Abstract
Background: Immunotherapies and targeted therapies are frequently associated with thyroid dysfunction, which is in contrast with the rare thyroid abnormalities induced by cytotoxic agents. Immunotherapy with NY-ESO-1, a tumor-associated antigen expressed by a number of malignancies, was reported to trigger hyperthyroidism or hypothyroidism in two HLA-A2 patients with ovarian cancer. We describe now a case of Graves' disease triggered by NY-ESO-1 in a HLA-A2–negative woman.
Patient Findings: A 32-year-old woman with a synovial sarcoma received radiotherapy, chemotherapy, and finally NY-ESO-1 vaccine. The patient was found to have HLA A11/A33(19), B13/B56(22), Cw3/−. One month after the beginning of immunotherapy, thyroid dysfunction was clinically suspected and Graves' disease was biochemically confirmed. Fearful of the antithyroid drugs' side effects, the patient was treated with a beta-blocker (propranolol, 80–20 mg/day). As hyperthyroidism progressively worsened, the patient underwent total thyroidectomy. We hypothesized that NY-ESO-1 shared partial homology with thyroid autoantigens (the so-called molecular mimicry mechanism) and that at least one pair of homologous sequences contained amino acid sequence binding motifs to a restricted number of HLA molecules. We used BLAST software to search amino acid sequence homologies between NY-ESO-1 and thyroid autoantigens (thyrotropin receptor [TSH-R], thyroperoxidase, and thyroglobulin), and the HLA ligand/motif database to look for HLA/T-cell receptor binding motifs in the regions of NY-ESO-1 and thyroid autoantigens that were homologous. We found 15 epitopic regions of NY-ESO-1 homologous to 15 regions of thyroid autoantigens, some of which epitopic: 5 of TSH-R, 8 of thyroglobulin, and 2 of thyroperoxidase. These homologous sequences contain binding motifs belonging to several HLA class I antigens, including HLA A2 and the patient's A11 and A33.
Summary: Genetically predisposed patients who receive NY-ESO-1 vaccination are at risk to develop thyroid dysfunction.
Conclusions: Considering the increasing use of NY-ESO-1, thyroid dysfunctions induced by NY-ESO-1 are expected to increase in cancer patients over the next years.
Introduction
Unlike cytotoxic agents, thyroid dysfunction is relatively frequent among toxicities associated with newer antineoplastic agents such as targeted therapies or immunotherapies (1). In a recent review on thyroid dysfunction caused by antineoplastic agents, cancer vaccination was overlooked (1). Vaccine immunotherapy for malignancies is based on the administration of tumor-associated antigens that are bound by specific HLA molecules. Tumor-associated antigens, which are proteins not expressed or underexpressed in normal cells (2), can elicit an immune response against tumoral cells in cancer patients (3). For instance, testicular cancer antigens can be expressed by a variety of nontesticular cells when they become malignant (4). One testicular cancer antigen is NY-ESO-1, which was discovered from an esophageal cancer cDNA library, and has been tested in over 30 clinical trials for a number of malignancies, such as esophageal, bladder, ovarian, prostate, non-small-cell lung cancer, melanoma, and sarcoma (5). Around 40% of patients with tumors expressing NY-ESO-1 produce antibodies against it (6). The case reported here demonstrates that HLA typization helps select responsive patients.
Vaccine immunotherapy with tumor-associated antigen administration is emerging as a standard treatment for several malignancies (5). However, immunotherapies and targeted therapies are frequently associated with thyroid dysfunction, which is in contrast with the rare thyroid abnormalities induced by cytotoxic agents (1). The case reported here is the first description of NY-ESO-1–induced Graves' disease in a HLA-A2–negative woman.
