of a vitronectin-derived peptide, which was previously shown to support the growth of human embryonic stem cells. Cells proliferated on peptide-conjugated beads in static culture but widespread detachment was observed after exposure to stirring. This prompted additional treatment of the microcarriers with a synthetic polymer commonly used to enhance cell adhesion. hPSCs were successfully cultivated on these microcarriers in stirred suspension vessels for multiple consecutive passages with attachment efficiencies close to 40%. Cultured cells exhibited on average a 24-fold increase in concentration per 6-day passage, over 85% viability, and maintained a normal karyotype and the expression of pluripotency markers such as Nanog, Oct4, and SSEA4. When subjected to spontaneous differentiation in embryoid body cultures or directed differentiation to the three embryonic germ layers, the cells adopted respective fates displaying relevant markers. Lastly, engineered microcarriers were successfully utilized for the expansion and differentiation of hPSCs to mesoderm progeny in stirred suspension vessels. Hence, we demonstrate a strategy for the facile engineering of xeno-free microcarriers for stirred-suspension cultivation of hPSCs. Our findings support the use of microcarrier bioreactors for the scalable, xeno-free propagation and differentiation of human stem cells intended for therapies.
Introduction
Human pluripotent stem cells (hPSCs), embryonic stem cells (ESCs), and induced pluripotent stem cells (iPSCs) are promising sources of cellular material for regenerative medicine and tissue engineering applications. Before the therapeutic potential of hPSCs can be realized however, their large-scale generation in a reproducible manner will be essential. Stirred-suspension bioreactors (SSBs)1–3 are an appealing culture modality for hPSC propagation and commitment given their scalability, robustly controlled operation, and widespread use in commercial production. hPSCs in these reactors can be grown as aggregates,1,4 after encapsulation5 or on microcarriers.6,7 In particular, microcarrier systems afford high surface-to-volume ratio, homogenous environment, simple operation and continuous monitoring, and control of the culture environment. hPSCs have been successfully expanded and differentiated to definitive endoderm, cardiomyocytes, and neural progenitor cells6,8,9 in stirred-suspension microcarrier vessels.
Despite success in cultivating hPSCs in microcarrier SSBs, the beads utilized in most studies are coated with animal-derived matrices such as Matrigel6,9–11 or collagen12 barring the applicability of this culture method from clinical settings. Similarly, the proposed use of rodent and human feeder cells for coating microcarriers10,13 raises issues with the downstream separation of multiple cell types and beads in addition to the expression of nonhuman immunogens by hPSC derivatives.14 Considerable progress has been noted in developing chemically defined, xeno-free media for hPSC culture15–19 some of which are commercially available.20–22 Nonetheless, research on three-dimensional (3D) substrates free of xenogeneic factors is still to bear simple solutions for the long-term culture of hPSCs at a reasonable cost. The disparate and sometimes conflicting results from comparative analyses of commercially available microcarrier types,7,23 which are suitable for the culture of non-hPSC lines (e.g., CHO cells, Vero cells, etc.), make increasingly clear that these microcarriers are not optimal for the culture of hPSCs. Recent studies on the cultivation of hPSCs on two-dimensional (2D) xeno-free surfaces featuring recombinant extracellular matrix (ECM) proteins like fibronectin,17 laminin,16,24 vitronectin,22,25 and synthetic polymer- or peptide-conjugated surfaces26–31 have garnered optimism for the scalable cultivation of stem cells and their progeny. Nonetheless, the fundamental differences between 2D and 3D surfaces (e.g., substrate curvature and elasticity affecting stem cell shape, spreading, and eventually commitment32–34), and static versus stirred-suspension cultures (e.g., agitation-induced shear in SSBs) hinder the direct translation of these findings to the hPSC expansion/differentiation in microcarrier SSBs.
Current protocols also rely on seeding hPSCs as clumps on microcarriers for SSB cultivation. This is due to the dramatic decrease in cell viability when hPSC colonies are completely dissociated into single cells. Cluster seeding, however, creates a bottleneck in the process due to the inefficient attachment of cells and the uneven colonization of the microcarriers. To that end, we set out to investigate the seeding of single dispersed hPSCs on microcarriers thereby boosting the attachment efficiency and the initial number of cells available for cultivation. Enhanced cell survival during the microcarrier “loading” phase was maintained with the use of a Rho-associated kinase (ROCK) inhibitor.35
More importantly, we demonstrate here the propagation of hPSCs over multiple successive passages and their directed differentiation on xeno-free microcarriers in stirred-suspension cultures with defined media. For this purpose, compact microcarriers were engineered by surface conjugation of a synthetic peptide derived from vitronectin. This peptide was previously shown to support the long-term self-renewal of human ESCs (hESCs) and their cardiogenic differentiation on flat surfaces.31 Our analysis revealed that peptide-conjugated microcarriers supported the growth of hPSCs in static cultures but extensive cell detachment was observed when the beads were suspended in spinner flasks. This was ameliorated upon treatment of peptide-conjugated microcarriers with poly-l-lysine (pLL), a synthetic polymer promoting cell attachment onto surfaces. hPSCs on those microcarriers consistently proliferated over multiple passages without loss of their pluripotency and normal karyotype. Additionally, cells on microcarriers were successfully subjected to differentiation toward mesoderm. These findings evidence the feasibility of cultivating hPSCs in xeno-free microcarrier systems and further support the use of such systems for the scalable generation of therapeutically useful progeny.
Materials and Methods
hPSC culture
hESCs [H9(WA09); passages 30–50] and human iPSCs [iPSC(IMR90)-4 thereafter IMR90; passages 30–40] were obtained from the WiCell Research Institute and their use was approved by the Committee for Stem Cell Research Oversight at SUNY-Buffalo. Cells cultured in dishes coated with Matrigel (BD Biosciences) and in mTeSR1 or TeSR2 medium (StemCell Technologies) were maintained in 5% CO2/95% air at 37°C. While both mTeSR1 and TeSR2 are chemically defined media, mTeSR1 contains bovine serum albumin (BSA) while TeSR2 is free of animal proteins. Medium was replaced every day, and the cells were passaged every 5–6 days by enzymatic dissociation with collagenase type IV (Life Technologies).
Viable cells were counted in a hemocytometer after Trypan Blue staining (Sigma-Aldrich). Alternatively, cells were stained with 20 mg/mL fluorescein diacetate (FDA-live cells; Sigma-Aldrich) in phosphate-buffered saline (PBS) for 5 min and after being washed twice with PBS, they were analyzed by fluorescence microscopy or flow cytometry (see below).
Lactate dehydrogenase (LDH) activity was determined in culture samples with an LDH cytotoxicity detection assay (Roche) according to the manufacturer's instructions as described.6,36
Microcarrier seeding and passaging
Collagen-coated polystyrene microcarriers (SoloHill) were processed as described.6 Briefly, beads were equilibrated in PBS for 30 min, autoclaved, and coated with Matrigel at room temperature for 1 h. Coated microcarriers were equilibrated in culture medium supplemented with 10 μM ROCK inhibitor (Y-27632; Enzo Biochem) for 1 h before cell seeding. Beads at 0.5 g (∼180 cm2 surface area)/50 mL medium were used.
Microcarriers subjected to peptide conjugation (see below) were equilibrated in medium (TeSR2 or mTeSR1) or PBS as noted for 30 min before use. The amount of peptide-conjugated microcarriers was adjusted to maintain a constant ratio of bead surface area-to-medium volume among experiments.
