Abstract
Essential cellular functions are often lost under culture in traditional two-dimensional (2D) systems. Therefore, biologically more realistic three-dimensional (3D) cell culture systems are needed that provide mechanical and biochemical cues which may otherwise be unavailable in 2D. For the present study, an alginate-based hydrogel system was used in which cells in an alginate solution were seeded onto dried alginate foams. A uniform distribution of NIH:3T3 and NHIK 3025 cells entrapped within the foam was achieved by in situ gelation induced by calcium ions integrated in the foam. The seeding efficiency of the cells was about 100% for cells added in a seeding solution containing 0.1–1.0% alginate compared with 18% when seeded without alginate. The NHIK 3025 cells were allowed to proliferate and form multi-cellular structures inside the transparent gel that were later vital stained and evaluated by confocal microcopy. Gels were de-gelled at different time points to isolate the multi-cellular structures and to determine the spheroid growth rate. It was also demonstrated that the mechanical properties of the gel could largely be varied through selection of type and concentration of the applied alginate and by immersing the already gelled disks in solutions providing additional gel-forming ions. Cells can efficiently be incorporated into the gel, and single cells and multi-cellular structures that may be formed inside can be retrieved without influencing cell viability or contaminating the sample with enzymes. The data show that the current system may overcome some limitations of current 3D scaffolds such as cell retrieval and in situ cell staining and imaging.
Introduction
Acurrent goal in developing biomaterials for cell culture, drug development, and tissue regeneration is to mimic the natural extracellular matrix (ECM) bridging the gap between in vitro and in vivo conditions.1 The approaches are highly diverse and aim at several aspects of creating environments for cells that reproduce, or mimic, what is found in nature. In the body, nearly all tissue cells reside in an ECM that consists of a complex three-dimensional (3D) fibrous meshwork of collagen and elastic fibers embedded in a highly hydrated gel-like material of glycosaminoglycans, proteoglycans, and glycoproteins, all together providing complex biochemical and physical signals.2
Despite the major differences compared with these 3D cell environments, most cell culture studies are performed using cells cultured as monolayers (two dimensional [2D]) on hard plastic surfaces because of the ease, convenience, and high cell viability associated with this culture method. However, forcing cells to adapt to an artificial flat and rigid surface can alter cell metabolism and change or reduce functionality, thereby providing results that may not be similar to expected behavior in vivo.2 Hence, a need for more realistic and controllable 3D cell culture that supports cell growth, organization, and differentiation is evident. Due to the large range of tissues being modeled with a variety of physical architectures and mechanical characteristics, the applicability of several different types of materials has been investigated and the formats are diverse.
Formats of alternative scaffolds to culture cells on 2D plastics include beads, fibers, membranes, meshes, foams, or hydrogels of different shapes and sizes.2,3 Even 2D cell culture onto different types of scaffolds show benefits compared with plastics, and valuable information about cellular responses to matrix stiffness and incorporated cell signaling factors has been obtained from such experiments.4–7 Some macroporous scaffolds, typically meshes, fibrous patches, or foams, enable cell seeding throughout the thickness of the matrix and cells may be spatially organized. Such systems are, however, considered semi-3D or 2.5D,2,8 as the initial cell-matrix interaction will be more similar to what is found in 2D, with cells on the surface of fibers or pore walls. A major challenge with such scaffolds may be cell seeding efficiency and cell distribution, as the pores are often either too small to let cells in or too large to retain cells inside. A variety of approaches have been investigated to overcome this challenge by utilizing, for example, cell seeding devices,9 bioreactors,10 centrifugal force,11 and vacuum.12,13
As referenced by others2,8 and in the present study, 3D cell culture can be defined as when cells are embedded in a hydrogel and signals from the scaffold and surrounding cells can be received from all directions. This requires that cells are first suspended in a hydrogel precursor solution and next entrapped by a gel initiation reaction forming covalently or noncovalently linked molecules.14,15 Hydrogels from both synthetic (for example, poly(ethylene glycol) (PEG), poly(hydroxyethyl methacrylate) (polyHEMA), and polyvinyl alcohol [PVA]) and natural polymers (for example, alginate, chitosan, hyaluronan, and dextran) are widely explored, including animal-derived materials from de-cellularized tissue and isolated proteins such as collagen and fibrin. Components of animal tissue are naturally recognized by cells due to the presence of cell binding ligands16 and have been considered good materials for scaffolds. However, these materials are less attractive because of a reduced degree of experimental control due to batch-to-batch variations as a result of their inherent diversity in material composition, the potential risk of pathogen transmission, immunogenicity, limited availability, and serious concerns for obtaining regulatory approval for use in the clinic.8 Despite the homogeneous nature of synthetic polymers, their use as cell-entrapping materials has to some extent been avoided, due to harsh polymerization conditions.2 However, some initiator systems for photopolymerization of, for example, PEG-diacrylates are suitable for cell-based hydrogel formation while considering cytotoxicity, cross-linking efficiency, and cross-linking kinetics.17 Several find natural hydrogels of nonanimal origin of great interest because of their outstanding biocompatibility and mild gelation conditions, although limited control of gelation kinetics, uncontrolled material composition, and limited control over mechanical properties have been reported.2
Alginates were introduced in the 1980s as a material for immobilizing living cells and creating an artificial organ.18 Culture of cells in alginate gel beads is well known,19 and a standard guide describing cell encapsulation in alginate is available from ASTM International.20 Alginates comprise a family of linear polysaccharides that can be extracted from brown seaweed and some bacteria. Alginates consist of (1→4) linked β-D-mannuronate (M) and α-L-guluronate (G) monomers. The content and distribution vary considerably between different alginates, enabling a structural basis for tailoring alginate-based materials.21 Alginates form hydrogels in the presence of certain divalent cations such as calcium and strontium, and blocks of consecutive G-residues (G-blocks) present in the polymer chain are the key structural elements of ionically cross-linked alginates. The mechanical properties of alginate-based hydrogels depend on the monomer composition of the alginate, its molecular weight, amount, and the gelling ions used and their concentrations.6,22,23 Well-characterized alginates with high purity may be used to prepare scaffolds with consistent mechanical properties for cell encapsulation and implantation with functionality as ECM.24–26 Such scaffolds are nonimmunogenic and can be made to have adjustable bioresorption properties that are strongly influenced by the amount and type of gelling ions initially bound to the alginate, and the alginate molecular weight.26–31 Bioresorption can also be modified by the use of hydrolytically susceptible oxidized alginates26,27,32–34 and incorporation of cross-links cleavable by local proteolysis by matrix metalloproteinases.35 Mammals do not have the capability to enzymatically degrade alginate molecules; however, a slow cleavage of the glycosidic bonds will take place due to alkaline β-elimination and acid hydrolysis, which may occur simultaneously under physiological conditions,36 although the former seems to dominate at pH 7.4.34 The rate constant of cleavage of glycosidic bonds can be estimated to about 3×10−6 h−1.34 Renal clearance has been shown for alginates below approximately 50 kDa.37
The present work describes the use of dried calcium alginate foams as a scaffold and source of gelling ions for an applied alginate solution that fills the pores of the foam and forms a gel in situ. In situ gelation is initiated by calcium ions that diffuse from the foam as it becomes rehydrated by the alginate solution, enabling entrapment and even distribution of cells and other molecules throughout the scaffold. A transparent composite hydrogel structure is formed, comprising a framework of rehydrated alginate foam filled by an alginate gel. The recent study describes a time-efficient and simplified system for 3D cell culture, where cell entrapment and cell retrieval is performed at conditions that are physiologically relevant for the cells. The characteristics of gelation rate and rigidity of the gels were evaluated by the influence of the concentration of applied alginate, and the type and concentration of gelling ions. Distribution of cells and seeding efficiency of murine fibroblasts (NIH:3T3) were compared and investigated for cell seeding solutions without alginate and with different alginate concentrations. Further, cell proliferation, formation of multi-cellular structures, and retrieval of cells and cellular structures were demonstrated using a human cervical carcinoma cell line (NHIK 3025).
