Abstract
The development of engineered microvessels with clinically relevant characteristics is a critical step toward the creation of engineered myocardium. Alignment is one such characteristic that must be achieved, as it both mimics native capillary beds and provides natural inlet and outlet sides for perfusion. A second characteristic that is currently deficient is cross-sectional lumen density, typically under 100 lumens/mm2; the equivalent value for human myocardium is 2000 lumens/mm2. Therefore, this study examined the effects of gel compaction and interstitial flow on microvessel alignment and lumen density. Strong microvessel alignment was achieved via mechanically constrained cell-induced fibrin gel compaction following vasculogenesis, and high lumen density (650 lumens/mm2) was achieved by the subsequent application of low levels of interstitial flow. Low interstitial flow also conferred microvessel barrier function.
Introduction
Most previous research on engineered microvessels has been in isotropic systems;1–3 however, aligned microvessels would be advantageous both in terms of mimicking alignment in native tissue (e.g., myocardium4), and in terms of establishing efficient perfusion of the microvessels, as alignment creates natural inlet and outlet sides. Recent work has shown that contact guidance in response to aligned fibrin fibrils is sufficient to align sprouts from blood outgrowth endothelial cell (BOEC)5 spheroids, and that cell-induced gel compaction resulted in both aligned fibrils and sprouts.6 We harnessed this mechanism here to engineer microvessels formed by self-assembly of dispersed BOECs in fibrin gel, showing that cell-induced gel compaction also aligns such microvessels.
In many engineered tissues, such as myocardium, a high capillary density will be required to support the highly metabolic cells. Indeed, in native adult myocardium, the average capillary density is 2000 capillaries/mm2, and the average intercapillary distance is around 20 μm.7,8 To date, no engineered tissue has achieved this density. Reported values range from 2 to 100 lumens/mm2 for tissues prior to implantation,9–12 although not all reports of engineered microvessels quantified cross-sectional lumen density. Total capillary density increases after implantation, with most studies reporting 200–600 lumens/mm2.2,13,14 In the studies reported here, we demonstrate vastly increased microvessel density in vitro using the same constrained gel compaction technique used to achieve aligned microvessels, attaining values similar to reported postimplantation values.
Interstitial flow occurs physiologically due to pressure differences between the vascular and lymphatic systems and is estimated to occur at flow rates of 0.1–2 μm/s.15 Previous reports have suggested that interstitial flow through engineered tissues containing ECs at superficial velocities of 4–13 μm/s increases microvessel density.16,17 The shear stresses applied via interstitial flow at these velocities were quite low (on the order of 0.001 Pa [0.01 dyne/cm2]), leading the previous investigators to attribute improvements to local morphogen gradients created by flow rather than shear stresses.16 In addition to the increased microvessel density, some alignment of microvessels in the direction of interstitial flow was observed in an initially isotropic construct.16 However, these results were achieved by applying flow prior to any microvessel formation; it is unclear from previous work whether or not interstitial flow can modulate existing microvessels, which we show here is possible.
The permeability of EC monolayers is affected by shear stresses. Acute shear stress (h) on the order of 0.1–1 Pa (1–10 dyne/cm2) increased permeability of EC monolayers.18,19 Chronic shear stress (days), however, reduced permeability.19 However, the majority of studies in this area have observed EC monolayers under laminar flow; it is unclear if the results hold for engineered microvessels exposed to interstitial flow. We show here that shear forces two orders of magnitude lower than those reported in the literature decrease microvessel permeability.
Shear forces are also known to affect the arterial or venous phenotype of ECs, as indicated by ephrinB2 and ephB4 levels, respectively.20–22 In stem and progenitor cells, shear stress increases ephrinB2 expression,23 whereas in more mature cells, it has the opposite effect.24 However, again previous work has examined EC monolayers rather than engineered microvessels.