Patient
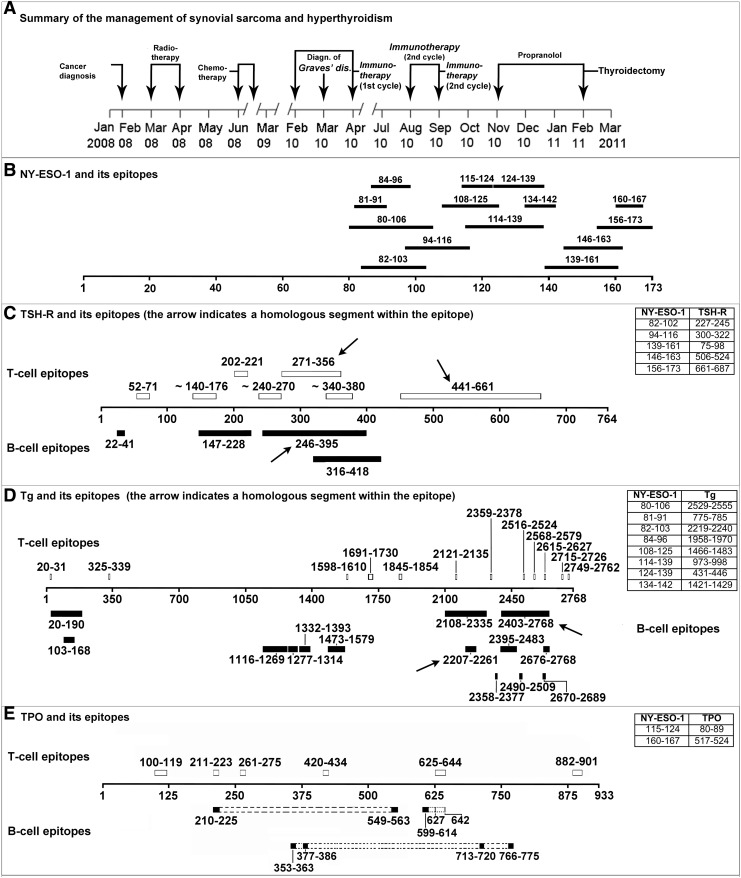
A 32-year-old Chinese-American woman was found to have a synovial sarcoma of the right infratemporal fossa in February 2008. Intensity-modulated radiotherapy to the head and neck (total dose 66 Gy) from March to April 2008 was given, followed by chemotherapy (doxorubicin plus ifosfamide plus mesna, for five cycles) from June to October 2008 (Fig. 1). Her general condition remained fair (Eastern Cooperative Oncology Group [ECOG] score grade 1). From February through April and from August through September 2010, she received two cycles of NY-ESO-1 combined with immune adjuvant Poly-ICLC emulsified in Montanide ISA-51, each cycle consisting of five administrations. To establish the eligibility for NY-ESO-1 vaccination, the oncologists performed class I HLA antigens typing by the microlymphocytotoxicity test. The patient was found to have HLA A11/A33(19), B13/B56(22), Cw3/−. HLA class II typization was not performed. One month after the beginning of immunotherapy, thyroid dysfunction was clinically suspected. Indeed, biochemical assays indicated mild hyperthyroidism caused by Graves' disease, with an elevated free triiodothyronine (4.8 pg/mL [7.4 pmol/L], normal range 2.3–4.3 pg/mL [3.5–6.6 pmol/L]), a subnormal thyrotropin (TSH; 0.13 mU/L, normal range 0.34–4.82 mU/L), and elevated thyroid-stimulating immunoglobulins (TSI; 152%, normal value <140%). Unwilling to risk side effects of antithyroid drugs, she elected to be treated with a beta-blocker (propranolol, 80–20 mg/day). Hyperthyroidism progressively worsened, in parallel with a progressive increase of serum TSI and thyroid size. In December 2010, her serum free thyroxine was >50 pg/mL (60 pmol/L; normal range 5.9–16.1 pg/mL [7.6–20.7 pmol/L]), her free triiodothyronine was >20 pg/mL (30 pmol/L), and the TSI was >500%. In February 2011, the patient underwent total thyroidectomy, and the diagnosis of Graves' disease was histologically confirmed (Fig. 1).
