Abstract
Extensive damage to the limbal region of the cornea leads to a severe form of corneal blindness termed as limbal stem cell deficiency (LSCD). Whereas most cases of corneal opacity can be treated with full thickness corneal transplants, LSCD requires stem cell transplantation for successful ocular surface reconstruction. Current treatments for LSCD using limbal stem cell transplantation involve the use of murine NIH 3T3 cells and human amniotic membranes as culture substrates, which pose the threat of transmission of animal-derived pathogens and donor tissue-derived cryptic infections. In this study, we aimed to produce surface modified therapeutic contact lenses for the culture and delivery of corneal epithelial cells for the treatment of LSCD. This approach avoids the possibility of suture-related complications and is completely synthetic. We used plasma polymerization to deposit acid functional groups onto the lenses at various concentrations. Each surface was tested for its suitability to promote corneal epithelial cell adhesion, proliferation, retention of stem cells, and differentiation and found that acid-based chemistries promoted better cell adhesion and proliferation. We also found that the lenses coated with a higher percentage of acid functional groups resulted in a higher number of cells transferred onto the corneal wound bed in rabbit models of LSCD. Immunohistochemistry of the recipient cornea confirmed the presence of autologous, transplanted 5-bromo-2′-deoxyuridine (BrdU)-labeled cells. Hematoxylin staining has also revealed the presence of a stratified epithelium at 26 days post-transplantation. This study provides the first evidence for in vivo transfer and survival of cells transplanted from a contact lens to the wounded corneal surface. It also proposes the possibility of using plasma polymer-coated contact lenses with high acid functional groups as substrates for the culture and transfer of limbal cells in the treatment of LSCD.
Introduction
The corneal epithelium is constantly renewed throughout life. The corneal epithelial stem cells reside at the limbus, a distinct anatomical structure at the corneoconjunctival junction.1–5 In cases of mild corneal surface damage, the limbal stem cells are activated, proliferate, and migrate to the central cornea assisting tissue regeneration and homeostasis. In cases of deep central corneal wounding, the eyes can be treated by penetrating keratoplasty (PKP). However, if the damage involves the limbal region, the corneal epithelium fails to regenerate and the conjunctiva invades the corneal surface resulting in discomfort and vision loss, often accompanied by severe inflammation leading to permanent corneal scarring.2,6 This condition termed as limbal stem cell deficiency (LSCD) can arise from a variety of etiologies, both inherited and acquired,6 most commonly by burns and acid and alkali injuries.7 As the epithelium of donor corneas has a short lifespan, LSCD patients cannot be successfully treated by PKP.8–12
Unilateral LSCD can be successfully treated with autologous keratolimbal grafts of 2–3 clock hours size (about 25% of the limbus) taken from the healthy fellow eye. However, larger grafts may involve the risk of inducing donor-site LSCD. In addition, transplantations of allogenic keratolimbal grafts for the bilateral LSCD patients involve the risk of graft rejection even with the use of potent immunosuppressive medications11 and the long-term outcomes are often poor.13,14 Cultured limbal epithelial transplantation using in vitro engineered corneal epithelial tissues is an alternative to conventional limbal grafting. This technique requires only a smaller limbal biopsy (2×2 mm) followed by expansion of stem cells in culture, thereby reducing the risk to the donor eye.15–17
Various surfaces have been used for in vitro culture of limbal epithelial cells, such as the fibrin gels, intact or de-epithelialized human amniotic membrane (hAM), and temperature-responsive culture inserts.15,16,18–23 Some of these procedures use mitotically inactivated mouse NIH 3T3 cells as feeder layers and animal products, including fetal bovine serum.15,16,18,23 Xenobiotics involve the risk of transmission of animal pathogens, while the use of human biological materials like hAM involves the risk of donor–host transmission of cryptic infections. In addition, hAM is not easily accessible, and the quality may vary from lot to lot.24 Epithelial cells have been cultured without fetal bovine serum or a feeder cell layer,20,25,26 but a suitable replacement for hAM has not yet emerged.
Plasma polymerization is a method used to deposit pinhole-free coatings onto a variety of surfaces. This technique utilizes electrical plasma to fragment chemical vapors into highly charged components. The reactive components adhere well to materials and form disordered polymers on the surface. The degree of fragmentation can be controlled and functional groups in the chemical vapor can be retained. Thus, this technique can be used to change the surface chemistry of materials. Plasma polymerization is currently used within the contact lens industry to improve wettability or to prevent lipid adhesion.27 It can be utilized to deposit functional groups that promote cell growth. This has previously been demonstrated through the use of acid-functionalized surfaces that promoted the growth of keratinocytes.28,29 The surface concentration of the acid groups can be controlled by the addition of diluents to the plasma process, which, in turn, can affect how strongly cells adhere to these surfaces, allowing the cells to transfer from the surface onto a wound bed.30
This study investigates the use of plasma polymer-coated contact lenses as substrates for in vitro culture and also as carriers for in vivo transfer of limbal epithelial cells in the treatment of LSCD. Unlike the established hAM-based techniques, where a contiguous sheet of epithelium supported by a membrane is sutured or glued onto the corneal surface using a fibrin glue, the contact lens-based approach transfers only the cells. Deshpande et al. have demonstrated that acid-functionalized surfaces deposited by plasma polymerization are able to support human and rabbit corneal epithelial cell proliferation in vitro.30 The current study demonstrates the suitability of this technique for successful transfer of cultured corneal epithelial cells onto the wounded corneal surface and confirms that transferred labeled cells survive, proliferate, and undergo stratification in vivo.
Materials and Methods
Animal experiments had the approval of the St. Vincent's Hospital, Melbourne Animal Ethics Committee and were consistent with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research.