Materials and Methods
Alginate foams and alginate for in situ gelation
Preparation of ionically gelled alginate foams by mechanical incorporation of air into an alginate solution, gelation, and subsequent air drying has been previously described.38 A few modifications were made to achieve a foam structure optimized for in situ gelation and cell seeding. Briefly, 2.0% (w/w) alginate (PRONOVA UP LVG, FG: 0.68, NG>1: 15.0, MW: 219 000 g/mol, NovaMatrix; FMC BioPolymer) was selected for the wet foam composition. A 4% aqueous dispersion of CaCO3 (0.43%, HuberCal 500 Elite; J. M. Huber Corp.) was sonicated (40 Hz, Branson 200) for 3×10 s to prevent agglomeration of particles.39,40 The amount of plasticizers in the wet foam formulation was 5.6% sorbitol (BioUltra; Sigma-Aldrich) and 2.4% glycerin (UltraPure; Invitrogen). 1.5% hydroxypropyl methyl cellulose (HPMC, Pharmacoat 603; Shin-Etsu) was used as the only foaming agent. Slowly hydrolyzing glucono-δ-lactone (GDL, 1.53%, Glucono delta lactone T; Roquette) was added to induce gelation by a transient lowering of pH and associated dissolution of CaCO3 particles. The molar ratio of GDL:calcium was 2:0.8 to ensure complete dissolution of the CaCO3 particles. Calcium was added to saturate 68% of the alginate monomers, where 100% saturation equals a molar ratio between the alginate monomers and calcium of 2:1. The wet foam was cast at 2 mm thickness and gelled for 4 h before drying. Disks (diameter: 14.5 mm) were cut from sheets of dry foam and placed in separate wells of 24-well cell culture plates (Costar; Corning, Inc.). The plates containing foam disks were sterilized by gamma (γ)-irradiation by a dose of 20 kGy at the Institute for Energy Technology. The foam is a component of NovaMatrix®-3D cell culture kit (www.novamatrix-3D.com).
The foam used for the cell proliferation experiment described later had a molar ratio of GDL to calcium of 2, and calcium was added to saturate 85% of the alginate monomers. The formulation was later changed as described earlier to ensure complete dissolution of the CaCO3 particles.
Alginates applied to dry alginate foam disks and used for in situ gelation are presented in Table 1 with regard to chemical composition (FG and NG>1), and weight average molecular weight (MW).
Table 1.
Weight Average Molecular Weight (MW) and Chemical Composition of Alginates Used for In Situ Gelation (Data from Supplier)
| Alginate (PRONOVA UP) | Molecular weight, MW (g/mol) | Fraction of G monomers, FG | Average G-block length, NG>1 |
|---|---|---|---|
| MVG | 207, 000 | 0.69 | 12.3 |
| LVM | 128, 000 | 0.43 | 5.4 |
| LVGa | 114, 000 | 0.69 | 15.5 |
Component of NovaMatrix®-3D. Vial of 50 mg micro-filtered (0.22 μm) and lyophilized alginate.
LVG, PRONOVA UP LVG; MVG, PRONOVA UP MVG; LVM, PRONOVA UP LVM.
Oscillatory rheology
A Physica MCR 300 rheometer (Anton Paar) was used to evaluate the in situ gelation kinetics of different alginates (Table 1) (0.5% (w/v) solutions). The alginates were dissolved in Dulbecco's-modified Eagle's medium (DMEM; Sigma-Aldrich) supplemented with 10% heat-inactivated Fetal Bovine Serum (FBS; Sigma-Aldrich), 1% Penicillin/Streptomycin (pen/strep 10, 000 units/mL; Biochrom AG), and 1% MEM nonessential amino acid solution (100×) (Sigma-Aldrich). Two dry foam disks were placed on top of each other on the serrated platform, and 200 μL of alginate solution were added. The foams were allowed to absorb the alginate solution for 1 min before the probe was placed into the measuring position. Polydimethylsiloxane (Xiameter PMX-200/10 cS, PDMSV; Dow Corning) was added to avoid drying of the gel, and the measurement started 1 min and 15 s after the addition of alginate solution. The structures were analyzed with a serrated probe (PP15, diameter 15 mm) at 37°C, 1 Hz frequency, 1% strain, and a constant gap of 0.7 mm. The structures were somewhat compressed to ensure good contact between the material and the serrated probe and platform. The values of storage modulus (G′), loss modulus (G′′), and phase angle (δ) were followed over time. Each type of alginate solution was tested in triplicate.
Young's modulus
The rigidity of alginate gels formed by in situ gelation was determined by compressing the gels with a Texture Analyser TA-XTplus (Stable Micro Systems) that was equipped with a 5 kg load cell and a cylindrical probe (diameter: 6.35 mm). The gels were compressed at a constant speed of 0.05 mm/s, and Young's modulus was calculated from the first linear region of nonzero slope, between 5% and 10% strain, of the stress–strain curve. The gap was kept constant at 2.00 mm, and the thickness of the gel was calculated from the distance the probe traveled before it came in contact with the gel.
Gels were first prepared to evaluate the influence of concentration of the alginate solution on in situ gelation. Solutions of PRONOVA UP LVG (LVG) alginate (Table 1) were prepared in complete DMEM within the concentration range of 0.10–1.25% (w/v). 100 μL LVG solution or DMEM only was added to alginate foams in 24-well plates and placed in an incubator with a humidified atmosphere, 37°C and 5% CO2. After 10 min, 1.5 mL DMEM at 37°C was added and incubation continued for 2 h before the gels were analyzed. Next, the effect of adding extra gelling ions on Young's modulus was evaluated. In brief, 1.5 mL of an isotonic calcium solution of 50 mM CaCl2 (Merck) and 250 mM mannitol (Sigma-Aldrich) or an isotonic strontium solution of 50 mM SrCl2 (Merck) and 250 mM mannitol at 37°C was added to gels 10 min after the addition of 0.5% and 1.0% LVG. After 10 min of incubation, the solutions with additional gelling ions were removed, the gels were washed with DMEM, and 1.5 mL new DMEM was added. The gels were incubated for another 2 h at 37°C before they were analyzed. Young's modulus was measured at different time points to evaluate the stability of gels over time. Gels were prepared with 0.5% LVG in DMEM and analyzed at day 0 (after 2 h incubation in DMEM), 3, 7, 14, and 21. DMEM was replaced thrice a week, and new gels were tested at each time point. Young's modulus is reported as the average of three to six replicate samples.
Cell types and maintenance of cells
NHIK 3025, an established cell line derived from a human cervical carcinoma in situ,41 and NIH:3T3, a cell line of murine fibroblasts from ATCC (CCL92), were kept in exponential proliferation by passaging thrice a week as monolayers in standard cell culture flasks (Nunc). NHIK 3025 and NIH:3T3 cells were cultured in Eagle's Minimum Essential Medium (MEM; Sigma-Aldrich) and DMEM, respectively, and both media were supplemented with 10% heat-inactivated FBS and 1% pen/strep (complete media). DMEM was additionally supplemented with 1% MEM nonessential amino acids. The cells were kept in a humidified atmosphere at 37°C and 5% CO2. Cells were detached from the culture flasks using a trypsin/ethylenediaminetetraacetic acid (EDTA) solution containing 0.05% (w/v) trypsin (Sigma-Aldrich) and 0.02% (w/v) EDTA (Sigma-Aldrich).