FIG. 1.
(A) Summary of the clinical management in this patient with synovial sarcoma and hyperthyroidism. (B) Scheme of the epitopes in NY-ESO-1. (C–E) Schemes of the epitopes in thyroid autoantigens and homologs with NY-ESO-1.
We looked for amino acid sequence homologies between NY-ESO-1 and thyroid autoantigens (TSH-receptor [TSH-R], thyroperoxidase, and thyroglobulin [Tg]) using BLAST software of the National Center for Biotechnology Information (7). Next, we used the HLA ligand/motif database (8) to search HLA/T-cell receptor binding motifs in the regions of NY-ESO-1 and thyroid autoantigens that were homologous. Fifteen regions of NY-ESO-1, all epitopic, were homologous to 15 regions of thyroid autoantigens: 5 segments with the TSH-R (3/5 containing 3 epitopes), 8 with Tg (2/8 containing 2 epitopes), and 2 with thyroperoxidase (none epitopic) (Fig. 1). These homologous segments contain binding motifs belonging to several HLA class I antigens, including the patient's A11 and A33. Furthermore, two homologous segments of NY-ESO-1 and TSH-R contain two HLA A2 binding motifs (Table 1).
Table 1.
Illustrative Homologies Between NY-ESO-1, the Thyrotropin Receptor, and HLA Antigens
| NY-ESO-1 vs. TSH-R | A2 | A11 | A26 (10) | A30 (19) | A33 (19) | DPw2 | DPw4 | DQ2 | DQ6 (1) | DQ7 (3) | DQ8 (3) | DR4 | DR9 | DR15 (2) | DR53 | DR51 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NY-ESO-1 82–102 TSH-R 227–245 |
2 1 |
3 6 |
1 1 |
1 1 |
||||||||||||
| NY-ESO-1 94–116 TSH-R 300–322 |
1 1 |
1 2 |
1 1 |
|||||||||||||
| NY-ESO-1 139–161 TSH-R 75–98 |
1 1 |
2 2 |
1 1 |
3 2 |
||||||||||||
| NY-ESO-1 146–163 TSH-R 506–524 |
1 1 |
1 1 |
1 2 |
1 1 |
4 4 |
1 1 |
||||||||||
| NY-ESO-1 156–179 TSH-R 661–687 |
1 3 |
1 1 |
1 1 |
1 2 |
4 2 |
1 2 |
3 4 |
1 2 |
1 1 |
The patient's HLA antigens are highlighted in gray.
TSH-R, thyrotropin receptor.
Discussion
Thyroid dysfunction after administration of newer antineoplastic agents, such as tyrosine-kinase inhibitors, is a relatively frequent side effect, since it can affect 20–50% of patients. Thyroid dysfunction is either monophasic (hypothyroidism more frequent than hyperthyroidism) or biphasic (transient hyperthyroidism followed by hypothyroidism) (1,9,10). The thyroid complications triggered by interferon-α, alemtuzumab, ipilimumab, and tremelimumab can range from destructive thyroiditis to Graves' disease (11,12). Rarely, hypothyroidism is caused by pituitary dysfunction (ipilimumab, tremelimumab, bexarotene) (1,13,14).
Although in a recent review, immunotherapies have been taken into account as a potential cause of thyroid dysfunction in cancer patients, vaccine immunotherapy is not mentioned (1). Actually, in a recent pilot study, NY-ESO-1 vaccination was reported to cause persistent hypothyroidism requiring levothyroxine therapy in one HLA-A2 patient after five doses of vaccine, and transient subclinical hyperthyroidism three months after completion of vaccination in another A2-positive patient (6).