For initial seeding on beads, stem cells on Matrigel-coated dishes were treated with 10 μM Y-27632 for 1 h and colonies were dissociated into single cells with Accutase (Innovative Cell Technologies). Dispersed hPSCs were transferred with microcarriers (cell-to-bead ratios as stated) to Petri dishes and placed in 5% CO2/95% air at 37°C for the period as noted.
Subsequently, the cell-laden beads were transferred to ProCulture spinner flasks (Corning), the total medium volume supplemented with Y-27632 was brought to 50 mL, and the agitation rate was set to 60 rpm for the duration of each run after the removal of floating cells. After the first day, the medium was replaced by medium without Y-27632. Subsequent medium changes were performed at half-volume every day. The cultures were maintained at 37°C in 5% CO2/95% air.
In preparation for passaging cells between spinner flasks, Y-27632 was added to the culture at a final concentration of 10 μM about 1 h prior to harvesting. Then, cells on microcarriers were collected from spinner flasks into centrifuge tubes, washed once with PBS, and incubated with Accutase for 10–15 min while gently mixing occasionally. Complete detachment from microcarriers as single cells was verified by microscopy. The cell/bead suspension was passed through a 100-μm mesh strainer (BD Biosciences) and harvested cells were inoculated onto fresh microcarriers as described for initial seeding above.
Preparation of microcarriers with peptide conjugation and pLL treatment
Polystyrene beads featuring –COOH groups on their surfaces (Rapp-Polymere GmbH) were incubated for 30 min with N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) (4:1 molar ratio; both from Thermo Scientific) in PBS for –COOH activation. After aspirating the EDC/NHS solution, the microcarriers were incubated with varying amounts of the synthetic peptide AcKGGPQVTRGDVFTMP31 (GenScript) derived from vitronectin in PBS for 2 h. The microcarriers were then immersed in ethanolamine (pH 8.5) for 1 h to quench any unreacted activated ester groups, washed thrice with PBS, and stored in 75% (v/v) aqueous ethanol. Prior to cell culture, peptide-conjugated microcarriers were washed thrice with sterile deionized water to eliminate traces of ethanol. For pLL coating, the beads were incubated with sterile 0.01% pLL solution (Sigma-Aldrich) for 5 min and dried.
The peptide density on the bead surface after conjugation and the efficiency of the amidation reaction were quantified using the FluoroProfile Protein Quantification assay (Sigma-Aldrich). Supernatant samples containing unreacted peptide were loaded in triplicates in black-wall 96-well plates (VWR) and fluorescence intensity was measured on a Synergy 4 Hybrid microplate reader (BioTek) with the Gen5 software (BioTek).
Reverse transcription–polymerase chain reaction and quantitative polymerase chain reaction
Total RNA was isolated using TRIzol (Invitrogen) according to the manufacturer's instructions, and reverse transcription was performed using the ImPromII reverse transcriptase (Promega) as described.6 Polymerase chain reaction (PCR) runs were performed with the resulting cDNA for 35 cycles at an annealing temperature of 58°C–60°C depending on the primer set (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/tea).
Quantitative PCR (qPCR) was performed on a CFX96 Real-Time PCR machine (Bio-Rad) using the DyNAmo™ SYBR Green qPCR Kit (Thermo Scientific): denaturation and polymerase activation at 95°C for 15 min; amplification for 40 cycles at 94°C for 10 s, 58°C–60°C for 20 s, and 72°C for 30 s. All reactions were run in triplicates. Amplification specificity was verified by the melting curve method and gel electrophoresis. Relative gene expression was calculated with the BioRad CFX software by normalizing to the endogenous β-actin (ACTB) expression. The CT for the housekeeping gene did not vary under different experimental conditions when equal amounts of RNA were used.
Flow cytometry
Cells were dissociated from microcarriers by incubation with TrypLE, passed through 100 μm mesh strainers (BD Biosciences) for bead removal, and pelleted by centrifugation at 200 g for 5 min. Cells were then fixed in a 3.7% formaldehyde solution (Sigma-Aldrich) for 10 min, washed with PBS, and permeabilized with Cytonin (Trevigen) for 30 min before blocking with 3% normal donkey serum (NDS; Jackson Immunoresearch Laboratories) for 20 min. The samples were subsequently incubated with primary antibodies including rabbit anti-OCT4 (cat. no. sc-9081; Santa Cruz Biotechnology), mouse anti-NANOG (cat. no. 560873; Millipore), and mouse anti-SSEA4 (cat. no. MC813; AbCam) for 1 h at room temperature. After washing thrice with 1% NDS, cells were incubated with appropriate DyLight secondary antibodies (Jackson Immunoresearch Laboratories) for 1 h at room temperature. The samples were washed again thrice with PBS and analyzed in a FACS Calibur flow cytometer with the CellQuest software (Becton Dickinson). Data were further analyzed with the FCS Express V4.0 suite (De Novo Software). Cells were considered as positive for a particular antigen if their emitted fluorescence level was higher than 99% of that of samples stained only with the corresponding secondary antibodies.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde (Sigma-Aldrich) in PBS, permeabilized/blocked in PBS with 0.1% Triton X-100 (Mallinckrodt Baker) and 1% BSA (Sigma-Aldrich) for 30 min, and incubated overnight at 4°C with primary antibodies: mouse anti-SSEA4 (cat no. MC813; AbCam), rabbit anti-OCT4 (cat. no. sc-9081; Santa Cruz Biotechnology), goat anti-SOX17 (cat. no. AF1924; R&D Systems), rabbit anti-FOXA2 (cat. no. 3143S; Cell Signaling Technologies), rabbit anti-MEOX1 (cat. no. NBP1-92134; Novus Biologicals), mouse anti-KDR (cat. no. ab9530; AbCam), mouse anti-NESTIN (cat. no. MAB1259; R&D Systems), and rabbit anti-TUBB3 (βIII-tubulin, cat. no. T3952; Sigma-Aldrich). After three washes with PBS, cells were incubated with DyLight secondary antibodies (Jackson Immunoresearch Laboratories) for 1 h at room temperature. Nuclear DNA was stained with DAPI (Vectashield; Vector Laboratories). Cells stained with antibodies against differentiated cell markers (undifferentiated hPSCs) or SSEA4 and OCT4 (293 human embryonic kidney cells) served as negative controls showing minimal or no immunoreactivity. Images were acquired with an inverted microscope with epifluorescence (Zeiss Axio Observer D1; Carl Zeiss) connected to a digital camera (Zeiss AxioCam MRm).
Karyotyping
Cells harvested from microcarrier cultures were replated on T-75 flasks and allowed to grow until ∼70% confluence before treatment with 30 ng/mL of KaryoMAX Colcemid Solution (Gibco) for 4 h at 37°C. Cells were then harvested, transferred to 15 mL conical tubes, centrifuged for 5 min at 250 g, and gently resuspended in cell hypotonic solution (CHS; 40 mM KCl, 20 mM HEPES, 0.5 mM EGTA, and 9 mM NaOH) for 1 h of incubation at 37°C. After centrifugation of the cell/CHS suspension at 250 g, the supernatant was removed and the cells were fixed with 1:3 (v/v) acetic acid-methanol solution. G-banding analysis was performed in the SKY/FISH facility at the Roswell Park Cancer Institute.
Embryoid body formation
Single dispersed hPSCs were cultured in AggreWell plates (StemCell Technologies) according to a manufacturer's protocol to induce embryoid body (EB) formation. Harvested EBs were transferred to Petri dishes and maintained in Dulbecco's modified Eagle's medium/Ham's F12 medium (DMEM/F12) (Life Technologies), supplemented with 20% FBS (PAA Laboratories). Medium was replenished every 2 days until analysis of the EBs.