Alginate gels—cell seeding, culture, and retrieval
LVG alginate (Table 1) was dissolved in complete DMEM (NIH:3T3) or MEM (NHIK 3025) medium to obtain 1.00% or 1.25% (w/v) solutions. Cells from monolayer culture were detached and counted using a Scepter™ 2.0 cell counter (MilliPore). A cell seeding solution to be applied onto the foams was prepared by mixing cells in DMEM or MEM medium with the alginate solution. 100 μL of the cell seeding solution was then applied dropwise to each foam disk in the 24-well plate. The foam absorbed the solution completely and became fully hydrated. The disks were incubated at 37°C for 10 min before approximately 1.5 mL MEM/DMEM was added to the wells and the cells were cultured further. MEM/DMEM was replaced thrice per week, and disks were transferred with a spatula to new wells if cells grew out of the gels and formed monolayers on the bottom of the wells.
Cells and multi-cellular structures that formed inside the foams were retrieved by dissolving the alginate disks. Each gel disk was transferred with a spatula to a micro centrifuge tube containing 1.5 mL isotonic solution at 37°C of 50 mM trisodium citrate tribasic dihydrate (Sigma-Aldrich) and 104 mM NaCl (Merck) for dissolution. The tubes were gently agitated or turned during the de-gelling process, which took approximately 2 min. The tubes were then centrifuged at 320 g for 5 min, the supernatants were aspirated, and the cell pellets were resuspended in DMEM/MEM. For evaluation spheroids as described later, the isotonic citrate solution was added to disks in a petri dish and the spheroids were released from the gel.
Cell seeding efficiency and cell distribution
The influence of alginate concentration in the cell seeding solution on cell seeding efficiency and cell distribution was evaluated by preparation of different cell seeding solutions containing NIH:3T3 cells. Monolayer NIH:3T3 cells were first vital stained with a 2 μM Calcein AM solution prepared from 10 μL of a 1 mM Calcein AM stock solution (Ex/Em: 495/516 nm; Invitrogen Molecular probes) and 5 mL Hanks' balanced salt solution (H8264; Sigma-Aldrich). The cells were covered with the Calcein AM solution and kept dark at room temperature for 45 min.
Cell seeding solutions were then prepared with the stained cells (5.0×105 cells/mL) that were suspended in supplemented DMEM only, 0.1%, 0.5%, or 1.0% (w/v) LVG alginate dissolved in DMEM. The exact cell density in each solution was then determined (Scepter 2.0 cell counter) in triplicate. 100 μL of the cell seeding solution was applied to each of three foam disks and after 10 min of incubation at 37°C, 1.0 mL DMEM was added and the cells were incubated further for 1 h. To remove cells that were potentially not entrapped inside the foam disks, the DMEM surrounding the gels was replaced, before the gels were transferred with a spatula to new wells, and 1.0 mL DMEM was added.
Images demonstrating cell distribution throughout the gels were obtained using a confocal laser scanning microscope (Nikon Modular confocal microscope system D-Eclipse C1 attached to Nikon Eclipse TE 2000-U microscope; Nikon Instruments) that was equipped with an argon laser and filter (Ex/Em: 488/515 nm). The images were processed using Nikon EZ-C1 software (v 3.30).
To quantify the entrapped cells and determine the seeding efficiency, the gels were de-gelled as described earlier, and the cells were counted using a Scepter 2.0 cell counter. The relative seeding efficiency was calculated from the total cell number found from the averaged value of two cell counts for each of the three disks.
Cell proliferation
A cell seeding solution was prepared with NHIK 3025 cells (2.0×105 cells/mL) suspended in 0.5% (w/v) LVG alginate dissolved in MEM. The cells were entrapped, cultured, and retrieved by dissolution of the disks after 6, 12, and 19 days of culture and counted using a Countess® automated cell counter (Life Technologies). At day 19, large spheroids had formed inside the gels. The spheroids were disaggregated by treatment with trypsin/EDTA to produce a suspension of single cells. The action of trypsin was stopped by adding FBS. The total cell number at each time point was calculated as the average value of two cell counts for three disks. Data were then normalized to the initial cell seeding at day 0.
Spheroid formation
A cell seeding solution was prepared with NHIK 3025 cells (2.5×105 cells/mL) suspended in 0.5% (w/v) LVG alginate dissolved in MEM. The cells were entrapped, cultured and at different time points, viable cells were stained while being inside the gel. Gel disks were then transferred to separate small petri dishes (diameter: 3.6 cm), and entrapped cells were stained using Calcein AM (in Hanks') or CellTrace™ CFSE cell proliferation kit (Molecular Probes; Ex/Em: 492/517 nm). Staining of viable cells using Calcein AM was performed as described earlier. For CellTrace CFSE, a stock solution of 5 mM was prepared by addition of 18 μL DMSO and then, the working solution of 10 or 20 μM was prepared by dilution with Hanks'. The disks were covered with 1 mL working solution and incubated at 37°C for 15 min before the working solution was replaced with Hanks' and incubated further for 30 min. All disks with stained cells were washed thrice with Hanks' before imaging. Cells were stained with 10 μM CellTrace CFSE at day 0, with 20 μM CellTrace CFSE at days 6 and 10, with 2 μM Calcein AM at day 16, and with 4 μM Calcein AM at days 23 and 30. The concentration was increased due to the increase in cell densities, and better images were obtained when staining with Calcein AM compared with CellTrace CFSE at higher cell densities.
Distribution of spheroids within the alginate gel disks was evaluated using confocal laser scanning microscopy before isolating stained spheroids from two dissolved gel disks. About five images of cells from each disk were used to manually determine the diameters of 50 spheroids using the software with the confocal microscope. At day 0, the disks contained only single cells, and data were obtained from 30 cells from one gel disk. Not all of the formed multi-cellular structures were perfectly spherical, and the vertical and horizontal diameters were measured and averaged for each multi-cellular structure. The growth rate of spheroids was followed by calculating the volume of the spheroids.
Statistical analysis
Statistical analysis was carried out using single-factor ANOVA. *p<0.05, **p<0.01, and ***p<0.001. The data were fitted by GraFit 7.0.2 (Erithacus Software Limited). All data are presented as mean±standard deviation (SD).
Results
In situ gelation
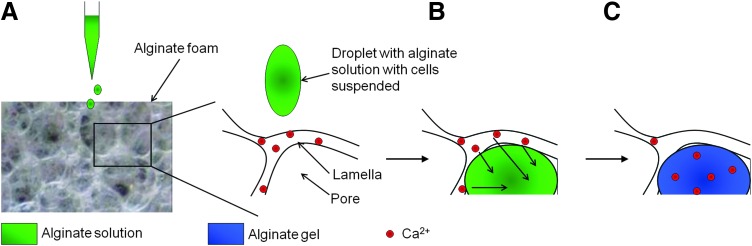
A continuous hydrogel was formed immediately after the applied alginate solution had been absorbed by the dry alginate foam disk. A stepwise description of the in situ gelation reaction is shown in Figure 1 and includes absorption of a sodium alginate solution by the calcium alginate foam (Fig. 1A) and the subsequent gelation of the alginate solution filling the pores as calcium ions diffuse from the hydrated lamellas of the foam (Fig. 1B, C). The transparent hydrogel structure that is formed comprises a framework of hydrated alginate foam with a hydrogel filling its pores.