Preparation of plasma copolymer-coated contact lenses
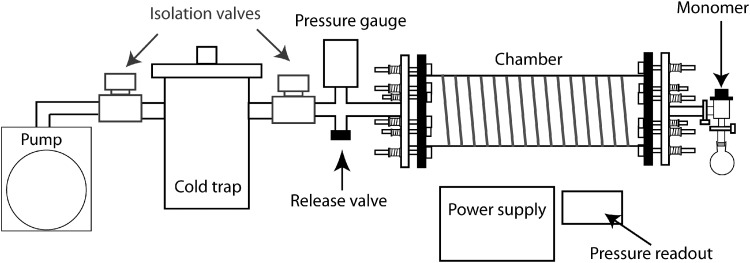
The plasma reactor consists of a 2-L cylindrical sample chamber with an external wire coil. Power was inductively supplied to the coil at 2 W and the sample chamber was kept under vacuum using a rotary pump. Plasma was sustained by a radio frequency (13.56 MHz) generator and was inductively coupled to the end flanges of the sample chamber. A schematic of the plasma reactor is shown in Figure 1. The base pressure within the reactor was typically within the range of 1.0×10−3–3.0×10−3 mbar.
FIG. 1.
Schematic of plasma reactor.
Before the plasma polymerization, the acrylic acid (AA; Sigma Aldrich) and 1,7-octadiene (OCT; Merck) monomers were processed with three freeze–thaw cycles to remove any dissolved gas. Silicone hydrogel contact lenses (Efrofilcon A; Contamac) were placed concave side facing upward within the chamber on a culture plate as a support base. A cleaned glass coverslip was also inserted for X-ray photoelectron spectroscopy (XPS) analysis of the deposited plasma polymer layer. AA was used to provide the acid functionality (–COOH), while OCT was used as the diluent to reduce the concentration of acid groups deposited onto the surfaces. These two chemicals are termed monomers and they were introduced into the chamber using a fine needle valve at various ratios at a combined flow rate of 2 sccm. Ratios of the monomers explored (AA:OCT) were 100:0, 75:25, 50:50, and 0:100 OCT. Plasma was sustained at a power of 2 W over a period of 15 min. Uncoated Efrofilcon A and Contamac lenses are used as negative controls and Focus Night and Day lens and CIBA Vision are used as positive control lenses.
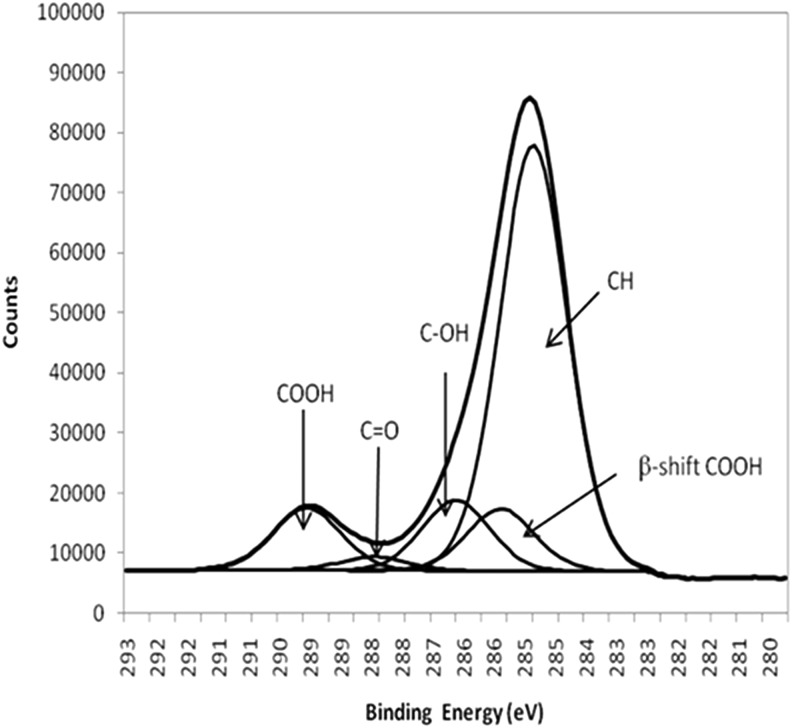
XPS was used to analyze the chemical composition of the plasma polymer deposited layer. A SAGE XPS system (SPECS) with a Phoibos 150 hemispherical analyzer and a MCD-9 detector was used. All the results presented in this study corresponded to the use of nonmonochromated MgKα (hν 1253.6 eV), operated at 10 kV and 20 mA (200 W). Survey spectra (0–1000 eV binding energy) were collected at a pass energy of 100 eV with a step size of 0.5 eV. Narrow scans of the C1s core level peak were collected at a pass energy of 20 eV with a step size of 0.1 eV. The XPS spectra were analyzed using CasaXPS (Neil Fairley) using supplied relative sensitivity factors. For curve fitting of the narrow scan, C1s core level peaks, a full width at half maximum of 1.55 eV, were used throughout and were used to identify relative amounts of –COOH for each sample (Fig. 2)
FIG. 2.
C1s spectra of acrylic acid (AA) and curve fitted peaks. Area under the –COOH fitted curve was used to calculate the percentage of functional –COOH groups present on the surface.
Explant culture of rabbit corneal epithelium
Explant culture of the rabbit corneal epithelium was performed as described previously.20 Rabbit limbal explants (1×2 mm) were obtained from dissected autologous limbal tissues of experimental animals. Contact lenses arrived dehydrated and were rehydrated overnight in corneal epithelial growth media (45% Dulbecco's modified Eagle's medium [DMEM], 45% Ham's F12 media [1:1 DMEM:F12; Gibco USA]), 10% fetal bovine serum, epithelial growth factor (10 ng/mL; Invitrogen), insulin-transferring-selenium supplement (5 μg/mL-5 μg/mL-5 ng/mL; Invitrogen), and antibiotic–antimycotic (100 U/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL, and amphotericin B; Invitrogen). Explant cultures were established on the concave side of lenses placed in six-well plates. For in vitro organ culture and characterization studies, a single explant was placed at the center of the contact lens. For in vivo studies on cell transfer, four explants were placed peripherally on each lens. The cultures were incubated overnight in 500 μL of corneal epithelial growth media to allow attachment of explants to the lens surface, and then the volume was increased to 2 mL. The cultures were then maintained in an incubator at 37°C with 5% CO2 supply. Spent media were changed once in 2 days during the subsequent 3–4 days of culture period. For in vivo cell transfer studies, the limbal cultures on the lens were pulsed with 100 μM of 5-bromo-2′-deoxyuridine (BrdU; Sigma Aldrich) for 3 h. After the pulse period, the medium was removed, thoroughly washed, and further cultured for 2 h with BrdU-free corneal epithelial growth media before using the cells for transfer onto the rabbit eyes.