Definitive endoderm, mesoderm, and neuroectoderm differentiation
Cells harvested from microcarriers were replated on Matrigel-coated dishes. For bioreactor differentiation, the medium used for expansion of hPSCs was exchanged with differentiation medium keeping the total working volume constant. Differentiation to definitive endoderm, mesoderm, and neuroectoderm were performed according to previous reports.5,6,37,38 Detailed protocols are provided in Supplementary Materials and Methods section.
Statistical analysis
Data are expressed as mean±SD unless stated otherwise. ANOVA and the post hoc Tukey test were performed using Minitab (Minitab, Inc.). p-Values less than 0.05 were considered as significant.
Results
Single dispersed hESC seeding on Matrigel-coated microcarriers for stirred suspension culture
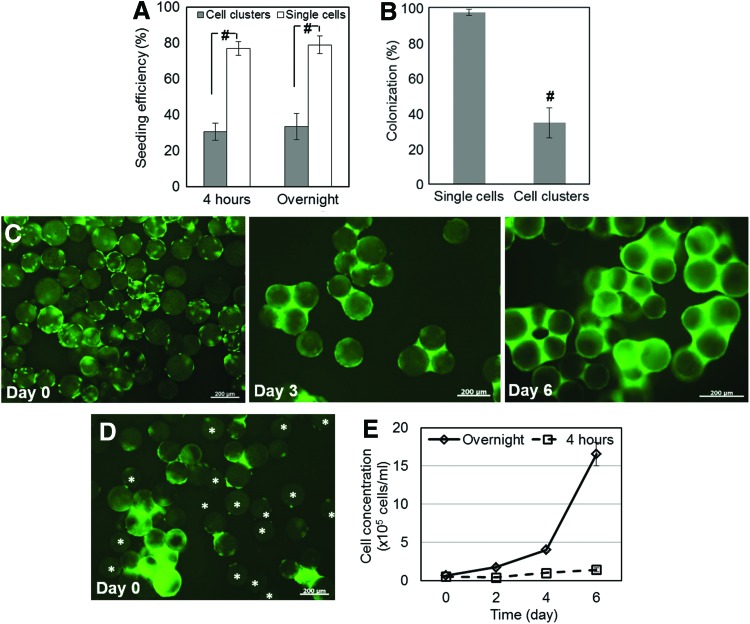
We previously reported that hESCs and hiPSCs seeded as clusters on Matrigel-coated microcarriers can be cultured in stirred-suspension vessels.1,6 Although hPSCs in clusters exhibit improved survival, this seeding method results in significant loss of viable cells and attachment efficiency of only ∼30%. Therefore, we investigated the seeding of dispersed hPSCs on microcarriers. hESCs were cultured with 10 μM ROCK inhibitor (which limits dissociation-induced apoptosis35) for 1 h on Matrigel-coated plates before harvested as single cells and loaded on Matrigel-coated microcarriers at 25 cells/bead. In parallel, cells were inoculated on Matrigel-coated beads as clusters at the same cell:bead ratio.6 Of the single hESCs, 77.0%±7.3% attached on the beads in the presence of ROCK inhibitor within 4 h compared to only 30.5%±4.8% of cells inoculated as clusters (Fig. 1A, p<0.05). The seeding efficiency was similar whether the cells were incubated with microcarriers for 4 h or overnight. Moreover, 97.2%±1.8% of beads were colonized with single cells versus only 34.7%±8.5% after cell cluster seeding (Fig. 1B). The distribution of hESCs seeded as dispersed cells on the beads was also more even and no large aggregates were observed after 6 days of culture compared to cultures of microcarriers seeded with cell clumps (Fig. 1C, D). Although “bridging” among beads was noted, the cells grew 25.1-fold (Fig. 1E, solid curve). Similar results were obtained for single dispersed hiPSCs and hiPSC clumps seeded onto Matrigel-coated microcarriers (Supplementary Fig. S1).
FIG. 1.
Seeding of human pluripotent stem cells (hPSCs) on Matrigel-coated microcarriers. (A) Efficiencies as fractions of seeded cells attached on the microcarrier surface are shown for seeded dispersed hPSCs or clusters. (B) Fraction of beads colonized after being seeded with dispersed cells or clusters. H9 human embryonic stem cells (hESCs) on Matrigel-coated beads stained with FDA after being seeded as (C) single cells or (D) small clusters. Microcarriers loaded with dispersed cells are shown at days 0, 3, and 6. Stars mark microcarriers with only a few or no cells. Scale bars: 200 μm. (E) Expansion of hESCs in spinner flasks after their seeding on Matrigel-coated microcarriers and 4 h (dashed curve) or overnight (solid curve) treatment with ROCK inhibitor. [#p<0.05 in (A, B).] Color images available online at www.liebertpub.com/tea
It should be noted that the above results were obtained after cell treatment with the ROCK inhibitor overnight. In contrast, when the inhibitor was removed after 4 h of seeding, cells exhibited a long lag phase and their concentration did not increase substantially (2.8-fold) under the same conditions (Fig. 1E, dashed curve). Keeping the cells with the beads either for 4 h or overnight during the loading phase did not affect the seeding efficiency (Fig. 1A) and this was ruled out as contributing to the difference in the fold expansion of hESCs during their subsequent culture in spinner flasks. When cells were seeded on Matrigel-coated beads without ROCK inhibitor treatment, little or no cell attachment on the beads was observed along with small clusters occasionally and very low survival as expected (Supplementary Fig. S2). We concluded therefore that overnight treatment with ROCK inhibitor was essential for successful expansion on microcarriers in stirred-suspension.
These findings prove that hPSCs can be loaded as single cells on microcarriers with higher seeding efficiency and are uniformly distributed in contrast to cluster seeding. Moreover, subsequent hPSC expansion in stirred-suspension microcarriers is dependent on the interval of hPSC treatment with ROCK inhibitor during and after seeding.