FIG. 1.
Schematic presentation of the steps of in situ gelation including (A) application of sodium alginate solution on top of a dry calcium alginate foam; (B) Rehydration of the foam by the alginate solution filling its pores, and diffusion of calcium ions from the foam lamellas to the absorbed alginate; (C) Formation of a calcium alginate hydrogel inside the pores of the foam. The alginate solution may contain cells or other materials that will be entrapped in the hydrogel that is formed.
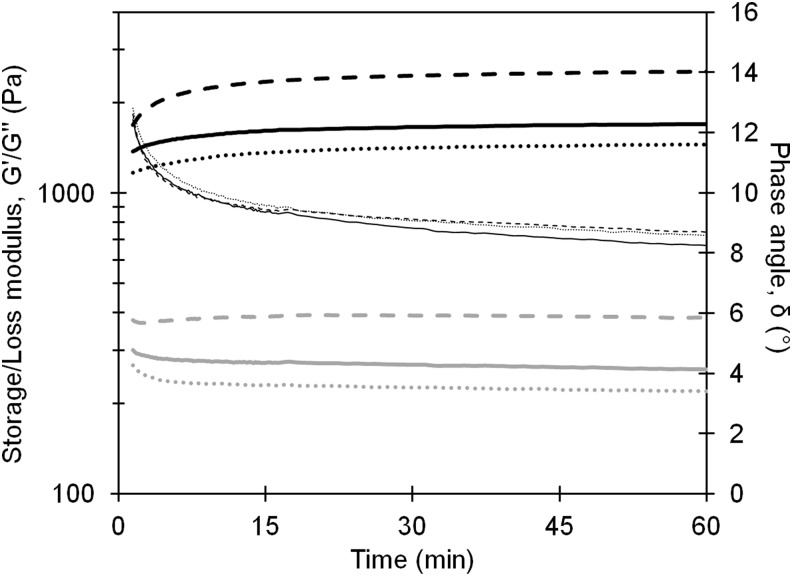
The in situ gelation reaction was monitored by oscillatory rheometry (Fig. 2). At all time points, the elastic properties of the materials dominated (G′>G′′). In addition, the ratio of G′:G′′ increased over time, showing that gel formation and gel rigidity increased as the added alginate cross-linked and gelled within the pores of the rehydrated foam. The increase in the dominating elastic properties of the gel was also confirmed by the decreasing phase angle (δ), defined as 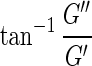 , where δ defines a Newtonian liquid at 90°, a Hookean solid at 0°, and viscoelastic materials in between with dominating elastic properties of the material for 0°<δ<45° that increase toward 0°. The highest G′ (2.6 kPa) was seen for gels with PRONOVA UP MVG (MVG), approximately 1.8 times higher than gels with PRONOVA UP LVM (LVM). The time derivative of the phase angle (dδ/dt) indicates a similar rate of gelation for the different types of alginate in this system.
, where δ defines a Newtonian liquid at 90°, a Hookean solid at 0°, and viscoelastic materials in between with dominating elastic properties of the material for 0°<δ<45° that increase toward 0°. The highest G′ (2.6 kPa) was seen for gels with PRONOVA UP MVG (MVG), approximately 1.8 times higher than gels with PRONOVA UP LVM (LVM). The time derivative of the phase angle (dδ/dt) indicates a similar rate of gelation for the different types of alginate in this system.
FIG. 2.
Gelation kinetics described by oscillatory rheometry after addition of 0.5% alginate solutions to dry alginate foam disks shown as storage modulus, G′ (thick black), loss modulus, G′′ (thick gray), and phase angle, δ (thin black). The gelation properties of PRONOVA UP LVG (solid line), PRONOVA UP MVG (dashed line), and PRONOVA UP LVM (dotted line) alginates in alginate foams are shown. The relative SD in the measurements was between 2–20%.
Mechanical properties of gel discs
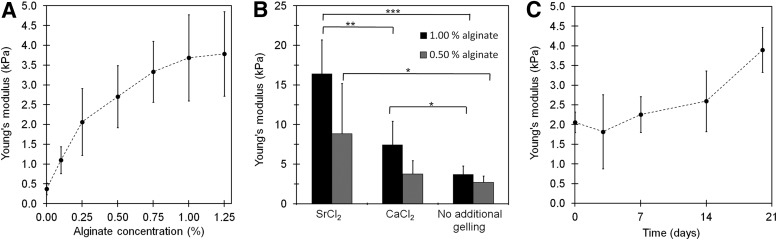
Compression testing was performed on gel disks added different concentrations of alginate solutions. Increased gel thicknesses and Young's moduli were seen for increasing LVG alginate concentrations within the range tested (0–1.25%) (Fig. 3A). The gel thickness increased from 0.93±0.07 mm for the foams-added DMEM only to 1.30±0.10 mm for the foams-added 1.25% LVG alginate. Further, addition of gelling ions by immersing the disks in isotonic gelling solutions of either SrCl2 or CaCl2 for a few minutes significantly influenced the rigidity of the disks-added 0.5% and 1.0% LVG alginate (Fig. 3B). Almost twice as rigid gels were achieved by immersing the disks in SrCl2 compared with CaCl2 (Fig. 3B). Disks with 0.5% LVG alginate that were kept in DMEM cell culture media which was replaced thrice per week showed stable and then slightly increasing Young's modulus over 3 weeks (Fig. 3C).
FIG. 3.
Young's modulus of gel disks as a function of the applied LVG alginate concentration (A). The effect on Young's modulus of gel disks added 1.0% or 0.5% LVG alginate followed by immersion in isotonic gelling solutions of either SrCl2 or CaCl2 (B). Young's modulus over time for gel disks-added 0.5% LVG alginate and kept in Dulbecco's-modified Eagle's medium (DMEM), which was replaced thrice per week (C). Characterization of gel disks in A, B, and C (Day 0) was accomplished on disks kept in DMEM for 2 h. Significance is considered between different treatments for the same concentration of added alginate. *p<0.05, **p<0.01, and ***p<0.001. Error bars present SD.
Cell seeding efficiency and distribution
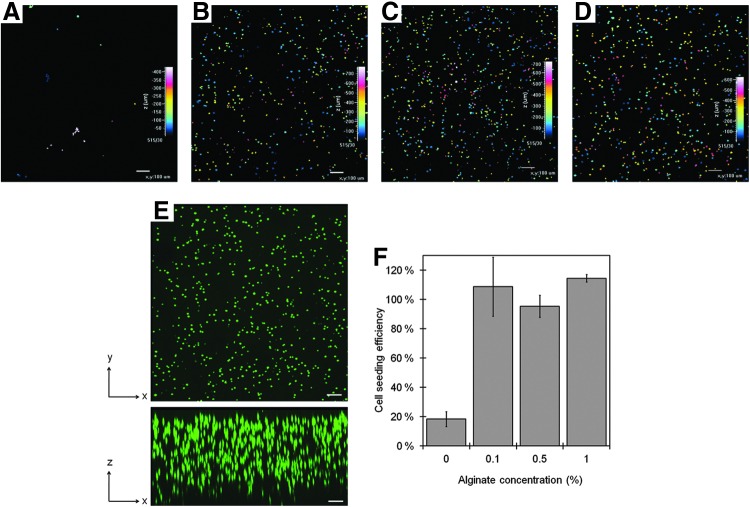
Images of fluorescently stained NIH:3T3 cells entrapped in gel disks show a striking effect of the presence of alginate in the cell seeding solution on cell seeding efficiency and cell distribution (Fig. 4A–D). Just a few cells were entrapped when they were applied to the foam suspended in DMEM only (Fig. 4A), whereas cells in 0.1%, 0.5%, and 1.0% alginate were uniformly distributed throughout 650 μm to 800 μm of the foam thickness (Fig. 4B–D). The uniform distribution of cells inside the gel, when applied to the foam suspended in 1.0% alginate, are evident on 3D images in xy- and xz- directions (Fig. 4E).