The studies reported here examined the effects of cell-induced gel compaction and interstitial flow on the characteristics of engineered microvessels in the resulting tissue-like sample. Constructs were formed in a custom chamber that enabled gel compaction followed by interstitial flow. Constructs were exposed to one of two interstitial flow rates for 3 or 6 days, and compared to both time-matched and baseline controls. A variety of microvessel properties were examined, including lumen density and alignment, permeability, mural cell recruitment, basement membrane deposition, and arterial/venous phenotype.
Materials and Methods
Cell culture
Human BOECs5 were supplied by Dr. Robert Hebbel at the University of Minnesota. BOECs were cultured in type I collagen-coated flasks in “BOEC medium,” which consisted of EGM-2 (Lonza) with an additional 8% fetal bovine serum (FBS) and 1% penicillin–streptomycin. Green fluorescent protein (GFP)-labeled human brain pericytes (PCs) were provided by Dr. George Davis at the University of Missouri. They were cultured in gelatin-coated flasks in DMEM with 10% FBS and 1% penicillin–streptomycin.
Construct preparation
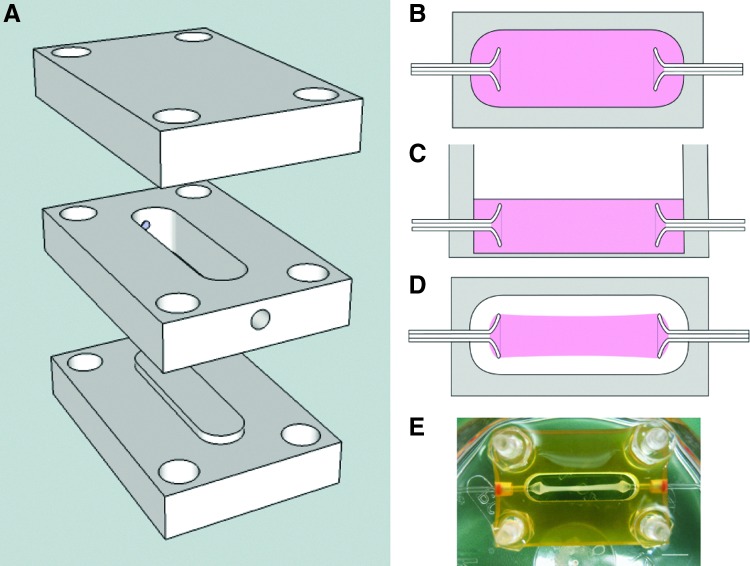
Constructs were cast in a custom chamber that included flared glass capillary tubes placed through the side walls (Fig. 1). At casting, BOECs and PCs were mixed with fibrinogen; thrombin; M199 medium; and three growth factors stromal-derived factor 1α, stem cell factor, and interleukin-3. The gel filled the bottom of the well and covered the glass capillary tubes. After gelation, the bottom of each well was removed, enabling nutrient transport from both sides. After 5 days of culture, some constructs were detached from the edges of the well using dental picks. The glass tubes served to anchor the gel during compaction of the fibrin gel and alignment of the fibrin fibrils (Fig. 1). Control constructs were harvested at days 5, 8, 11, and 14. For additional details on construct preparation and all methods, please refer to the Supplementary Data (Supplementary Data are available online at www.liebertpub.com/tea).
FIG. 1.