Rabbit corneal organ culture
Rabbit corneal organ culture was developed with slight modifications of the method described by Deshpande et al.30 Rabbit eyes were harvested from fresh cadavers, transported, and stored in phosphate-buffered saline (PBS) at −20°C. The eyes were then thawed and submerged in 20% methanol in PBS for 60 s. The corneal epithelium was removed by thorough scraping, including the limbal region, leaving the Bowman's membrane intact. Corneoscleral buttons, which include about 3–5 mm of scleral margin, were then dissected from the eyes and freeze thawed at −20°C three times. This method of corneal preparation destroys any residual cells so that the corneal surface provides only a suitable wound bed for cell transfer. The corneas were then placed in a sterile dish with the concave side facing upward, then filled with 320 μL of filler media (1:1 DMEM:F12 with 35% sterile agar), and allowed to set for 10 min. The corneas were then turned upright, transferred to a culture dish with 2 mL of media, and incubated at 37°C with 5% CO2 supply overnight to inspect for contamination before using them as in vitro cornea models.
Corneal explants were cultured on the concave surface of contact lenses for 4 days before the explants were removed using sterile forceps. The contact lens was then transferred to the de-epithelialized surface of the recipient cornea models. The contact lens surface was maintained at the air–liquid interface and the surface was wetted by applying 100 μL of medium to the lens every 12 h. The organ cultures were further maintained for 3 days. This was repeated a number of times using different plasma polymer-coated contact lenses. In cases where the explants had failed to attach, the experiment was discontinued.
Resazurin assay for cell proliferation
The resazurin solution (Sigma) is nontoxic and has been used for corneal cells and organ-cultured corneas.31,32 A stock solution of resazurin was dissolved in PBS (880 μM), filter sterilized, and stored in the dark at 4°C. For the assay, the spent medium was removed from the culture wells and 1950 μL of fresh corneal media and 50 μL of resazurin stock solution were added. A no-cell control well was also prepared in parallel. Cultures were incubated for 6 h at 37°C and 5% CO2. Media were collected from each well and the absorbance at 570 nm was determined with a Nanodrop spectrophotometer (Thermo Fisher Scientific), blanked against a no-cell control (n=6). A standard curve, derived from twofold serial dilutions from 500,000 limbal cells, is used to determine cell numbers.
Cultured corneal epithelial cell transfer in a rabbit model of LSCD
Surgical removal of the limbus in rabbits results in a model with features of LSCD, including vascularization and conjuctivalization of the corneal surface.1,2 New Zealand white rabbits (weighing 2–2.5 kg) were given an intramuscular general anesthetic (ketamine 35 mg/kg, xylazine 5 mg/kg, acepromazine 0.75 mg/kg) and topical oxybupivicaine 0.4%. Rabbits were then subjected to a 360° conjunctival limbal peritomy starting 2 mm posterior and extending anterior from the corneal limbus using blunt scissors. The debridement was performed by topical application of methanol and mechanical debridement of residual limbal and central epithelia followed by irrigation with PBS. The nictitating membrane was then sutured in its normally retracted position within the medial canthus using 1 mattress suture of 4-0 silk. Suture knots were placed on the dermal surface and the sutures were buried within the third eyelid itself to avoid risk of ocular abrasion. Following surgery, the eyes were treated with topical chloramphenicol drops four times daily for 7 days, corticosteroid; prednisolone acetate and phenylephrine hydrochloride drops twice daily for the duration of experiment, topical cycloplegic; atropine 1% daily for 1 week, and topical oxybupivicaine Pro re nata (i.e., as needed). At 4 days postinjury, the contact lens with (6 rabbits) or without (3 control rabbits) autologous cultured corneal epithelium cells were applied.
At 3 days after transfer (7 days postinsult), the lenses were removed. At 26 days after transfer (30 days postinsult), the corneal surface was examined after applying 1% fluorescein sodium and the ocular surface was examined using a slit lamp microscope with a cobalt blue filter. The animals were then euthanized and the corneas were excised for histological examination.
Immunofluorescence and histochemistry
For immunofluorescence, the cells grown on contact lenses for 4 days were pulsed with BrdU (100 μM) for 30 min, fixed with 4% paraformaldehyde for 10 min, washed with PBS, permeabilized with 0.5% Triton X100 for 10 min, washed and blocked with 10% goat serum. The cells were then incubated with primary antibodies (rabbit anti-Keratin-K12 [H-60, 1:50], rabbit anti-p63α [H-129, 1:50]; Santa Cruz Biotechnology, Inc., mouse anti-BrdU [ZBU-30; Zymed]) for 1 h at room temperature. After three PBS washes, the cells were incubated with secondary antibodies at 1:500 dilution (anti-mouse or anti-rabbit Alexa 488/Alexa-594 conjugates; Molecular Probes) for 45 min at room temperature. The cells were counterstained with either DAPI or PI. After washes, the whole mounts were imaged using an epifluorescent microscope (Olympus, IX71).
For immunohistochemistry (IHC), the rabbit corneas were fixed for 1 h at 4°C with 4% paraformaldehyde and then processed into paraffin blocks. Five-micrometer-thick sections were mounted on glass slides, dewaxed with xylene, and permeablized with ice-cold 70% methanol for 10 min. Antigen retrieval and DNA denaturation was performed using 2 N HCl for 30 min at room temperature before washing with PBS and neutralizing in the borate buffer (0.1 M). Sections were quenched for endogenous peroxidase using 3% H2O2 for 5 min, washed in PBS, and then blocked with 10% goat serum. The sections were then incubated with a mouse anti-BrdU monoclonal antibody (Clone ZBU-30; Zymed)33 at 1:800 dilution overnight at 4°C. After three washes with PBS, a goat anti-mouse biotin (DAKO) secondary antibody at 1:200 dilution was added and incubated for 30 min at room temperature, washed with PBS, and followed by incubations with the streptavidin horseradish peroxidase conjugate (DAKO). After washing, the sections were developed by incubating with 3,3′-diaminobenzidine (Thermo Fisher) for 10 min and counterstained with hematoxylin. Normal control rabbit corneal sections were used as negative controls.