FIG. 4.
Distribution of NIH:3T3 cells in gel disks and cell seeding efficiency influenced by the alginate concentration in the cell seeding solution 2 h after seeding. Cell localization throughout the thickness of the gel indicated by colors when cells were suspended in cell seeding solutions containing alginate concentrations of 0% (A), 0.1% (B), 0.5% (C), and 1.0% (D). Cell distribution in gels showed for xy- and xz-directions (E). Percent cell seeding efficiency as a function of alginate concentration in the cell seeding solution (F). Magnification: ×40. Scale bars: 100 μm, Color bars: foam thickness 0–400 μm (A), 0–700 μm (B, D) and 0–600 μm (C). The error bars depict SD.
The high seeding efficiency was confirmed by counting of the cells retrieved from the foam when the seeding solution contained any concentration of alginate. Overall, 95–115% of the cells seeded in alginate was retrieved from the gel disks, whereas only 18% of the cells seeded without alginate became entrapped (Fig. 4F).
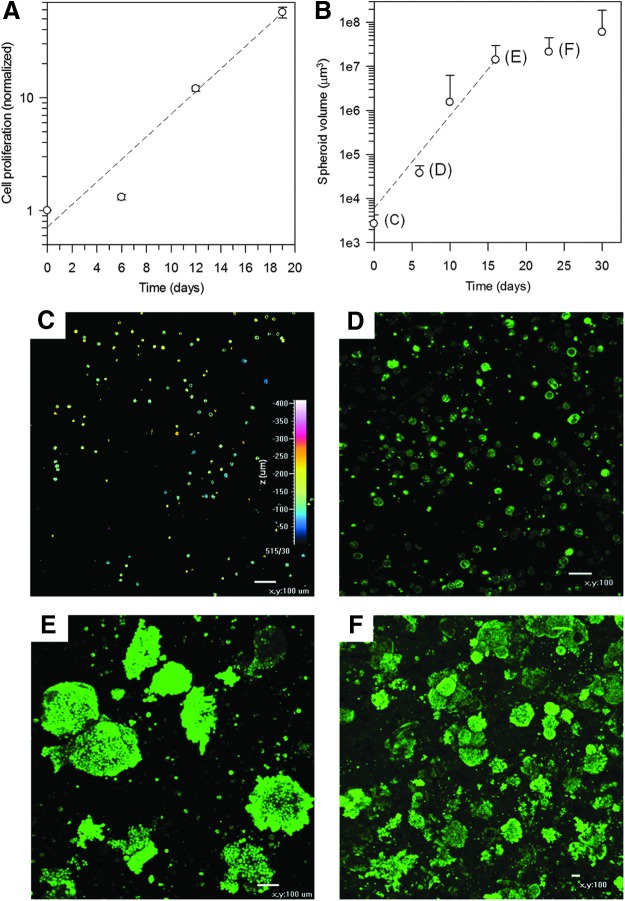
Cell proliferation, spheroid formation, and isolation
The proliferation of NHIK 3025 cells cultured in gel disks was followed for almost 3 weeks (Fig. 5A). Minor changes in total cell number were seen after 1 week of culture, whereas a 12-fold and 57-fold increase was measured after 12 and 19 days, respectively. The cells showed formation of multi-cellular aggregates as spheroids with increasing volumes over 30 days (Fig. 5B), with an average diameter of the spheroids after 30 days of 372±211 μm. Images of cells retrieved from the gels and inside the gel at different time points are shown in Figure 5C–F.
FIG. 5.
Proliferation of NHIK 3025 cells normalized to initial cell seeding when cultured in alginate gel disks as a function of time (A). An approximate exponential increase in cell number was found with a cell doubling time of 3.0±0.3 days. Volume of spheroids as a function of time, with an almost exponential increase between days 0 and 16 before it levels off (B). The calculated time to obtain double volume was based on the exponential fit of data between days 0 and 16 and was found to be 1.43±0.05 days. The images show distribution of calcein-stained cells throughout the thickness of the gel at the day of seeding (C), inside the gel after 6 days of culture (D), isolated after 16 days of culture (E), and inside the gel after 23 days of culture (F). Magnification: ×40 (C–E) and ×100 (F). Scale bars: 100 μm. Error bars present SD.
Discussion
The first aim of this study was to demonstrate the principles of in situ gelation. The delayed gelation enables the foam to be filled with the alginate solution before calcium ions diffuse from the rehydrated foam, thereby inducing gelation of the absorbed solution. A study by Herlofsen et al.42 demonstrated preparation of self-gelling alginate hydrogel disks by mixing a suspension of calcium alginate particles with a solution of sodium alginate and human bone marrow-derived mesenchymal stem cells for chondrogenic differentiation. Besides this study, there have been no other reports of the use of calcium alginate foam as the source of gelling ions for alginate solutions containing biologics and no previous reports utilizing predefined shapes such as the foams described here. The gelation kinetics and mechanical properties of the gels were influenced by the type of alginate that was applied to the foams (Figs. 2 and 3A). Previous studies39,43 demonstrated an increased strength and elasticity of alginate hydrogels by increasing the G-content of the alginate and by increasing the alginate concentration in a similar manner to what is demonstrated here. In this study, however, the calcium-to-alginate ratio was decreased by increasing the alginate concentration, whereas it was kept constant in the other studies. The slight flattening of the curve in Figure 3A for the highest alginate concentrations may be explained by the reduced amount of calcium ions available to gel the added alginate.
The ability to tune the mechanical properties of the gel is a positive attribute, as the foams contain a preselected concentration of gelling ions. Differentiation of stem cells and behavior of differentiated cells have been shown to be influenced by the mechanical properties of alginate hydrogels,6,44 and alginate hydrogels can be prepared within the elasticity range of most tissues.4 The system presented here matches the elasticity of soft tissues,4 and the demonstrated variations were obtained by changing the applied alginate and alginate concentration. The foam itself may also influence the resulting gel structure elasticity, as it may be made from different types and concentrations of alginate and contain different types and concentrations of gelling ions.38 The foams used in this study were sterilized by γ-irradiation, which induce alginate degradation and therefore loss of mechanical integrity of the foam itself.38 The selection of γ-sterilization doses will have an influence on the mechanical properties and also bioresorption rates of implantable alginate structures.23,28 Relative to other studies,38 the lower γ-dose of 20 kGy was used to retain better mechanical integrity.