Multi-passage suspension culture of hPSCs seeded as dispersed cells on Matrigel-coated microcarriers
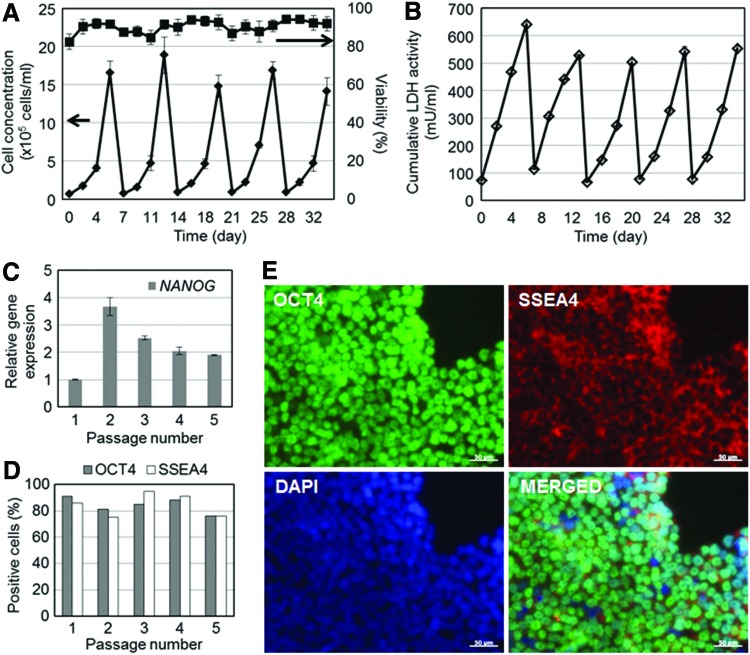
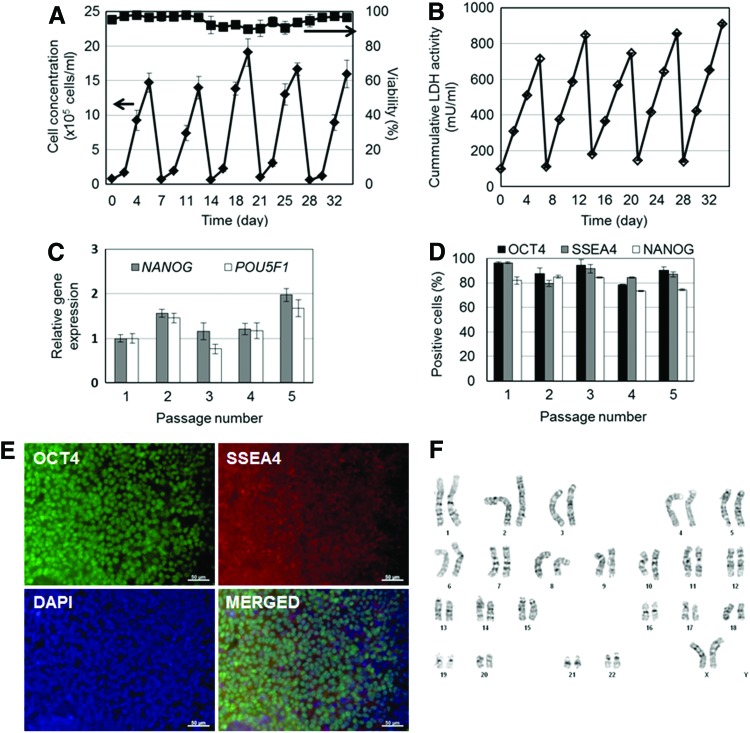
Next, we addressed the question of whether hPSCs seeded as single cells on Matrigel-coated beads can be cultured for multiple passages. Indeed, H9 hESCs were successfully cultured for five 6-day passages on microcarriers. Cell proliferation was consistent among passages with an average increase of 20.7±4.0-fold to concentrations of 1.5–1.9×106 cells/mL (Fig. 2A). Cumulative LDH activity in the medium was kept at low levels (peak values <650 mU/mL) similar to microcarrier cultures after hESC cluster seeding6 (Fig. 2B). After each passage, cells harvested from microcarriers were probed for the expression of pluripotency markers by qPCR, flow cytometry, and immunostaining. NANOG expression was the highest after the second passage and consistently higher in all passages when compared with passage 1 (Fig. 2C). The low expression of NANOG after the initial passage may be in part due to the adaptation of cells to the bioreactor environment. When analyzed by flow cytometry, the majority of cells (>75%) also retained the expression of OCT4 and SSEA4 over successive passages (Fig. 2D). These results are corroborated by immunostaining for the same markers of cells harvested from the last passage (Fig. 2E). Taken together, our data indicate that hESCs seeded as single cells with ROCK inhibitor can be propagated on microcarriers in a stirred-suspension vessel without loss of pluripotency marker expression.
FIG. 2.
Multi-passage expansion of H9 hESCs on Matrigel-coated microcarriers. (A) Growth profile and viability of microcarrier-attached cells cultured for five passages. (B) Corresponding cumulative lactate dehydrogenase (LDH) activity. Cells were probed for the expression of pluripotency markers by (C) quantitative polymerase chain reaction (qPCR), (D) flow cytometry, and (E) immunostaining (scale bars: 50 μm). Color images available online at www.liebertpub.com/tea
Culture of hPSCs on peptide-conjugated microcarriers
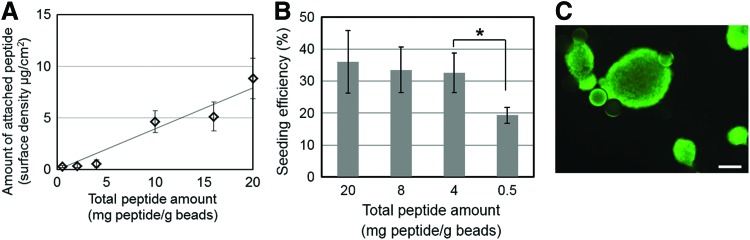
With an improved protocol in place for the efficient seeding of beads with hPSCs, we proceeded to engineer the microcarrier surface by replacing the mouse sarcoma-derived Matrigel matrix with synthetic substrates facilitating the adhesion of hPSCs without affecting their pluripotency. For this purpose, polystyrene microcarriers with surface –COOH groups were decorated with an arginine–glycine–aspartic acid (RGD)-containing vitronectin fragment shown to promote hESC adhesion and growth in static cultures.31 The peptide was conjugated to the –COOH groups via an EDC/NHS catalyzed amidation reaction. The –COOH concentration was constant (1.09 mmol/g beads) and the amounts of conjugated peptide and peptide used (per gram of beads) in the coupling reaction were proportional (Fig. 3A) allowing for control of the peptide density on the bead surface. Carboxyl group activation by EDC/NHS was critical for the subsequent coupling reaction as no peptide was detected on beads when the EDC/NHS treatment was skipped.
FIG. 3.
Peptide surface density of microcarriers and seeding efficiency of hPSCs. (A) Surface peptide density versus the total amount of peptide used in the amidation reaction. (B) Seeding efficiency of IMR90 cells on microcarriers conjugated with different amount of peptides. Cells were cultured in TeSR2 medium. (C) IMR90 cells seeded on CP beads and cultured in spinner flasks failed to spread on the beads and formed aggregates. Scale bar: 200 μm. Color images available online at www.liebertpub.com/tea
Microcarriers with different peptide surface densities were incubated with IMR90 hiPSCs in TeSR2 medium to evaluate cell attachment. At or above 4 mg peptide/g bead used for the amidation reaction there were no statistically significant differences in seeding efficiency, which was at 30%–36% (Fig. 3B). At 0.5 mg peptide/g bead the efficiency decreased to 19%. We therefore selected 4 mg peptide/g bead (600 ng peptide/cm2) as the concentration for engineering microcarriers in subsequent experiments. The cell-to-bead ratio was set to 50 to keep the same number of attached cells on peptide-conjugated and Matrigel-coated microcarriers.
IMR90 cells seeded on peptide-conjugated (CP) beads were cultured in spinner flasks for 6 days. However, cells did not spread on the beads and extensive aggregate formation was observed (Fig. 3C). Hence, despite attachment on CP microcarriers in static culture and in 2D surfaces coated with same peptide,31 the adhesion of cells was not sufficiently strong to maintain them on the beads under stirring.