Results
XPS characterization of plasma polymer surfaces
Whereas it is known that plasma polymers with hydrophilic surfaces can support cell adhesion, we hypothesized that a critical mix of hydrophilic and hydrophobic surfaces may not only promote cell adhesion and proliferation, but also enable better cell transfer, when an ideal substrate is provided, such as the in vivo wound bed. In this study, we have prepared and analyzed four different plasma polymer surfaces using 100% AA, 100% OCT, and combinations of both (75:25 and 50:50 of AA:OCT).
The C1s narrow scan was utilized to calculate the percentage of acid groups deposited onto the surface (Fig. 2). Table 1 is a summary of the concentration of acid group percentages calculated for each of the treated surfaces. As the ratio of AA to OCT changed, the acid peak increased in correlation with a decrease in OCT, where hereafter, the surfaces will be referred to as high, mid, and low acid-functionalized surfaces. In samples that were not plasma polymerized with AA, an acid peak in the C1s was not detected. After 15 min of plasma deposition time, the chemistry of the underlying contact lens material was no longer evident, indicating that a plasma layer was thicker than the XPS analysis depth of 10 nm.
Table 1.
Percentage of Acid Groups Calculated for Each Surface Type (n=9)
| Surface chemistry | Average –COOH% | Standard deviation |
|---|---|---|
| AA only | 18.01 | 2.8 |
| 75:25 AA:OCT | 7.59 | 1.1 |
| 50:50 AA:OCT | 3.7 | 0.5 |
| OCT only | Not detected | N/A |
AA, acrylic acid; N/A, not applicable; OCT, octadiene.
Assessment of cell adhesion using limbal explant cultures
To assess the ability of different plasma polymer surfaces to promote cell adhesion, the proportion of rabbit limbal explants that remain adhered on the lens surface at 3 days of culture was compared among different plasma polymer chemistries as shown in Table 2. As the concentration of acid functional groups on the contact lens surface increased, the attachment of the explants improved. Whereas high and mid acid-functionalized surfaces enabled 75% explant attachment, only 50% of the explants remain attached on low acid-functionalized surfaces. As expected, none of the explants remained attached on OCT-only surfaces. The commercially available siloxane hydrogel contact lenses (Focus Night and Day lens [CIBA Vision]), which were used previously for the culture and transplantation of the corneal epithelium in human patients,34 were used as positive controls and showed about 81% explant attachment.
Table 2.
Attachment Rates of Rabbit Corneal Epithelium Explants to Different Plasma Polymer Coatings in Contact Lenses
| Surface | OCT only | 50:50 AA:OCT | 75:25 AA:OCT | AA only | Night&Day™ |
|---|---|---|---|---|---|
| Explant attachment | 0/5 | 5/10 | 6/8 | 6/8 | 13/16 |
| % attachment | 0 | 50 | 75 | 75 | 81 |
Assessment of cell proliferation and transfer in vitro
To assess for the ability of different plasma polymer-coated contact lenses to support cell transfer, after 4 days of culture, the contact lenses with the limbal cells were transferred onto the surface of in vitro corneal organ culture models as described in the methods. After 3 days of transfer, the lenses were removed and cell numbers on the recipient corneal surfaces and the lens surfaces were determined by the resazurin assay. The total viable cell numbers and the percentage cell transfer were calculated from the resazurin data.
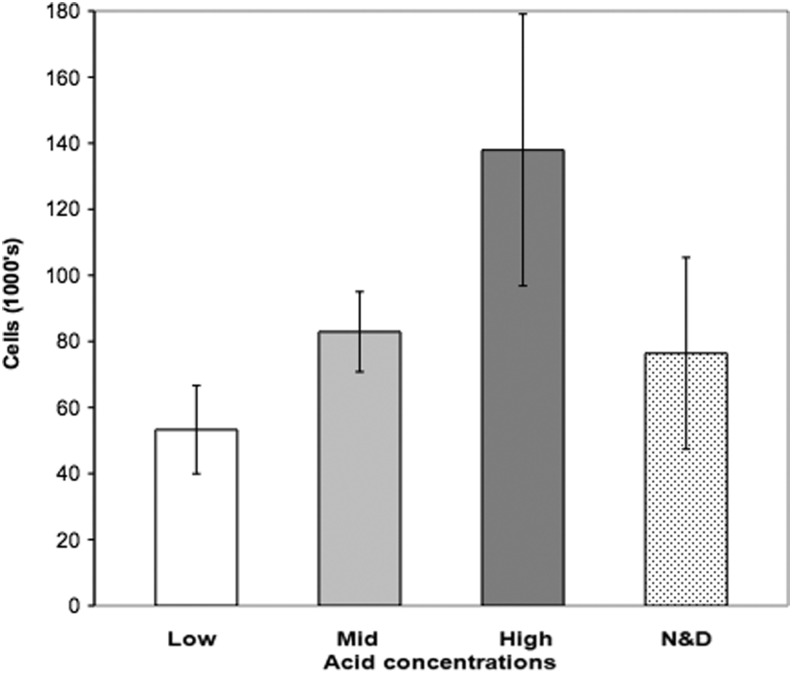
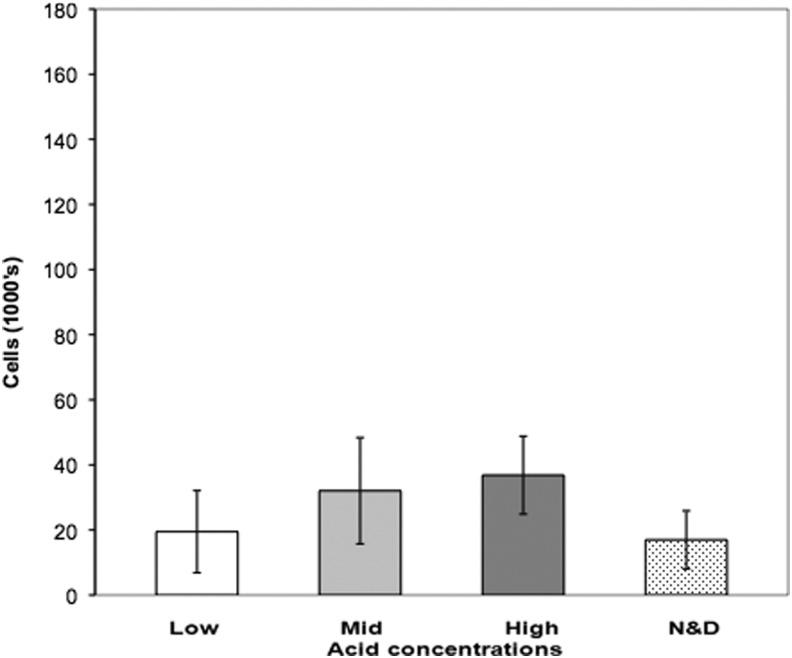
Although not statistically significant, a trend of higher total cell viability correlating with a higher concentration of acid functional groups was observed as shown in Figure 3.