A further increase in rigidity of the gels was achieved by adding extra gelling ions (Fig. 3B) by immersing the gelled structures in solutions of SrCl2 or CaCl2. An increased rigidity of strontium alginate gels compared with calcium alginate gels is ascribed to the higher affinity of G-rich alginates for strontium.19,45 A comparison of gels formed by cross-linking solutions of strontium, zinc, and calcium salts for bone tissue engineering31 showed increased stability of hydrogels by increasing alginate concentration, G-content, and strontium as the gelling ion. The same study also showed that increased rates of released strontium ions enhanced osteogenic differentiation. In addition, the ability to tune biodegradation may influence cellular behavior and formation of new tissue in response to mechanical stimuli. The present technology would enable utilization of the approaches previously described to tune the rate of degradation and to improve the quality and amount of newly formed tissue.26–33,35
A potential limitation to the use of ionically gelled alginates for cell culture is the sensitivity toward calcium-chelating compounds such as phosphate, citrate, and lactate, or nongelling cations such as sodium or magnesium. The gels prepared here showed no reduction in rigidity over 3 weeks when kept in DMEM (Fig. 3C). Cell culture media such as DMEM and MEM contain 1.8 mM Ca2+, which is sufficient to retain gel stability in this study, whereas other culture media such as Roswell Park Memorial Institute medium (RPMI, 0.4 mM Ca2+) and some other model physiologic solutions may need to be supplemented with Ca2+.46 Alternatively, increased stability can be achieved by regular immersion of the gels in solutions with gelling ions. Preparation of strontium alginate foams38,47 for in situ gelation may also increase the stability of the gels. An alternative approach is to form covalently cross-linked hydrogels,48 but later dissolution of the gels for cell retrieval may be challenging without the use of alginate lyases.42
The second aim of this study was to demonstrate the utilization of the two-component alginate system for uniformly seeding cells into the foam structure, followed by prolonged 3D cell culture, and retrieval of cells or multi-cellular structures by subsequent dissolution of the gels. The highly interconnected foams fully absorbed the cell seeding solutions of cells suspended in alginate solution or DMEM. Only a few cells were entrapped in the foams when seeded in DMEM only (Fig. 4A), whereas the in situ gelation ensured uniform distribution of NIH:3T3 cells throughout the thickness of the gels (Fig. 4B–E). Furthermore, neither cell distribution nor seeding efficiency was influenced by the concentration of alginate in the cell seeding solution from 0.1% to 1.0% alginate (Fig. 4B–D). The importance of alginate in the cell seeding solution was demonstrated by 95–115% cell seeding efficiency with alginate compared with 18% without alginate (Fig. 4F). There is, however, most likely an upper limit in viscosity for the seeding solution, as a too high solution viscosity would impair absorption by the foam and thus have an impact on structure homogeneity and cell distribution. The viscosity of the alginate will be influenced by both the concentration and molecular weight of the alginate.
A similar approach using a porous alginate-chitosan scaffold and an alginate-containing cell seeding solution has been demonstrated.49 In that study, the scaffold was first soaked in cell culture medium for at least 24 h, and the medium was removed by vacuum before the cells were seeded in the alginate solution. In contrast to the present study, diffusion of externally added calcium ions was used to gel the applied alginate. This will also expectedly give a structural difference compared with this system, as a predetermined amount of calcium will be evenly distributed inside the foam upon rehydration. The foams presented here may, therefore, be used to obtain isotropic gels with an adjustable degree of ionic cross-linking. A different approach of in situ gelation was presented by Weinstein-Oppenheimer et al.,50 who initiated a gelling reaction by mixing cells with thrombin and fibrinogen before the suspension was added to a porous scaffold. A fibrin gel with cells entrapped was later formed inside the pores. Ushida et al.51 entrapped cells in a scaffold of nonwoven poly-L-lactic acid by thermal gelation of an applied solution of collagen and cells. High seeding efficiency and uniform distribution of cells were achieved; however, retrieval of the cells from the device was not demonstrated. The ability to retrieve cells may enable further examination of cells and cellular structures by, for example, immunohistochemistry, gene and protein expression studies, or flow cytometry. The dissolution step did not seem to influence cell viability or disrupt the multi-cellular structures (Fig. 5E).
Cells entrapped in alginate hydrogels are mainly surrounded by an aqueous environment giving cells the space and ability to proliferate and form multi-cellular structures. This was confirmed here by NHIK 3025 cells entrapped in hydrogels of 99.5% aqueous medium and 0.5% alginate (Fig. 5A). This indicates a slower proliferation rate compared with a cell cycle time of 23.6 h reported for culture of these cells in monolayers with the same culture media.52 The cell cycle time of NHIK 3025 cells cultured as spheroids in culture media on nonadherent culture flasks has been reported to depend on the distance from the spheroid surface.53 Compared with 2D culture, the cell cycle traverse takes about 1.7 and 2.2 times longer if they are in the outer parts or the inner region of the spheroid, respectively.53 An increasing volume of multi-cellular structures was seen over the entire period of 30 days (Fig. 5B–F). After an approximated exponential growth between days 0 and 16, the growth seemed to level off, most likely inhibited by the high number of large spheroids (Fig. 5F). After 6 days of culture, only a minor increase in cell number was observed, while the volume of cells and cellular structures over the same period increased 10-fold. This may be explained by an initial formation of spheroids, but this should be investigated further, and staining of the DNA of each cell may better indicate the number of cells in each spheroid. The experiments were not conducted at the same time and with the exact same foam formulation.
The rapid formation of spheroids by NIHK 3025 cells (Fig. 5B, D–F) suggests that the technology may be a relevant tumour model for cancer drug development.1,8,54 Spheroid growth curves similar to Figure 5B can be made to evaluate drug and dose efficiency in a manner more relevant than current 2D studies. In addition, such systems are highly desirable as a replacement for toxicity testing in animals and in preclinical assays for the selection of appropriate drug candidates. Hydrogels may also be used as engineered xenografts containing structures to grow and deliver entrapped cancer cells in vivo to potentially increase the take rate (cell survival) and increase tumour formation.55 The lack of cell-ECM interactions with alginate may stimulate cell–cell interaction and provide valuable information for studies of cell–cell communication in tumor development.1 However, studies of cell–ECM interactions are also compatible with this system, as alginates may be covalently modified to contain known types and densities of cell adhesion molecules. Either the foam and/or the seeding solution may contain modified alginates with covalently attached signaling factors such as ligands that are recognized by integrins56,57 or syndecan cell receptors58.
The cell density should be optimized for the specific cell type and application, as it may influence cellular behavior such as proliferation rate, viability, deposition of newly formed ECM, and differentiation. Guidance of optimal cell densities using this system can be adapted from the literature where this parameter is investigated in comparable scaffolds for specific cell types such as chondrocytes,59,60 keratinocytes,61 hepatocytes,62 and intervertebral disk cells.63 The scaffold design such as porosity and dimensions are also crucial to ensure sufficient flow of oxygen, nutrients, and cellular waste products. This flow may be impaired by very large scaffolds and high polymer concentration, and approaches that are utilized to improve this parameter include identification of critical scaffold dimensions, use of macroporous scaffolds, and use of perfusion bioreactor systems.2,61,64,65
In addition to cells, the current system may be used to deliver drugs and signaling molecules from either the foam66,67 or the gelled alginate solution. The release kinetics of molecules entrapped in alginate gels is influenced by their size and/or charge and can be released by diffusion through the pores of the gel network (5 to 200 nm) or as the gel degrades.68 The release rate of fluorescent dextrans entrapped by in situ gelation in alginate foams showed a 30% slower release of the dextran of 70 kDa compared with 10 kDa.47 The diffusivity can be adjusted by type and concentration of alginate in addition to type and concentration of gelling ions.29,68,69 Alginates as immobilization matrices for cells and the release of therapeutic molecules take advantage of this control over diffusivity, as relatively small molecules such as nutrients, oxygen, proteins, and waste molecules freely diffuse in and out of the hydrogel, whereas the cells may be protected from the immune cells of the host.70
The nonanimal origin of the alginate-based system presented here likely offers less batch to batch variations that are inherently found in animal-derived materials and also reduced risk of pathogen transmission. This, therefore, also makes the system more relevant for in vivo use. In addition, it may provide a 3D platform for fundamental studies of cell mechanobiology and additional biomimetic approaches to tissue engineering such as incorporation of signaling molecules, which warrant further investigation.