hPSC expansion on peptide-conjugated, pLL-treated microcarriers
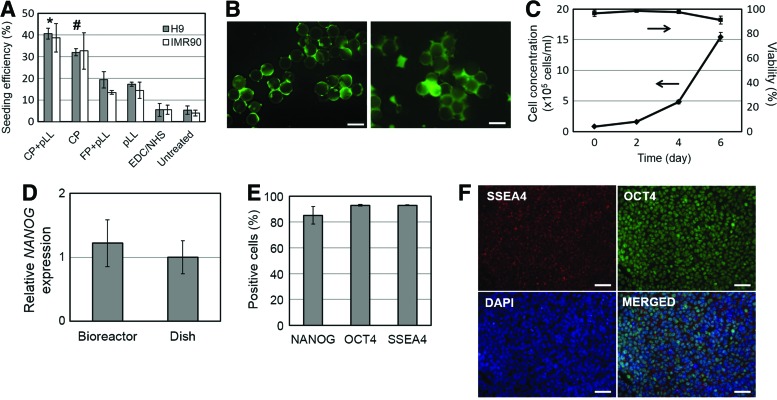
The extensive aggregation and loss of surface adhesion observed for hiPSCs cultured on CP microcarriers in stirred-suspension suggested the need for devising ways to strengthen the cell-to-bead attachment. To that end, microcarriers were coated with pLL, which is a positively charged synthetic polymer enhancing cell attachment,39 and seeded with hPSCs at 50 cells/bead in TeSR2 medium (Fig. 4A). The highest seeding efficiency was measured on CP microcarriers coated with pLL (CP+pLL) at 38.7%±6.6% compared to 32.7%±8.3% for peptide-conjugated microcarriers without pLL (CP). Cells seeded on microcarriers coated with only pLL (pLL) or a mixture of pLL and free peptide (without EDC/NHS coupling reaction; FP+pLL), exhibited significantly lower adhesion with efficiencies between 14.5%±3.8% and 13.5%±0.9%. Further, microcarriers treated with only EDC/NHS (untreated) displayed poor cell attachment indicating that this step per se does not contribute to the adhesiveness of the bead surface. Interestingly enough, when IMR90 hiPSC clusters were seeded onto CP+pLL beads at the same cell-to-bead ratio, the seeding efficiency was 16.5%±3.2%, that is, significantly lower than inoculating dispersed single cells. Moreover, minimal cell attachment was observed in the absence of ROCK inhibitor as expected (Supplementary Fig. S2). It should be also noted that equilibration of microcarriers in TeSR2 or PBS had no effect on the seeding efficiency of hPSCs (Supplementary Fig. S3).
FIG. 4.
Coating of peptide-conjugated microcarriers with poly-l-lysine (pLL) for hPSC culture in xeno-free medium. (A) Seeding efficiency of IMR90 and H9 cells on microcarriers featuring different surface treatments: CP+pLL: beads with conjugated peptide and pLL coating; CP: beads with conjugated peptide; FP+pLL: beads with free (unconjugated) peptide and pLL coating; pLL: beads with pLL coating only; N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC)/N-hydroxysuccinimide (NHS): beads subjected to the coupling reaction without peptide; Untreated: plain beads without any treatment. *p<0.05: CP+pLL versus pLL and CP+pLL versus FP+pLL, #p<0.05: CP versus EDC/NHS and CP versus untreated. Mean values are compared among different microcarriers for the same cell type (H9 or IMR90). (B) FDA-stained IMR90 cells on CP+pLL beads right after the seeding phase (left) and during spinner flask culture (right). (C) Time course of the concentration and viability of IMR90 human induced pluripotent stem cells (hiPSCs) cultured on CP+pLL beads in stirred suspension. (D) Relative expression of NANOG in IMR90 cells cultured for 6 days in a microcarrier suspension (bioreactor). The corresponding gene expression of IMR90 cells maintained in dishes is also shown. (E) Analysis of cultured cells (day 6) by flow cytometry for the expression of stem cell markers. Values are shown as mean±SD (n ≥3). (F) Immunostaining of cells after 6 days of expansion on CP+pLL beads in the bioreactor. Cells in (A–F) were cultured in TeSR2 medium. Scale bars in (B): 200 μm, (F): 50 μm. Color images available online at www.liebertpub.com/tea
Dispersed hiPSCs were then seeded onto CP+pLL beads and cultured in spinner flask with TeSR2 medium for 6 days. In contrast to forming aggregates when cultured on CP beads, cells cultured on CP+pLL beads attached and spread and no floating EB-like clusters were observed (Fig. 4B). The cells on CP+pLL microcarriers grew 18.8-fold in 6 days of stirred suspension cultivation to 15.5×105 cells/mL with viability over 90% (Fig. 4C). Cells examined after their culture expressed stem cell markers at similar levels as those cells maintained in parallel in dishes (Fig. 4D). Over 85% of the cells were positive for NANOG, OCT4, and SSEA4 by flow cytometry (Fig. 4E) and these results were in line with immunostaining data (Fig. 4F). Thus, the hiPSCs cultured on CP+pLL beads in spinner flasks with TeSR2 medium were successfully expanded without significant loss of pluripotency marker expression.
Given the higher cost of the xeno-free TeSR2 medium, we conducted a multi-passage culture experiment of hiPSCs on CP+pLL beads in chemically defined mTeSR1. Cells were continuously cultured for five 6-day passages. A 23.3±5.3-fold increase in cell concentration peaking at 1.4–1.9×106 cells/mL was noted per passage corresponding to a doubling time of 31 h compared with 34.3 h for Matrigel-coated dish cultures (Fig. 5A). Cells exhibited cumulative LDH release patterns similar to H9 cells cultured on Matrigel-coated beads (Fig. 5B). Moreover, cells maintained the expression of NANOG, POU5F1 (OCT4), and SSEA4 mRNA and corresponding proteins at each passage (Fig. 5C, D). After five passages, cells plated on Matrigel-coated dishes were positive for pluripotency markers (Fig. 5E). These cells were also karyotypically normal based on G-banding analysis (Fig. 5F).
FIG. 5.
Human PSCs cultured for multiple passages on CP+pLL microcarriers in stirred-suspension vessels. (A) Growth profile, viability, and (B) cumulative LDH activity of IMR90 hiPSCs cultured for five passages on microcarriers in spinner flasks. After each passage, the expression of pluripotency markers was characterized by (C) qPCR and (D) flow cytometry. (E) After the last passage, cells were plated and stained for pluripotency markers (scale bars: 50 μm). (F) Karyotyping results for IMR90 cells after their culture for five passages on CP+pLL beads in spinner flasks. Color images available online at www.liebertpub.com/tea
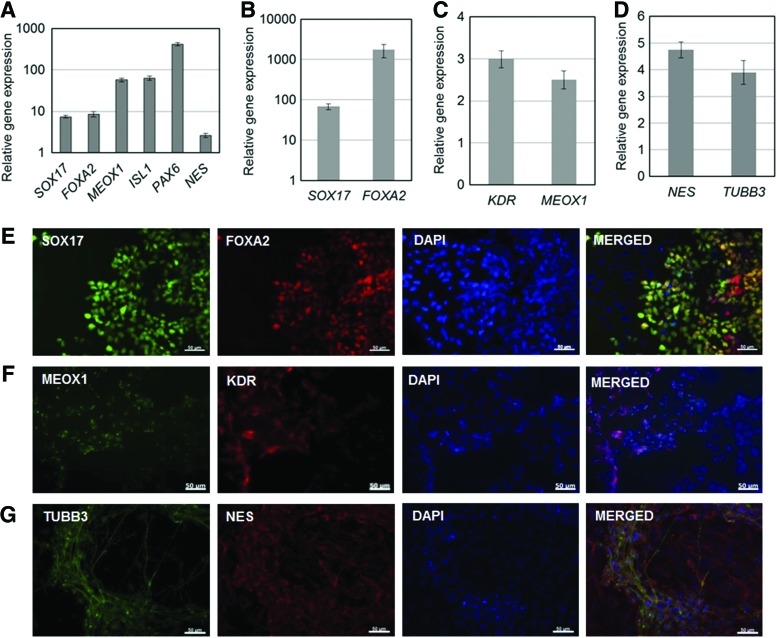
Formation of EBs is routinely employed to assess the potential of hPSCs for spontaneous multi-lineage differentiation. Cells propagated for five passages on CP+pLL microcarriers in spinner flasks were then cultured as EBs. RNA isolated from these EBs contained transcripts of genes characteristic of the three germ layers: definitive endoderm (SOX17, FOXA2),40,41 mesoderm (ISL1, MEOX1),42,43 and ectoderm (PAX6, NES)38 (Fig. 6A).