FIG. 3.
Proliferation of corneal epithelial cells in organ culture. Rabbit corneal epithelial explants are grown on different contact lenses and total cell numbers were determined by resazurin assay. Low, mid, and high: concentration of acid group functionality. Error bars are SEM. N&D, commercial grade Focus Night and Day lenses.
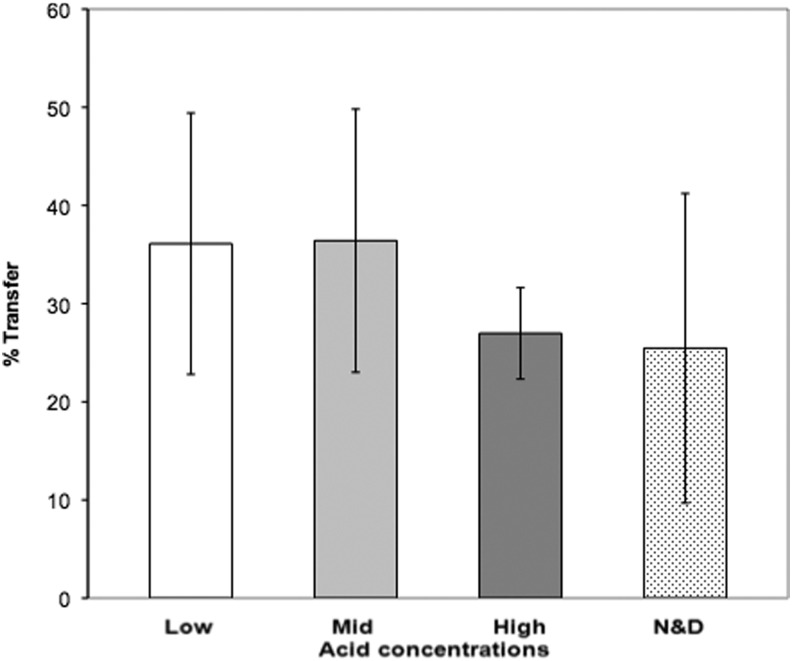
Cell transfer was initially assessed using in vitro organ culture by comparing cell numbers on the recipient corneal surface to the total cell number on the lens at 6 days (Fig. 3). As hypothesized, the majority of the cells remained well adhered to the highly hydrophilic high acid-functionalized surface and showed a lower percentage of cell transfer (27%) than the surfaces that are less hydrophilic such as the mid and low acid-functionalized surfaces (both 36%). The commercial contact lenses showed the lowest transfer rate (25%) suggesting a highly hydrophilic nature (Fig. 4). However, there were no statistically significant differences due to biological variability.
FIG. 4.
Transfer rates of corneal epithelium cells from plasma polymer-coated contact lenses in rabbit corneal organ culture. Rabbit corneal epithelium explant cultures were grown on different contact lenses and the percentage of the total cell number transferred to the wound determined by resazurin assay (mean data). Low, mid, and high: different concentration of acid functional groups. Error bars are SEM.
When we compared the total cell numbers transferred on to the recipient corneal surface, high acid-functionalized surface enabled the highest number of cells transferred (mean 36,800 cells) despite a lower transfer percentage (Fig. 5). Whereas mid acid-functionalized surface also enabled the transfer of a high number of cells (mean 32,000 cells), the low acid-functionalized surface and the Day and Night lens had the lowest number of cells transferred (mean 19,500 and 17,000 cells, respectively).
FIG. 5.
Transfer of corneal epithelial cells from plasma polymer-coated contact lenses onto rabbit corneal organ culture. Rabbit limbal explant cultures were grown on different contact lenses and transferred onto a rabbit corneal organ culture model. Cell numbers that got transferred were determined by resazurin assay. Low, mid, and high: different concentration of acid functional groups. Note that the scales are kept identical to Figure 3 to allow for direct comparison. Error bars are SEM.
Characterization of limbal cells grown on 100% AA-coated lenses
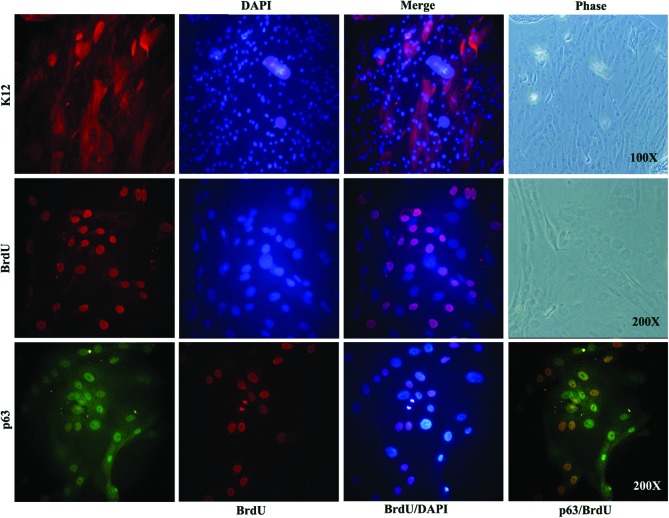
Since 100% AA enabled better cell growth and also allowed more number of cell transfer, we have finalized to use this surface to carry out the proof-of-concept trial for cell transfer in rabbit models of LSCD. To validate the limbal cells grown on 100% AA-coated lenses, we did immunostaining to check for the expression of K12 (mature corneal marker) and p63α (epithelial stem cell marker) on cultures grown for 4 days. As shown in Figure 6, the cultured cells are a heterogeneous pool consisting of both the mature cells and stem cells. In addition, we found that about 20–30% cells incorporated the BrdU label within a 30-min pulse, suggesting their active proliferative state. Therefore, for transplantation onto rabbit eyes, we have pulsed the cells with BrdU for 3 h before transfer to track the transplanted autologous cells in vivo.