Conclusions
The in situ gelation technique enables tailored mechanical properties of the foams utilizing the macromolecular properties of different alginates, in addition to the concentration of alginate and amount and type of gelling ions. The seeding efficiency of cells was 95–115% when cells were added in a seeding solution containing 0.1% to 1.0% alginate compared with 18% when seeded without alginate. A uniform distribution throughout the scaffold was achieved when the seeded cells were suspended in alginate. The cells were allowed to proliferate and form multi-cellular structures inside the gel that were vital stained and evaluated by confocal microscopy. Intact multi-cellular structures were isolated by dissolution of the gels for determination of spheroid growth rate, suggesting applicability as a tumor model.
The in situ gelation of alginate solutions within alginate foams containing cross-linking ions represents a new way of preparing transparent foam-hydrogel structures with a variable degree of cross-linking and mechanical properties. Furthermore, cells or other constituents may easily be evenly entrapped into the gels, and cell viability and proliferation may easily be followed by confocal microscopy. In addition, dissolution of the gel disk structures enabled easy retrieval of the containing cells and/or multi-cellular structures.
Acknowledgment
Portions of this work were supported by grant 22241 METOXIA from the European Union 7th Framework Programme.
Disclosure Statement
No competing financial interests exist.
References
- 1.Pampaloni F., Reynaud E.G., and Stelzer E.H.The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8,839, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Lee J., Cuddihy M.J., and Kotov N.A.Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev 14,61, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Justice B.A., Badr N.A., and Felder R.A.3D cell culture opens new dimensions in cell-based assays. Drug Discov Today 14,102, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Buxboim A., Ivanovska I.L., and Discher D.E.Matrix elasticity, cytoskeletal forces and physics of the nucleus: how deeply do cells “feel” outside and in? J Cell Sci 123,297, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reilly G.C., and Engler A.J.Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech 43,55, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Huebsch N., Arany P.R., Mao A.S., Shvartsman D., Ali O.A., Bencherif S.A., Rivera-Feliciano J., and Mooney D.J.Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 9,518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowley J.A., and Mooney D.J.Alginate type and RGD density control myoblast phenotype. J Biomed Mater Res 60,217, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Prestwich G.D.Simplifying the extracellular matrix for 3-D cell culture and tissue engineering: a pragmatic approach. J Cell Biochem 101,1370, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Soletti L., Nieponice A., Guan J., Stankus J.J., Wagner W.R., and Vorp D.A.A seeding device for tissue engineered tubular structures. Biomaterials 27,4863, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Papadimitropoulos A., Riboldi S.A., Tonnarelli B., Piccinini E., Woodruff M.A., Hutmacher D.W., and Martin I.A collagen network phase improves cell seeding of open-pore structure scaffolds under perfusion. J Tissue Eng Regen Med 7,183, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Dar A., Shachar M., Leor J., and Cohen S.Cardiac tissue engineering—Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol Bioeng 80,305, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Solchaga L.A., Tognana E., Penick K., Baskaran H., Goldberg V.M., Caplan A.I., and Welter J.F.A rapid seeding technique for the assembly of large cell/scaffold composite constructs. Tissue Eng 12,1851, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen M., Michaud H., and Bhowmick S.Controlled vacuum seeding as a means of generating uniform cellular distribution in electrospun polycaprolactone (PCL) scaffolds. J Biomech Eng 131,074521, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Drury J.L., and Mooney D.J.Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24,4337, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Place E.S., George J.H., Williams C.K., and Stevens M.M.Synthetic polymer scaffolds for tissue engineering. Chem Soc Rev 38,1139, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Rosso F., Giordano A., Barbarisi M., and Barbarisi A.From cell-ECM interactions to tissue engineering. J Cell Physiol 199,174, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Mironi-Harpaz I., Wang D.Y., Venkatraman S., and Seliktar D.Photopolymerization of cell-encapsulating hydrogels: crosslinking efficiency versus cytotoxicity. Acta Biomater 8,1838, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Lim F., and Sun A.M.Microencapsulated islets as bioartificial endocrine pancreas. Science 210,908, 1980 [DOI] [PubMed] [Google Scholar]
- 19.Smidsrød O., and Skjåk-Bræk G.Alginate as immobilization matrix for cells. Trends Biotechnol 8,71, 1990 [DOI] [PubMed] [Google Scholar]
- 20.ASTM International F 2315 Standard Guide for Immobilization or Encapsulation of Living Cells or Tissue in Alginate Gels. In Annual Book of ASTM Standards, Volume. West Conshohocken: ASTM International, 2003 [Google Scholar]
- 21.Andersen T., Strand B.L., Formo K., Alsberg E., and Christensen B.E.Alginates as biomaterials in tissue engineering. In: Rauter A.P., ed. Carbohydrate Chemistry: Chemical and Biological Approaches—Glycosciences for Human Health and Disease. London: Royal Society of Chemistry, 2012, pp. 227–258 [Google Scholar]
- 22.Draget K.I., Smidsrod O., and Skjak-Braek G.Alginates from algae. In: Vandamme E.J., De Baets S., and SteinBüchel A., eds. Biopolymers, Polysaccharides II: Polysaccharides from Eukaryotes. Weinheim: Wiley, 2002, pp. 215–244 [Google Scholar]
- 23.Kong H.J., Smith M.K., and Mooney D.J.Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 24,4023, 2003 [DOI] [PubMed] [Google Scholar]
- 24.ASTM International F 2064 Standard Guide for Characterization and Testing of Alginates as Starting Materials Intended for Use in Biomedical and Tissue-Engineered Medical Products Application In: in Annual Book of ASTM Standards. West Conshohocken: ASTM International, 2000 [Google Scholar]
- 25.Dornish M., Kaplan D., and Skaugrud O.Standards and guidelines for biopolymers in tissue-engineered medical products—ASTM alginate and chitosan standard guides. Ann Ny Acad Sci 944,388, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Lee K.Y., and Mooney D.J.Alginate: properties and biomedical applications. Prog Polym Sci 37,106, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boontheekul T., Kong H.J., and Mooney D.J.Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 26,2455, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Alsberg E., Kong H.J., Hirano Y., Smith M.K., Albeiruti A., and Mooney D.J.Regulating bone formation via controlled scaffold degradation. J Dent Res 82,903, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Simmons C.A., Alsberg E., Hsiong S., Kim W.J., and Mooney D.J.Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone 35,562, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Kong H.J., Alsberg E., Kaigler D., Lee K.Y., and Mooney D.J.Controlling degradation of hydrogels via the size of cross-linked junctions. Adv Mater 16,1917, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Place E.S., Rojo L., Gentleman E., Sardinha J.P., and Stevens M.Strontium- and zinc-alginate hydrogels for bone tissue engineering. Tissue Eng Part A 17,2713, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Bouhadir K.H., Lee K.Y., Alsberg E., Damm K.L., Anderson K.W., and Mooney D.J.Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog 17,945, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Jeon O., Alt D.S., Ahmed S.M., and Alsberg E.The effect of oxidation on the degradation of photocrosslinkable alginate hydrogels. Biomaterials 33,3503, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kristiansen K.A., Tomren H.B., and Christensen B.E.Periodate oxidized alginates: Depolymerization kinetics. Carbohydr Polymers 86,1595, 2011 [Google Scholar]