FIG. 6.
Differentiation potential of hPSCs after their propagation on CP+pLL microcarriers for five passages. (A) IMR90 hiPSCs were subsequently cultured as embryoid bodies and expression of markers of the three embryonic germ layers was assessed. The expression of undifferentiated IMR90 cells was used for normalization. Alternatively, propagated cells were plated and subjected to directed differentiation toward (B, E) definitive endoderm (DE), (C, F) mesoderm, and (D, G) neuroectoderm. The expression was evaluated by qPCR (B–D) and immunostaining (E–G). Gene expression was normalized to IMR90 cells differentiated in basal medium without differentiation factors. Scale bars in (E–G): 50 μm. Color images available online at www.liebertpub.com/tea
Cells cultured for five passages in CP+pLL microcarriers were also transferred onto Matrigel-coated dishes and subjected to directed differentiation to definitive endoderm (DE), mesoderm (MS), and neuroectoderm (NE) by applying previously established protocols.5,6,37,38 Differentiation to each lineage was evident by the expression of relevant markers as assessed by qPCR (Fig. 6B–D) and immunostaining (Fig. 6E–G). Cells treated with the same basal media but without differentiation agents served as controls.
These findings demonstrate that CP+pLL microcarriers support the expansion of hPSCs without adversely affecting their pluripotency and karyotype. Cells are subsequently capable of multilineage commitment after application of either spontaneous (EB) or directed differentiation protocols.
Directed differentiation of hPSCs on CP+pLL microcarriers in stirred-suspension culture
Our results showed that hPSCs seeded as dispersed cells on CP+pLL beads can be propagated over multiple passages in a xeno-free stirred suspension culture without loss of their pluripotent state. Then, we questioned whether the same system can be employed to integrate the expansion of hPSCs with their differentiation in a single scalable process.
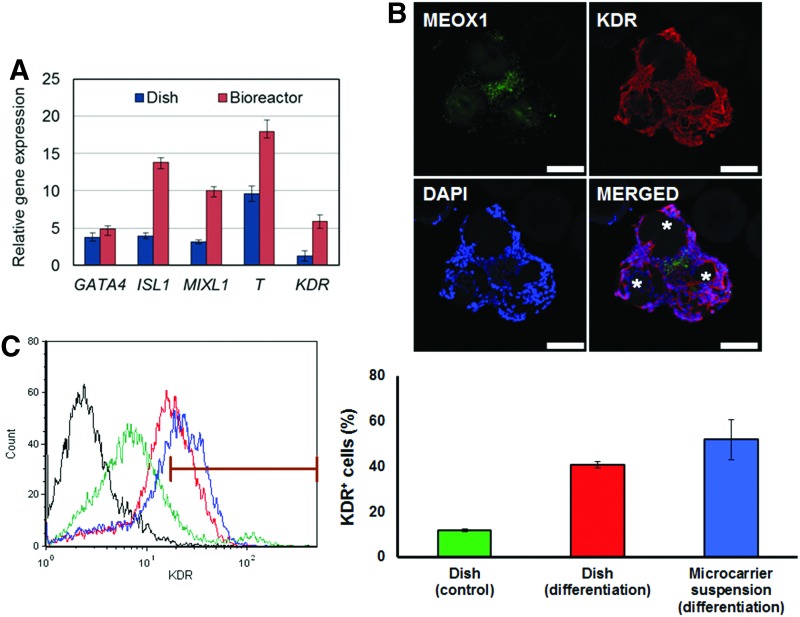
For this purpose, stem cells expanded on CP+pLL microcarriers in stirred-suspension were directly subjected to MS differentiation in spinner flasks. In parallel experiments, cells were also dissociated from beads, transferred to dishes, and coaxed to MS. After 5 days of differentiation, MS progeny emerged expressing relevant markers as evidenced by qPCR and immunostaining (Fig. 7A, B). The fraction of KDR+ cells (indicating cardiovascular progenitors44) in each culture modality was quantified by flow cytometry as a measure of differentiation efficiency (Fig. 7C). Differentiation in spinner flasks yielded 52%±8.8% KDR+ cells compared to 41%±1.29% in dishes, whereas cells cultured in basal medium without differentiation stimuli exhibited 12%±0.6% KDR+ cells. It should be noted that the concentration of cells significantly decreased during the first day of commitment both in dish and spinner flask cultures (on average ∼50%) similar to our previous observations for DE commitment.6 Taken together, these data support that the xeno-free microcarrier system developed here can accommodate both the expansion and directed differentiation of hPSCs.
FIG. 7.
Directed differentiation of hPSCs cultured on CP+pLL microcarriers in a stirred-suspension bioreactor. (A) Expression of MS genes after IMR90 hiPSC differentiation in the bioreactor. Gene expression was normalized to hiPSCs differentiated in basal medium without factors. (B) Immunostaining of differentiated cells on beads. Stars denote the position of microcarriers. Scale bars: 100 μm. (C) Flow cytometric analysis of KDR expression. A flow cytometry graph is shown from a representative experiment. Curves correspond to samples of negative control (black), cells in dishes incubated in basal media without differentiation factors (green; dish control), cells differentiated in dishes (red) and cells differentiated in microcarrier suspension culture (blue). The gate excludes 99% of the negative control cells. Results are summarized in the bar graph (n=3) as mean±SD.
Discussion
The development of scalable platforms for the propagation and differentiation of hPSCs is essential for stem cell-based therapies to become a reality. Significant advancements have been made toward culturing stem cells in environments without xenogeneic substrates. The work herein describes the facile engineering of xeno-free microcarriers suitable for the expansion and directed differentiation of hPSCs. hESCs and hiPSCs on peptide/pLL-decorated beads proliferated in chemically defined medium at similar or higher levels compared to those for hPSCs grown on commercially available microcarriers coated with Matrigel. Expanded cells maintained a normal karyotype, high viability, and the expression of stemness markers. When subjected to differentiation either in static culture or stirred-suspension culture, these cells promptly committed to lineages of the three embryonic germ layers.
Defined substrates for supporting the growth of undifferentiated stem cells have been explored in several studies (e.g., Refs.24,26,31). Many of the proposed substrates mimic ECMs to which cell adhesion is mediated via integrins.25,45 ECM proteins featuring the integrin-binding RGD motif,46 such as laminin, fibronectin, collagen, and vitronectin, and derivative peptides have been considered as candidate substrates for hPSC culture. For instance, stem cells have been cultured on RGD-containing ECM proteins/peptides, which were either physically adsorbed or covalently conjugated on 2D surfaces.16,17,20,22,24,25,28,31 However, information has been limited on synthetic 3D surfaces supporting hPSC expansion. More importantly, most studies entail static cultures in which the cells are dispensed from the shear due to agitation experienced in stirred vessels. Indeed, the vitronectin fragment utilized here supports the propagation of undifferentiated hESCs and their differentiation toward cardiac mesoderm on static flat surfaces.31 We also observed that hPSCs attach and spread well on CP beads in static culture. Yet, the cells come off promptly and/or form aggregates when the beads are placed in agitated suspension. In fact, others also observed reduced hESC growth on (full-length) vitronectin-coated microcarriers compared with flat tissue culture plates layered with the same protein.7 Therefore, findings based on the culture of cells on microcarriers in static culture do not readily translate to a bioreactor environment.