FIG. 6.
Immunostaining of limbal cells cultured on 100% AA-coated lenses. Limbal cells grown on 100% AA-coated contact lenses were fixed and single stained for the corneal epithelial marker, K12 (red) and cell proliferation marker, 5-bromo-2′-deoxyuridine (BrdU) (red). The cells were also dual stained for the stem cell marker, p63α (green) and BrdU (red). The cells counted stained with DAPI (blue). A phase image of the same field shows the cell morphology. Color images available online at www.liebertpub.com/tea
Transplantation of autologous limbal cells and assessment of in vivo cell transfer, survival, and stratification
The size of rabbit corneas are comparable to that of humans and are compatible with the commercially available contact lenses intended for human use.30 In addition, they share structural similarities with that of humans, including the presence of the Bowman's layer. Therefore, rabbits have been used extensively for studying corneal epithelial cells.20,30,35,36 In this study, we have generated rabbit LSCD models and used them to assess the transfer of autologous cultured cells from the lens surface to the corneal wound bed.
To track the cells transferred on the recipient eyes, the autologous epithelial cells cultured on the lenses were pulsed with BrdU in vitro for a short period before transplantation. All proliferating cells in culture would incorporate BrdU into their DNA. When chased for long periods, the highly proliferating and differentiating cells would lose the label and the slow dividing stem cell population would retain BrdU. This not only enables tracking of the transplanted cells, but also helps to assess whether the stem cells are retained on the recipient corneal surface.
The lens surface that carried the highest percentage of acid functional groups and enabled the transfer of highest number of cells (as shown in Fig. 5) was chosen for the in vivo cell transfer study. The contact lenses with (tests) or without (sham controls) the cultured and BrdU-labeled autologous cells were applied to the wound bed of the recipient corneal surface. The lenses were allowed to remain for 3 days to enable cell transfer before they were removed. At 26 days post-transfer, the corneas were examined by fluorescein staining before the animals were euthanized and the corneas were excised for IHC using the anti-BrdU antibody.
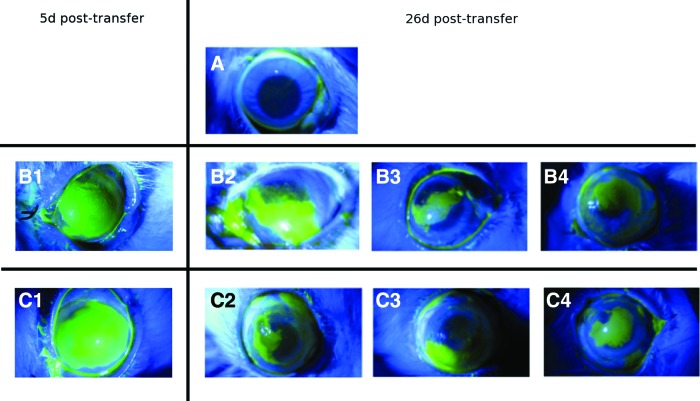
Upon surface examination using fluorescein staining, the recipient eyes showed only partial reconstruction of the corneal surface and were not noticeably different from the sham controls (Fig. 7). However, hematoxylin staining of the IHC sections of the recipient corneas of test animals revealed the presence of patches of a stratified three to four layered epithelium, suggesting that the transplanted cells survived, proliferated, terminally differentiated, and stratified in vivo. In addition, some of the basal and suprabasal cells retained the BrdU label, indicating successful cell transfer and retention of transplanted stem cells in vivo (Fig. 8).
FIG. 7.
Fluorescein staining of experimental animal eyes. (A) Untreated eye, no surgery. (B1) Sham surgery, with no cells on contact lenses at 5 days post-transfer. (B2–B4) Sham surgery, with no cells on contact lenses at 26 days post-transfer. (C1) Eyes that received contact lens with cultured autologous limbal epithelial cells at 5 days post-transfer. (C2–C4) Eyes that received contact lens with cultured autologous limbal epithelial cells at 26 days post-transfer. (B1) and (B2) are the same animal, (C1) and (C2) are another animal, all other eyes are biological repeats. Color images available online at www.liebertpub.com/tea
FIG. 8.
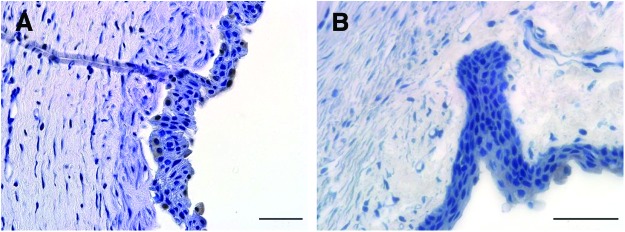
Transfer of corneal epithelial cells in vivo: Different contact lenses with BrdU pulsed and washed corneal cells applied to the wounded rabbit corneal surface to allow in vivo cell transfer. After 30 days post-transfer, animals were sacrificed and corneas were excised for immunohistochemistry to detect the retention of BrdU label. (A) Corneal section showing BrdU label retaining cells both at the basal and apical layers of the regenerated corneal epithelium. (B) Normal healthy rabbit corneal sections without BrdU labeling were used as negative controls. Color images available online at www.liebertpub.com/tea
Discussion
Plasma polymerization has been utilized successfully in the past to deposit acid functional groups onto bandages to promote the growth and transfer of cultured keratinocytes into a skin wound bed.28,29 Consistent with the observations of primary corneal epithelial cells here, AA-derived surfaces have previously been shown to promote keratinocyte attachment and a greater number of cells transferred onto de-epithelialized dermis culture in vitro.28 The presence of acid functional groups on a surface is known to promote cell attachment.37–39 If the cells are adhered too well, they are less likely to get transferred to the wound bed.28 Therefore, we investigated the transfer efficiency of surfaces with various concentrations of acid functionalities.