Because NY-ESO-1 is expressed in 80% of patients with synovial sarcoma, NY-ESO-1 is used when this malignancy has become refractory to all standard treatments (15). Tumor cells process the NY-ESO-1 protein generating peptides, whose epitopes are recognized by HLA-restricted T-cells. These peptides, currently used as vaccines, are administered in HLA-restricted patients, in order to increase immunogenicity of NY-ESO-1 (6). Hence, all cancer patients who are candidates for NY-ESO-1 immunotherapy have to be typed for HLA loci to select responsive patients. For instance, the NY-ESO-1 peptides 60–72, 92–100, 87–100, 95–107, 124–134, 157–165, and 157–170 are recognized by the following HLA-restricted T cells: A2, B7, Cw3, DRB1*0901, DQB1*0401, DRB1*0803, and DP4 (16–19).
Autoimmune thyroid disease can be triggered by exposure to proteins sharing at least partial homology with thyroid autoantigens (the so-called molecular mimicry mechanism) in individuals genetically predisposed (e.g., by having certain HLA alleles) (20).
We hypothesized that NY-ESO-1 contained segments of amino acid sequence homology with one or more thyroid autoantigens and that at least one pair of homologous sequences contained amino acid sequence binding motifs to a restricted number of HLA molecules for the corresponding T-cell receptor. Because of the major role of the TSH-R in the pathogenesis of Graves' disease through its activation by autoantibodies (TSI) and subsequent stimulation of both thyroid growth and function, we hypothesized that one autoantigen involved could be TSH-R. We found that NY-ESO-1 shared epitopic homologous regions with at least two major thyroid autoantigens, the TSH-R and Tg. These homologous sequences contain HLA class I and class II binding motifs. NY-ESO-1 sequences 82–103 and 146–163 and their homologous counterparts Tg 2219–2240 and TSH-R 506–524 contain one HLA A11 binding motif each. The NY-ESO-1 sequence 156–179 and the homologous TSH-R sequence 661–687 contain one HLA A33 binding motif, respectively. Indeed, our patient was HLA A11/A33 positive. Moreover, several homologous segments contain binding motifs within other HLA molecules (A2, DP4, DR9), which are known to present NY-ESO-1 peptides (Tables 1 and 2).
Table 2.
Illustrative Homologies Between NY-ESO-1, Thyroglobulin, and HLA Antigens
| NY-ESO-1 vs. Tg | A11 | A24 (9) | A30 (19) | B62 (15) | B27 | B39 (16) | B53 | B54 (22) | DPw2 | DPw4 | DQ2 | DQ6 (1) | DQ7 (3) | DR1 | DR17 (3) | DR4 | DR9 | DR15 (2) | DR53 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NY-ESO-1 84–96 Tg 1958–1970 |
2 1 |
1 1 |
2 1 |
||||||||||||||||
| NY-ESO-1 82–103 Tg 2219–2240 |
1 1 |
1 1 |
2 1 |
1 2 |
2 1 |
3 4 |
2 1 |
1 1 |
1 1 |
||||||||||
| NY-ESO-1 114–139 Tg 973–998 |
1 1 |
1 1 |
2 1 |
2 1 |
1 1 |
2 1 |
|||||||||||||
| NY-ESO-1 80–106 Tg 2529–2555 |
1 1 |
1 2 |
3 2 |
1 2 |
1 2 |
||||||||||||||
| NY-ESO-1 134–142 Tg 1421–1429 |
|||||||||||||||||||
| NY-ESO-1 108–125 Tg 1466–1483 |
1 1 |
||||||||||||||||||
| NY-ESO-1 124–139 Tg 431–446 |
1 1 |
1 2 |
2 2 |
1 1 |
1 1 |
1 1 |
|||||||||||||
| NY-ESO-1 81–91 Tg 775–785 |
1 1 |
1 1 |
The patient's HLA antigens are highlighted in gray.
Tg, thyroglobulin.
Since vaccines are emerging as a standard treatment for malignancies, vaccine-induced thyroid dysfunctions are expected to increase in genetically predisposed patients over the next years. Therefore, physicians should be alerted not only to targeted therapies but also to cancer vaccination as a possible cause of thyroid abnormalities. Because of the symptomatology (e.g., asthenia, nervousness, insomnia, weight loss), cardiac consequences or complications (tachyarrhythmias, ischemic heart disease), the possibility of thyroid storm with an associated mortality as high as 75% (21), and because of the side effects of antithyroid drugs (particularly hematological and hepatic), Graves' disease is not a desirable complication in these seriously ill patients.