- 35.Fonseca K.B., Bidarra S.J., Oliveira M.J., Granja P.L., and Barrias C.C.Molecularly designed alginate hydrogels susceptible to local proteolysis as three-dimensional cellular microenvironments. Acta Biomater 7,1674, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Holme H.K., Davidsen L., Kristiansen A., and Smidsrod O.Kinetics and mechanisms of depolymerization of alginate and chitosan in aqueous solution. Carbohydr Polymers 75,656, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Al-Shamkhani A., and Duncan R.Radioiodination of alginate via covalently-bound tyrosinamide allows monitoring of its fate in vivo. J Bioact Compat Pol 10,4, 1995 [Google Scholar]
- 38.Andersen T., Melvik J.E., Gåserød O., Alsberg E., and Christensen B.E.Ionically gelled alginate foams: physical properties controlled by operational and macromolecular parameters. Biomacromolecules 13,3703, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Draget K.I., Østgaard K., and Smidsrød O.Homogeneous alginate gels: a technical approach. Carbohydr Polymers 14,159, 1991 [Google Scholar]
- 40.Moe S.T., Draget K.I., Skjåk-Bræk G., and Smidsrød O.Alginate. In: Stephen A., ed. Food Polysaccharides and Their Applications. New York: Marcel Dekker, 1995, pp. 245–286 [Google Scholar]
- 41.Nordbye K., and Oftebro R.Establishment of four new cell strains from human uterine cervix, II. Exp Cell Res 58,459, 1969 [Google Scholar]
- 42.Herlofsen S.R., Kuchler A.M., Melvik J.E., and Brinchmann J.E.Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in self-gelling alginate discs reveals novel chondrogenic signature gene clusters. Tissue Eng Part A 17,1003, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuo C.K., and Ma P.X.Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials 22,511, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Banerjee A., Arha M., Choudhary S., Ashton R.S., Bhatia S.R., Schaffer D.V., and Kane R.S.The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 30,4695, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mørch Y.A., Donati I., Strand B.L., and Skjåk-Bræk G.Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 7,1471, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Kuo C.K., and Ma P.X.Maintaining dimensions and mechanical properties of ionically crosslinked alginate hydrogel scaffolds in vitro. J Biomed Mater Res A 84,899, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Gaserod O., Andersen T., Melvik J.E., Dornish M., and Riley P.J.Gelled composite. US20080033392,2008
- 48.Jeon O., Bouhadir K.H., Mansour J.M., and Alsberg E.Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials 30,2724, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Li Z.S., Gunn J., Chen M.H., Cooper A., and Zhang M.Q.On-site alginate gelation for enhanced cell proliferation and uniform distribution in porous scaffolds. J Biomed Mater Res A 86A,552, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Weinstein-Oppenheimer C.R., Aceituno A.R., Brown D.I., Acevedo C., Ceriani R., Fuentes M.A., Albornoz F., Henriquez-Roldan C.F., Morales P., Maclean C., Tapia S.M., and Young M.E.The effect of an autologous cellular gel-matrix integrated implant system on wound healing. J Transl Med 8,59, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ushida T., Furukawa K., Toita K., and Tateishi T.Three-dimensional seeding of chondrocytes encapsulated in collagen gel into PLLA scaffolds. Cell Transplant 11,489, 2002 [PubMed] [Google Scholar]
- 52.Ostgaard K., Wibe E., Lindmo T., and Eik-Nes K.B.Effects of steroids and different culture media on cell cycle of the androgen-sensitive human cell line NHIK3025. J Cell Sci 48,281, 1981 [DOI] [PubMed] [Google Scholar]
- 53.Wibe E.Resistance to vincristine of human cells grown as multicellular spheroids. Br J Cancer 42,937, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Labarbera D.V., Reid B.G., and Yoo B.H.The multicellular tumor spheroid model for high-throughput cancer drug discovery. Expert Opin Drug Discov 7,819, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Prestwich G.D., Liu Y., Yu B., Shu X.Z., and Scott A.3-D culture in synthetic extracellular matrices: new tissue models for drug toxicology and cancer drug discovery. Adv Enzyme Regul 47,196, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Rowley J.A., Madlambayan G., and Mooney D.J.Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 20,45, 1999 [DOI] [PubMed] [Google Scholar]
- 57.West J.L.Bioactive hydrogels: mimicking the ECM with synthetic materials. In: Ma P.X., and Elisseeff J., eds. Scaffolding in Tissue Engineering. Boca Raton: CRC Press, Taylor and Francis Group, 2006, pp. 275–281 [Google Scholar]
- 58.Sapir Y., Kryukov O., and Cohen S.Integration of multiple cell-matrix interactions into alginate scaffolds for promoting cardiac tissue regeneration. Biomaterials 32,1838, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Yen C.N., Lin Y.R., Chang M.D., Tien C.W., Wu Y.C., Liao C.J., and Hu Y.C.Use of porous alginate sponges for substantial chondrocyte expansion and matrix production: effects of seeding density. Biotechnol Prog 24,452, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Talukdar S., Nguyen Q.T., Chen A.C., Sah R.L., and Kundu S.C.Effect of initial cell seeding density on 3D-engineered silk fibroin scaffolds for articular cartilage tissue engineering. Biomaterials 32,8927, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Acevedo C.A., Weinstein-Oppenheimer C., Brown D.I., Huebner H., Buchholz R., and Young M.E.A mathematical model for the design of fibrin microcapsules with skin cells. Bioprocess Biosyst Eng 32,341, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Dvir-Ginzberg M., Gamlieli-Bonshtein I., Agbaria R., and Cohen S.Liver tissue engineering within alginate scaffolds: effects of cell-seeding density on hepatocyte viability, morphology, and function. Tissue Eng 9,757, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Stephan S., Johnson W.E., and Roberts S.The influence of nutrient supply and cell density on the growth and survival of intervertebral disc cells in 3D culture. Eur Cell Mater 22,97, 2011 [DOI] [PubMed] [Google Scholar]
- 64.Shvartsman I., Dvir T., Harel-Adar T., and Cohen S.Perfusion cell seeding and cultivation induce the assembly of thick and functional hepatocellular tissue-like construct. Tissue Eng Part A 15,751, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Grayson W.L., Martens T.P., Eng G.M., Radisic M., and Vunjak-Novakovic G.Biomimetic approach to tissue engineering. Semin Cell Dev Biol 20,665, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hegge A.B., Andersen T., Melvik J.E., Kristensen S., and Tønnesen H.H.Evaluation of novel alginate foams as drug delivery systems in antimicrobial photodynamic therapy (aPDT) of infected wounds—an in vitro study: studies on curcumin and curcuminoides XL. J Pharm Sci 99,3499, 2010 [DOI] [PubMed] [Google Scholar]
- 67.Hegge A.B., Andersen T., Melvik J.E., Bruzell E., Kristensen S., and Tønnesen H.H.Formulation and bacterial phototoxicity of curcumin loaded alginate foams for wound treatment applications: studies on curcumin and curcuminoides XLII. J Pharm Sci 100,174, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Wee S., and Gombotz W.R.Protein release from alginate matrices. Adv Drug Deliv Rev 31,267, 1998 [DOI] [PubMed] [Google Scholar]
- 69.Kuo C.K., and Ma P.X.Controlling diffusion of solutes through ionically crosslinked alginate hydrogels designed for tissue engineering. Mater Res Soc Symp Proc 662,2001 [Google Scholar]
- 70.Schmidt J.J., Rowley J., and Kong D.J.Hydrogels used for cell-based drug delivery. J Biomed Mater Res A 87A,1113, 2008 [DOI] [PubMed] [Google Scholar]