The total number of cells cultured on plasma polymerized contact lenses in our study is comparable with a previous in vitro study using rabbit organ culture models.30 However, Deshpande et al. seeded suspended corneal epithelial cells that require 3T3 feeder cells,30 which are less desirable for clinical use. Previous studies have demonstrated feeder-free explant culture of corneal epithelium on commercial siloxane-hydrogel lenses in vitro25 and a pilot study on humans produced clinical improvement in three patients. However, transfer of cells from the lens to the corneal surface was not conclusively demonstrated.34 We used the same commercial siloxane-hydrogel contact lenses as control surfaces. We confirmed that corneal epithelium can be cultured on siloxane-hydrogel contact lenses. However, our high acid-functionalized contact lenses promoted greater cell proliferation than these commercial contact lenses. Cell transfer was reduced as the proportion of AA used increased, however, greater cell numbers on the high acid surface resulted in the highest number of cells transferred despite lower transfer efficiency.
This study demonstrates that plasma polymer-coated contact lenses can be used as a culture surface for the corneal epithelium. The cultured limbal cell population was a heterogeneous pool consisting of both the stem cells and mature cells and were actively proliferating. The detection of BrdU-labeled cells on the corneal surface transferred from the lens, with a high acid-functionalized surface 26 days after transfer, demonstrates that successful cell transfer has occurred. The transferred cells contributed to the partial reconstruction of the recipient corneal surface.
Future trials may benefit from longer periods of culture to produce larger cell populations, which would then result in greater number of cells being transferred. A previous study found that transfer of cells from a suspension culture was poor in vitro after >5 days of culture.30 For this reason, we limited our outgrowth time to 3–4 days here. Although not statistically significant, the results of this study indicate a greater amount of epithelial cell proliferation on lenses with a high acid-functionalized surface that resulted in greater number of cells transferred despite lower transfer efficiency. In human patients, whose behavior involves better hygiene than rabbits, longer transfer times could be utilized and this may improve the transfer rate by allowing more time for cells to transfer or providing a protected environment to prevent the loss of transferred cells. While rabbits have been used with commercial contact lenses,30 the radius of anterior corneal curvature in the rabbit (7.26±0.26 mm) is smaller than found in humans (7.7–7.8 mm).40 A contact lens designed for the smaller curvature of the rabbit may improve cell transfer to the recipient cornea. Despite the transfer of cells, the clinical outcome for these rabbits was comparable to no-cell sham control animals. This ambiguity could be avoided by allowing more time (4–6 weeks) for the corneal surface to heal and to observe pannus growth for confirming the LSCD model before using them in cell transplantation studies.30 In our experiments, the cells grown on contact lenses were applied immediately at 3 days after creating the LSCD model. Therefore, it is possible that, either the controls had an incomplete LSCD or the prevailing inflammation had resulted in reduced transplanted cell survival. Nevertheless, the BrdU-labeled cells, which got transferred from the lens to the rabbit corneal surface, had survived for 30 days post-transfer and also resulted in partial reconstruction of the corneal surface.
Plasma polymerized contact lenses are used commercially; therefore, our contact lenses will be clinically acceptable if further trials prove its efficacy in humans. They have good storage properties and can be ethylene oxide sterilized to a surgical standard,28 as opposed to hAM, which requires storage at −70°C and cannot be sterilized. Our contact lens-based approach utilizing cultured corneal epithelial stem cells avoids the need for sutures and can be a suitable replacement for hAM. Combined with autologous cells and a culture system free of feeder cells and bovine serum, the risk of donor-derived cryptic infections could be avoided. However, this technique can also be used for allogenic applications using donor limbal tissues.
In summary, we found that a high concentration of acid functional groups on the plasma polymerized contact lens produced surfaces that encouraged greater cell attachment, proliferation, and transfer than the lens surfaces with lower concentrations of acid functional groups. Using such a contact lens surface, we were able to demonstrate that the cultured limbal cells can be transferred to the rabbit corneal surface. The transferred cells could also survive for up to 26 days post-transfer and could help in partial reconstruction of the recipient corneal surface. This is the first proof of principle experimentation that provides evidence for successful in vivo cell transfer from a contact lens surface on to rabbit eyes.
Acknowledgments
This project is supported by the Australian and Indian Government under the Australia-India Strategic Research Fund—a component of the Australian Scholarships initiative, the Ophthalmic Research Institute of Australia, and the Department of Biotechnology (DBT), Government of India. The Centre for Eye Research Australia and the O'Brien Institute receive Operational Infrastructure Support from the Victorian Government.
Disclosure Statement
No competing financial interests exist.