Hence, complete HLA typization in cancer patients who receive NY-ESO-1 vaccination is important not only to predict the effectiveness of immunotherapy but also to identify patients who are at high risk to develop thyroid dysfunction. We predict that NY-ESO-1–treated patients carrying any of the HLA antigens A2, A11, A24(9), A26(10), A30(19), A33(19), B27, B39(16), B53, B54(22), B62(15), DP2, DP4, DQ2, DQ6(1), DQ7(3), DQ8(3), DR1, DR4, DR9, DR15(2), DR17(3), DR51, and DR53 (Fig. 1) are at risk of developing autoimmune hyperthyroidism or hypothyroidism. Among these, a number of HLA molecules (A2, DQ2, DQ3, DR3, DR4, DR9) are known to genetically predispose to Graves' disease (22–27).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hamnvik OP, Larsen PR, Marqusee E.2011Thyroid dysfunction from antineoplastic agents. J Natl Cancer Inst 103:1572–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turk MJ, Wolchok JD, Guevara-Patino JA, Goldberg SM, Houghton AN.2002Multiple pathways to tumor immunity and concomitant autoimmunity. Immunol Rev 188:122–135 [DOI] [PubMed] [Google Scholar]
- 3.Reuschenbach M, von Knebel Doeberitz M, Wentzensen N.2009A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother 58:1535–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YT, Old LJ.1999Cancer-testis antigens: targets for cancer immunotherapy. Cancer J Sci Am 5:16–17 [PubMed] [Google Scholar]
- 5.O'Meara MM, Disis ML.2011Therapeutic cancer vaccines and translating vaccinomics science to the global health clinic: emerging applications toward proof of concept. OMICS 15:579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diefenbach CSM, Gnjatic S, Sabbatini P, Aghajanian C, Hensley ML, Spriggs DR, Iasonos A, Lee H, Dupont B, Pezzulli S, Jungbluth AA, Old LJ, Dupont J.2008Safety and immunogenicity study of NY-ESO-1b peptide and montanide ISA-51 vaccination of patients with epithelial ovarian cancer in high-risk first remission. Clin Cancer Res 14:2740–2748 [DOI] [PubMed] [Google Scholar]
- 7.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ.1997Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402 (Software freely available at http://blast.ncbi.nlm.nih.gov/Blast.cgi Accessed January15, 2013) [DOI] [PMC free article] [PubMed]
- 8.Sathiamurthy M, Hickman HD, Cavett JW, Zahoor A, Prilliman K, Metcalf S, Fernandez Vina M, Hildebrand WH.2003Population of the HLA ligand database. Tissue Antigens 61:12–19 [DOI] [PubMed] [Google Scholar]
- 9.Van Doorn L, Eskens FA, Visser TJ, van der Lugt A, Mathijssen RH, Peeters RP.2011Sorafenib induced thyroiditis in two patients with epatocelular carcinoma. Thyroid 21:197–202 [DOI] [PubMed] [Google Scholar]
- 10.Sato S, Muraishi K, Tani J, Sasaki Y, Tokubuchi I, Tajiri Y, Yamada K, Suekane S, Miyajima J, Matsuoka K, Hiromatsu Y.2010Clinical characteristics of thyroid abnormalities induced by sutinib treatment in Japanese patients with renal cell carcinoma. Endocr J 57:873–880 [DOI] [PubMed] [Google Scholar]
- 11.Jonasch E, Haluska FG.2001Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist 6:34–55 [DOI] [PubMed] [Google Scholar]
- 12.CAMMS223 Trial Investigators, Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, Margolin DH, Norris K, Tandon PK. 2008Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 359:1786–1801 [DOI] [PubMed] [Google Scholar]