This also raises caution when screening several microcarrier types in static cultures (e.g., Petri dishes, multi-well plates) and may in part explain conflicting results reported for commonly used microcarriers, which best accommodate hPSCs in culture. For example, eight types of commercially available microcarriers were recently tested in static culture.23 Cytodex 1 beads were among those with the worst performance based on the total amount of hESCs recovered after 72 h of culture. However, Chen et al.7 examined a cohort of 10 different microcarrier types and noted that hESCs attached efficiently during seeding (attachment efficiency ∼80% 2 h postseeding) and grew to ∼7.7×105 cells/mL after at least two consecutive passages when cultured on Cytodex 1 beads. Coating the microcarriers with Matrigel or purified mouse laminin resulted in higher concentrations of hESCs cultured in mouse embryonic fibroblast-conditioned medium.
The expansion of hPSCs was supported on CP beads in dishes but not in spinner flasks. Increasing the peptide density on the microcarrier surface may strengthen the adhesion and boost the retention of cells under shear but we ruled out this conjecture. First, the seeding efficiency did not increase further with higher peptide densities (and amounts; Fig. 3) on the surface of microcarriers. Second, the density of 600 ng/cm2 is significantly higher than the threshold density of 250 ng absorbed vitronectin/cm2 on tissue culture plates to support the growth of undifferentiated hESCs.47 For the static culture of hESCs on polystyrene beads, a density of 450 ng vitronectin/cm2 was reported48 that is closer to the peptide density in our experiments. It should be mentioned that the whole vitronectin molecule (MW∼75 kDa or 459 amino acid residues) was used in the studies above in contrast to the smaller decapentapeptide (MW∼1.6 kDa) used here. Hence, the effective density of binding sites was significantly higher than previous reports but the CP beads still did not support cultured stem cells in stirred suspension.
The seeding protocol described here yields higher fractions of attached cells than the current practice of loading hPSCs as clumps on microcarriers for culture. For instance, hESCs are seeded on Matrigel-coated microcarriers as clumps with ∼30% efficiency versus almost 80% when seeded as single cells. Moreover, regular culture with the agitation at a set rate can be commenced after 4 h of inoculating the beads with cells. The nominal loss of cells during the “loading phase” and the reduced preparation time are important from a bioprocess development standpoint. In fact, the protocol timeframe is significantly shorter than a 3-day seeding preparation reported for hESC clumps on cationic microcarriers (Hillex II; SoloHill) with xeno-free medium in spinner flasks.23 In that study, cells were cultured for a single 11-day passage and therefore the applicability of the method could not be evaluated over longer-term cultivation entailing successive passages. We also found that incubation with the ROCK inhibitor for 24 h after seeding commences is necessary for the long-term culture of hPSCs in stirred-suspension. During this interval, the inhibitor may curtail apoptosis (e.g., from shear) as the cells adjust to the bioreactor environment.
The use of pLL with the vitronectin-derived RGD peptide for decorating the surface of microcarriers supported the adhesion of hPSCs and their growth under agitation. pLL is a positively charged synthetic polymer used to facilitate cell attachment while it also promotes the adsorption of ECM proteins to culture surfaces.39 To our knowledge, there are no reports of pLL alone supporting the maintenance of pluripotent hPSCs in culture. However, pLL enhances the attachment and spreading of mesenchymal stem cells on tissues culture flasks unlike other soluble polymers, which are neutral or negatively charged.49 Correspondingly, microcarriers with positive surface charges are better suited for the culture of hPSCs compared to beads with negative or no outer charge.7,23 We posit that the combination of pLL and RGD peptide is effective because the former enhances hPSC attachment by increasing the positive charge on the microcarrier surface while the latter provides proper binding sites for integrin-mediated cell adhesion on beads.
The fraction of dispersed cells attached on CP+pLL microcarriers was almost 50% of that of cells seeded on Matrigel-coated beads. A similar pattern was noted when comparing the seeding efficiencies of hPSC clusters on the two types of microcarriers. Although CP+pLL beads supported the proliferation and differentiation of IMR90 cells over multiple passages, there is still space for improving the seeding efficiency. Matrigel is a complex protein mixture primarily consisting of entactin, laminin, and collagen IV providing sites for cell adhesion.50 Thus, using an assortment of synthetic peptides featuring distinct cell adhesion sites (e.g., RGD, IKVAV, and YIGSR) rather than a single sequence may enhance the attachment and spreading of hPSCs on microcarriers for stirred-suspension cultivation.
The polystyrene microcarriers with surface COOH groups were utilized in our study to show that 3D surfaces can be engineered for the scalable culture of hPSCs by employing relatively simple chemistry for peptide conjugation. Going forward, however, other microcarriers may be utilized with lower specific gravity or different architecture (e.g., macroporous) to minimize potential effects due to shear-induced agitation. Moreover, use of biodegradable microcarriers will be advantageous for direct implantation of stem cell progeny reducing or eliminating steps of downstream separation of cells from beads and thus decreasing the overall cost. For instance, lactic/glycolic acid-based biomaterials featuring chemical groups for attaching adhesion molecules may be used to engineer such microcarriers. Lastly, nonspherical microcarriers amenable to the same surface functionalization can be considered as their use may confer advantages as others have indicated.7
In conclusion, our study demonstrates the facile engineering of microcarriers for long-term scalable culture of hPSCs under defined xeno-free conditions. hPSCs were successfully maintained on vitronectin peptide-conjugated/pLL-treated microcarriers in stirred-suspension vessels for multiple passages without compromising their pluripotency, proliferation, and karyotype. The microcarrier culture system described here is a step forward in the development of bioprocesses integrating expansion and directed differentiation of human stem cells for the production of therapeutically useful progeny.
Supplementary Material
Acknowledgments
Funding support has been provided by the National Institutes of Health (NHLBI, R01HL103709) and the New York Stem Cell Science Trust (NYSTEM, contracts C024355 and C026714) to E.S.T.
Disclosure Statement
No competing financial interests exist.