References
- 1.Chen J.J., and Tseng S.C.Corneal epithelial wound healing in partial limbal deficiency. Invest Ophthalmol Vis Sci 31,1301, 1990 [PubMed] [Google Scholar]
- 2.Riley M.V.Anion-sensitive ATPase in rabbit corneal endothelium and its relation to corneal hydration. Exp Eye Res 25,483, 1977 [DOI] [PubMed] [Google Scholar]
- 3.Cotsarelis G., Cheng S.Z., Dong G., Sun T.T., and Lavker R.M.Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells (abstract only). Cell 57,201, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Watanabe K., Nishida K., Yamato M., Umemoto T., Sumide T., Yamamoto K., et al. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett 565,6, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Majo F., Rochat A., Nicolas M., Jaoude G.A., and Barrandon Y.Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature 456,250, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Dua H.S., Saini J.S., Azuara-Blanco A., and Gupta P.Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol 48,83, 2000 [PubMed] [Google Scholar]
- 7.Baylis O., Figueiredo F., Henein C., Lako M., and Ahmad S.13 years of cultured limbal epithelial cell therapy: a review of the outcomes. J Cell Biochem 112,993, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Tsai R.J., and Tseng S.C.Human allograft limbal transplantation for corneal surface reconstruction. Cornea 13,389, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Ilari L., and Daya S.M.Long-term outcomes of keratolimbal allograft for the treatment of severe ocular surface disorders. Ophthalmology 109,1278, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Kenyon K.R., and Tseng S.C.Limbal autograft transplantation for ocular surface disorders. Ophthalmology 96,709; discussion 22, 1989. [DOI] [PubMed] [Google Scholar]
- 11.Tsubota K., Satake Y., Kaido M., Shinozaki N., Shimmura S., Bissen-Miyajima H., et al. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med 340,1697, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Holland E.J., and Schwartz G.S.The evolution of epithelial transplantation for severe ocular surface disease and a proposed classification system. Cornea 15,549, 1996 [PubMed] [Google Scholar]
- 13.Cauchi P.A., Ang G.S., Azuara-Blanco A., and Burr J.M.A systematic literature review of surgical interventions for limbal stem cell deficiency in humans. Am J Ophthalmol 146,251, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Solomon A., Ellies P., Anderson D.F., Touhami A., Grueterich M., Espana E.M., et al. Long-term outcome of keratolimbal allograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology 109,1159, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Tsai R.J., Li L.M., and Chen J.K.Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med 343,86, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Rama P., Bonini S., Lambiase A., Golisano O., Paterna P., De Luca M., et al. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation 72,1478, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Shortt A.J., Secker G.A., Notara M.D., Limb G.A., Khaw P.T., Tuft S.J., et al. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv Ophthalmol 52,483, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Pellegrini G., Traverso C.E., Franzi A.T., Zingirian M., Cancedda R., and De Luca M.Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 349,990, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Schwab I.R.Cultured corneal epithelia for ocular surface disease. Trans Am Ophthalmol Soc 97,891, 1999 [PMC free article] [PubMed] [Google Scholar]
- 20.Francis D., Abberton K., Thompson E., and Daniell M.Myogel supports the ex-vivo amplification of corneal epithelial cells. Exp Eye Res 88,339, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Koizumi N., Inatomi T., Suzuki T., Sotozono C., and Kinoshita S.Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology 108,1569, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Mariappan I., Maddileti S., Savy S., Tiwari S., Gaddipati S., Fatima A., et al. In vitro culture and expansion of human limbal epithelial cells. Nat Protoc 5,1470, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Schwab I.R., Reyes M., and Isseroff R.R.Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea 19,421, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Schwab I.R., Johnson N.T., and Harkin D.G.Inherent risks associated with manufacture of bioengineered ocular surface tissue. Arch Ophthalmol 124,1734, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Di Girolamo N., Chui J., Wakefield D., and Coroneo M.T.Cultured human ocular surface epithelium on therapeutic contact lenses. Br J Ophthalmol 91,459, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura T., Inatomi T., Sotozono C., Ang L.P., Koizumi N., Yokoi N., et al. Transplantation of autologous serum-derived cultivated corneal epithelial equivalents for the treatment of severe ocular surface disease. Ophthalmology 113,1765, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Lopez Alemany A., Compan V., and Refojo M.F.Porous structure of purevision (TM) versus focus (R) night & day (TM) and conventional hydrogel contact lenses. J Biomed Mater Res 63,319, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Haddow D.B., Steele D.A., Short R.D., Dawson R.A., and Macneil S.Plasma-polymerized surfaces for culture of human keratinocytes and transfer of cells to an in vitro wound-bed model. J Biomed Mater Res A 64,80, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Moustafa M., Simpson C., Glover M., Dawson R.A., Tesfaye S., Creagh F.M., et al. A new autologous keratinocyte dressing treatment for non-healing diabetic neuropathic foot ulcers. Diabet Med 21,786, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Deshpande P., Notara M., Bullett N., Daniels J.T., Haddow D.B., and MacNeil S.Development of a surface-modified contact lens for the transfer of cultured limbal epithelial cells to the cornea for ocular surface diseases. Tissue Eng Part A 15,2889, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Perrot S., Dutertre-Catella H., Martin C., Rat P., and Warnet J.M.Resazurin metabolism assay is a new sensitive alternative test in isolated pig cornea. Toxicol Sci 72,122, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Larson E.M., Doughman D.J., Gregerson D.S., and Obritsch W.F.A new, simple, nonradioactive, nontoxic in vitro assay to monitor corneal endothelial cell viability. Invest Ophthalmol Vis Sci 38,1929, 1997 [PubMed] [Google Scholar]
- 33.Khan S., Raza A., Petrelli N., and Mittleman A.In vivo determinations of labelling index of metastatic colorectal carcinoma and normal colonic mucosa using intravenous infusions of bromodeoxyuridine. J Surg Oncol 39,114, 1988 [DOI] [PubMed] [Google Scholar]
- 34.Di Girolamo N., Bosch M., Zamora K., Coroneo M.T., Wakefield D., and Watson S.L.A contact lens-based technique for expansion and transplantation of autologous epithelial progenitors for ocular surface reconstruction. Transplantation 87,1571, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Li Q.J., Ashraf F.M., Rana T.S., Tuli S., Mai E.L., Adler R.A., et al. Long-term survival of allogeneic donor cell-derived corneal epithelium in limbal deficient rabbits. Curr Eye Res 23,336, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Higa K., Shimmura S., Kato N., Kawakita T., Miyashita H., Itabashi Y., et al. Proliferation and differentiation of transplantable rabbit epithelial sheets engineered with or without an amniotic membrane carrier. Invest Ophthalmol Vis Sci 48,597, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Faucheux N., Schweiss R., Lutzow K., Werner C., Groth T., and Ltzow K.Self-assembled monolayers with different terminating groups as model substrates for cell adhesion studies. Biomaterials 25,2721, 2004 [DOI] [PubMed] [Google Scholar]
- 38.France R.M., Short R.D., Dawson R.A., and MacNeil S.Attachment of human keratinocytes to plasma co-polymers of acrylic acid octa-1,7-diene and allyl amine octa-1,7-diene. J Mater Chem 8,37, 1998 [Google Scholar]
- 39.Tidwell C.D., Ertel S.I., Ratner B.D., Tarasevich B.J., and Atre S.Endothelial cell growth and protein adsorption on terminally functionalized, self-assembled monolayers of alkanethiolates on gold. Langmuir 13,3404, 1997 [Google Scholar]
- 40.Bozkir G., Bozkir M., Dogan H., Aycan K., and Guler B.Measurements of axial length and radius of corneal curvature in the rabbit eye. Acta Med Okayama 51,9, 1997 [DOI] [PubMed] [Google Scholar]