- 13.Di Giacomo AM, Biagioli M, Maio M.2010The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin Oncol 37:499–507 [DOI] [PubMed] [Google Scholar]
- 14.Sherman SI, Gopal J, Haugen BR, Chiu AC, Whaley K, Nowlakha P, Duvic M.1999Central hypothyroidism associated with retinoid X receptor-selective ligands. N Engl J Med 340:1075–1079 [DOI] [PubMed] [Google Scholar]
- 15.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, Rosenberg SA.2011Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 29:917–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robson NC, McAlpine T, Knights AJ, Schnurr M, Shin A, Chen W, Maraskovsky E, Cebon J.2010Processing and cross-presentation of individual HLA-A, -B, or -C epitopes from NY-ESO-1 or an HLA-A epitope for Melan-A differ according to the mode of antigen delivery. Blood 116:218–225 [DOI] [PubMed] [Google Scholar]
- 17.Mizote Y, Taniguchi T, Tanaka K, Isobe M, Wada H, Saika T, Kita S, Koide Y, Uenaka A, Nakayama E.2010Three novel NY-ESO-1 epitopes bound to DRB1*0803, DQB1*0401 and DRB1*0901 recognized by CD4 T cells from CHP-NY-ESO-1-vaccinated patients. Vaccine 28:5338–5346 [DOI] [PubMed] [Google Scholar]
- 18.Jäger E, Chen YT, Drijfhout JW, Karbach J, Ringhoffer M, Jäger D, Arand M, Wada H, Noguchi Y, Stockert E, Old LJ, Knuth A.1998Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med 187:265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng G, Wang X, Robbins PF, Rosenberg SA, Wang RF.2001CD4(+) T cell recognition of MHC class II-restricted epitopes from NY-ESO-1 presented by a prevalent HLA DP4 allele: association with NY-ESO-1 antibody production. Proc Natl Acad Sci USA 98:3964–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guarneri F, Benvenga S.2007Environmental factors and genetic background that interact to cause autoimmune thyroid disease. Curr Opin Endocrinol Diabetes Obes 14:398–409 [DOI] [PubMed] [Google Scholar]
- 21.Mackin JF, Canary JJ, Pittman CS.1974Thyroid storm and its management. N Engl J Med 291:1396–1398 [DOI] [PubMed] [Google Scholar]
- 22.Dong RP, Kimura A, Okubo R, Shinagawa H, Tamai H, Nishimura Y, Sasazuki T.1992HLA-A and DPB1 loci confer susceptibility to Graves' disease. Hum Immunol 35:165–172 [DOI] [PubMed] [Google Scholar]
- 23.Ratanachaiyavong S, McGregor AM.1994HLA-DPB1 polymorphisms on the MHC-extended haplotypes of families of patients with Graves' disease: two distinct HLA-DR17 haplotypes. Eur J Clin Invest 24:309–315 [DOI] [PubMed] [Google Scholar]
- 24.Ofosu MH, Dunston G, Henry L, Ware D, Cheatham W, Brembridge A, Brown C, Alarif L.1996HLA-DQ3 is associated with Graves' disease in African-Americans. Immunol Invest 25:103–110 [DOI] [PubMed] [Google Scholar]
- 25.Orhan Y, Azezli A, Carin M, Aral F, Sencer E, Molvalilar S.1993Human lymphocyte antigens (HLA) and Graves' disease in Turkey. J Clin Immunol 13:339–343 [DOI] [PubMed] [Google Scholar]
- 26.Jacobson EM, Huber A, Tomer Y.2008The HLA gene complex in thyroid autoimmunity: from epidemiology to etiology. J Autoimmun 30:58–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeo PP, Chan SH, Thai AC, Ng WY, Lui KF, Wee GB, Tan SH, Lee BW, Wong HB, Cheah JS.1989HLA Bw46 and DR9 associations in Graves' disease of Chinese patients are age- and sex-related. Tissue Antigens 34:179–184 [DOI] [PubMed] [Google Scholar]