(A–D) Schematics of interstitial flow chamber design. (A) The chamber bottom snapped into the well for casting, at which point the well measured 20 mm (l)×4.8 mm (w)×4 mm (h). Flared glass capillary tubes (1 mm outer diameter, 0.58 mm inner diameter) were inserted into the small holes in the sides of the well. The chamber top was not used for casting. During culture, the chamber bottom was removed to allow nutrient transport from either side. The chamber bottom and top were used to enclose the gel during interstitial flow. The pieces were tightened together using a screw at each corner. (B) Top view schematic of a construct at casting. (C) Side view schematic of a construct at casting. The gel (pink) filled the entire well and covered the glass capillary tubes. (D) Top view schematic of a construct after compaction, prior to embedding with agarose gel. The construct was only adherent to the glass capillary tubes. (E) A compacted construct after 8 days of culture. Scale bar=5 mm. Color images available online at www.liebertpub.com/tea
Application of interstitial flow
On day 8 of culture, aligned constructs were embedded in 5% agarose gel, which was much less permeable than the constructs, and enclosed in the chamber. The glass capillary tubes were then connected to a flow circuit. The presence of the agarose gel prevented fluid from exiting the tissue. Flow rates of 0.015 μL/min (“low”) and 0.105 μL/min (“high”) were used, corresponding to superficial flow velocities of 0.33 and 2.33 μm/s, respectively. These flow rates were chosen because they fit within the range of shear stress conditions previously shown to increase lumen formation in engineered microvessels.16,17 Constructs (from three separate castings; n=3), including controls that were not embedded in agarose or exposed to flow, were harvested at days 11 (3 days of flow) and 14 (6 days of flow). Approximately 24 h prior to the cessation of flow, gold nanoparticles (GNP; 15 nm diameter) were introduced to the inlet flow to evaluate the fluid path through the tissue.
Hydraulic permeability measurement
Day 8 constructs (n=3) were embedded in agarose and connected to a flow circuit as described above, except that an in line pressure transducer was placed immediately proximal to the construct. Steady-state pressure and flow rate were compared for three flow rates on three constructs, and the hydraulic conductivity of each construct was calculated from Darcy's Law. The hydraulic permeability was obtained by multiplying hydraulic conductivity and fluid viscosity.
Viscosity measurement
The viscosity of BOEC medium was measured using a cone and plate viscometer according to the manufacturer's instructions. Briefly, BOEC medium was maintained at 37°C via a circulating water bath, and the viscosity was measured at three shear rates. Four separate samples of BOEC medium were tested.
Estimation of shear stress
The shear stress experienced by the ECs was estimated using two methods. The first method used a formula derived by Wang and Tarbell:25
 |
where τ is shear stress, μ is fluid viscosity, Q is volumetric flow rate, A is construct cross-sectional area, kp is the hydraulic permeability of the construct, and B is a factor that depends on the volume fraction of the cells from an equation specified by Wang and Tarbell.
Equation (1) assumes cells to be spheres, but in these constructs, the ECs were primarily arranged into microvessels when interstitial flow was applied. Therefore, shear stress was also estimated by modeling flow around a cylinder aligned with the flow direction and embedded in porous medium in COMSOL Multiphysics, which solved the Brinkman equations to obtain the velocity field.26 The average shear rate along the length of the cylinder was obtained from the COMSOL output, and the shear stress was calculated by multiplying by the fluid viscosity.
Histology/image analysis
Constructs were fixed in 4% paraformaldehyde and both longitudinal and cross sections were taken. Cross sections were taken from three regions (inlet, middle, and outlet; Fig. 2B) to assess variations in BOEC microvessel properties along the length of the constructs.
FIG. 2.