References
- 1.Kehoe D.E., Jing D., Lock L.T., and Tzanakakis E.M.Scalable stirred-suspension bioreactor culture of human pluripotent stem cells. Tissue Eng Part A 16,405, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodrigues C.A., Fernandes T.G., Diogo M.M., da Silva C.L., and Cabral J.M.Stem cell cultivation in bioreactors. Biotechnol Adv 29,815, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Sharma S., Raju R., Sui S., and Hu W.S.Stem cell culture engineering—process scale up and beyond. Biotechnol J 6,1317, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Krawetz R., Taiani J.T., Liu S., Meng G., Li X., Kallos M.S., et al. Large-scale expansion of pluripotent human embryonic stem cells in stirred-suspension bioreactors. Tissue Eng Part C Methods 16,573, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Jing D.Investigation of the cardiogenic differentiation of human pluripotent stem cells in static cultures and stirred-suspension bioreactors [Ph.D. 3423476]. State University of New York at Buffalo, New York, United States, 2010 [Google Scholar]
- 6.Lock L.T., and Tzanakakis E.S.Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue Eng Part A 15,2051, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen A.K., Chen X., Choo A.B., Reuveny S., and Oh S.K.Critical microcarrier properties affecting the expansion of undifferentiated human embryonic stem cells. Stem Cell Res 7,97, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Lecina M., Ting S., Choo A., Reuveny S., and Oh S.Scalable platform for human embryonic stem cell differentiation to cardiomyocytes in suspended microcarrier cultures. Tissue Eng Part C Methods 16,1609, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Bardy J., Chen A.K., Lim Y.M., Wu S., Wei S., Weiping H., et al. Microcarrier suspension cultures for high-density expansion and differentiation of human pluripotent stem cells to neural progenitor cells. Tissue Eng Part C Methods 19,166, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Nie Y., Bergendahl V., Hei D.J., Jones J.M., and Palecek S.P.Scalable culture and cryopreservation of human embryonic stem cells on microcarriers. Biotechnol Prog 25,20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh S.K., Chen A.K., Mok Y., Chen X., Lim U.M., Chin A., et al. Long-term microcarrier suspension cultures of human embryonic stem cells. Stem Cell Res 2,219, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Fernandes A.M., Marinho P.A., Sartore R.C., Paulsen B.S., Mariante R.M., Castilho L.R., et al. Successful scale-up of human embryonic stem cell production in a stirred microcarrier culture system. Braz J Med Biol Res 42,515, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Phillips B.W., Lim R.Y., Tan T.T., Rust W.L., and Crook J.M.Efficient expansion of clinical-grade human fibroblasts on microcarriers: cells suitable for ex vivo expansion of clinical-grade hESCs. J Biotechnol 134,79, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Martin M.J., Muotri A., Gage F., and Varki A.Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med 11,228, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Liu Y., Song Z., Zhao Y., Qin H., Cai J., Zhang H., et al. A novel chemical-defined medium with bFGF and N2B27 supplements supports undifferentiated growth in human embryonic stem cells. Biochem Biophys Res Commun 346,131, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Li Y., Powell S., Brunette E., Lebkowski J., and Mandalam R.Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol Bioeng 91,688, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Lu J., Hou R., Booth C.J., Yang S.H., and Snyder M.Defined culture conditions of human embryonic stem cells. Proc Natl Acad Sci USA 103,5688, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao S., Chen S., Clark J., Hao E., Beattie G.M., Hayek A., et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci USA 103,6907, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furue M.K., Na J., Jackson J.P., Okamoto T., Jones M., Baker D., et al. Heparin promotes the growth of human embryonic stem cells in a defined serum-free medium. Proc Natl Acad Sci USA 105,13409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludwig T.E., Levenstein M.E., Jones J.M., Berggren W.T., Mitchen E.R., Frane J.L., et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol 24,185, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Ludwig T.E., Bergendahl V., Levenstein M.E., Yu J., Probasco M.D., and Thomson J.A.Feeder-independent culture of human embryonic stem cells. Nat Methods 3,637, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Chen G., Gulbranson D.R., Hou Z., Bolin J.M., Ruotti V., Probasco M.D., et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods 8,424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marinho P.A., Vareschini D.T., Gomes I.C., Paulsen Bda S, Furtado D.R., Castilho Ldos R, et al. Xeno-free production of human embryonic stem cells in stirred microcarrier systems using a novel animal/human-component-free medium. Tissue Eng Part C Methods 19,146, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Rodin S., Domogatskaya A., Strom S., Hansson E.M., Chien K.R., Inzunza J., et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol 28,611, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Braam S.R., Zeinstra L., Litjens S., Ward-van Oostwaard D., van den Brink S., van Laake L., et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells 26,2257, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Villa-Diaz L.G., Nandivada H., Ding J., Nogueira-de-Souza N.C., Krebsbach P.H., O'Shea K.S., et al. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat Biotechnol 28,581, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brafman D.A., Chang C.W., Fernandez A., Willert K., Varghese S., and Chien S.Long-term human pluripotent stem cell self-renewal on synthetic polymer surfaces. Biomaterials 31,9135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klim J.R., Li L., Wrighton P.J., Piekarczyk M.S., and Kiessling L.L.A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat Methods 7,989, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mei Y., Saha K., Bogatyrev S.R., Yang J., Hook A.L., Kalcioglu Z.I., et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat Mater 9,768, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irwin E.F., Gupta R., Dashti D.C., and Healy K.E.Engineered polymer-media interfaces for the long-term self-renewal of human embryonic stem cells. Biomaterials 32,6912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melkoumian Z., Weber J.L., Weber D.M., Fadeev A.G., Zhou Y., Dolley-Sonneville P., et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol 28,606, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Kilian K.A., Bugarija B., Lahn B.T., and Mrksich M.Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci USA 107,4872, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engler A.J., Sen S., Sweeney H.L., and Discher D.E.Matrix elasticity directs stem cell lineage specification. Cell 126,677, 2006 [DOI] [PubMed] [Google Scholar]
- 34.McBeath R., Pirone D.M., Nelson C.M., Bhadriraju K., and Chen C.S.Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6,483, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Watanabe K., Ueno M., Kamiya D., Nishiyama A., Matsumura M., Wataya T., et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 25,681, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Kehoe D.E., Lock L.T., Parikh A., and Tzanakakis E.S.Propagation of Embryonic Stem Cells in Stirred Suspension without Serum. Biotechnol Prog 24,1342, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Wu J., and Tzanakakis E.S.Contribution of Stochastic Partitioning at Human Embryonic Stem Cell Division to NANOG Heterogeneity. PLoS One 7,e50715, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nat R., Nilbratt M., Narkilahti S., Winblad B., Hovatta O., and Nordberg A.Neurogenic neuroepithelial and radial glial cells generated from six human embryonic stem cell lines in serum-free suspension and adherent cultures. Glia 55,385, 2007 [DOI] [PubMed] [Google Scholar]
- 39.McKeehan W.L.Use of basic polymers as synthetic substrata for cell culture. In: Barnes D.W., Sirbasku D.A., Sato G.H., eds. Methods for Preparation of Media, Supplements, and Substrata for Serum-Free Animal Cell Culture. 1st ed., New York: Alan R. Liss, Inc., 1984. pp. 209–213 [Google Scholar]
- 40.Ang S.L., Wierda A., Wong D., Stevens K.A., Cascio S., Rossant J., et al. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development 119,1301, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Sasaki H., and Hogan B.L.Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development 118,47, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Lin L., Cui L., Zhou W., Dufort D., Zhang X., Cai C.L., et al. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci USA 104,9313, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petropoulos H., Gianakopoulos P.J., Ridgeway A.G., and Skerjanc I.S.Disruption of Meox or Gli activity ablates skeletal myogenesis in P19 cells. J Biol Chem 279,23874, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Kattman S.J., Huber T.L., and Keller G.M.Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell 11,723, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Xu C., Inokuma M.S., Denham J., Golds K., Kundu P., Gold J.D., et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol 19,971, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Ruoslahti E., and Pierschbacher M.D.New perspectives in cell adhesion: RGD and integrins. Science 238,491, 1987 [DOI] [PubMed] [Google Scholar]
- 47.Yap L.Y., Li J., Phang I.Y., Ong L.T., Ow J.Z., Goh J.C., et al. Defining a threshold surface density of vitronectin for the stable expansion of human embryonic stem cells. Tissue Eng Part C Methods 17,193, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Heng B.C., Li J., Chen A.K., Reuveny S., Cool S.M., Birch W.R., et al. Translating human embryonic stem cells from 2-dimensional to 3-dimensional cultures in a defined medium on laminin- and vitronectin-coated surfaces. Stem Cells Dev 21,1701, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Lu H., Guo L., Kawazoe N., Tateishi T., and Chen G.Effects of poly(L-lysine), poly(acrylic acid) and poly(ethylene glycol) on the adhesion, proliferation and chondrogenic differentiation of human mesenchymal stem cells. J Biomater Sci Polym Ed 20,577, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Hughes C.S., Postovit L.M., and Lajoie G.A.Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10,1886, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.