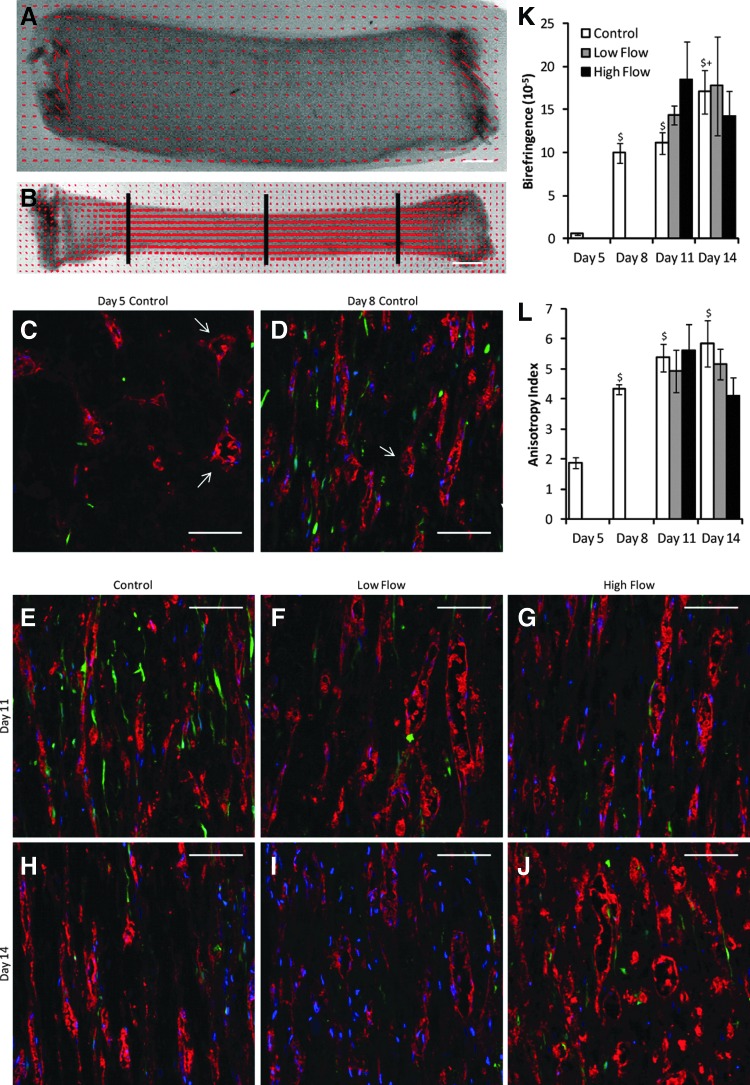
(A, B) Representative alignment maps of uncompacted (day 5; A) and compacted (day 8; B) constructs, in which the red lines indicate the local direction and strength of alignment. Scale bars=1 mm. The black lines in (B) indicate the approximate locations of inlet, middle, and outlet cross sections. (C–J) Representative longitudinal sections stained for CD31 (red) of control and flow constructs. Pericytes (PCs) were green fluorescent protein (GFP)-labeled (green), and nuclei were stained with Hoechst 33342 (blue). The axial direction is vertical. The arrows in (C, D) indicate some of the microvessels with lumens. Scale bars=100 μm. (K) Birefringence (a measure of fibril alignment), quantified from polarimetry, increased with compaction, but did not vary between the static and flow conditions, suggesting that flow did not have an effect on fibril alignment. (L) Microvessel anisotropy index, a measure of microvessel alignment obtained from images, also increased with compaction but did not vary between the static and flow conditions. $p<0.05 in comparison to day 5 control. +p<0.05 in comparison to day 8 control. Color images available online at www.liebertpub.com/tea
Cross sections were immunostained with an antibody for CD31 and imaged. A custom MATLAB code (Supplementary Fig. S1) detected EC structures (defined as connected areas of CD31+ staining) and lumens and measured various parameters of interest, including lumen density and average lumen diameter, which was calculated from the lumen area assuming a circular lumen. The code also measured cell density based on nuclear staining and determined the level of recruitment of the GFP-labeled PCs. A PC was considered recruited if any part of it was immediately adjacent to a microvessel.
Cross sections were also immunostained using antibodies for collagen IV, laminin, ephrinB2, and ephB4. Another custom MATLAB script calculated staining intensity. Basement membrane data were normalized to the average nonlumen area of each sample, as differences were seen in total lumen area between conditions. Because both PCs and BOECs expressed ephrinB2, the co-localization of ephrinB2 staining in red and the GFP-labeling of PCs were used to separate expression levels for each cell type. The BOEC ephrinB2 expression was normalized to the average fraction of each section positively stained for CD31. EphrinB2 and ephB4 staining was also performed on BOEC monolayers created from cells identically grown as for gel entrapment and on HUVEC monolayers as a positive control.
Longitudinal sections of aligned constructs were stained with the CD31 antibody and imaged. The average angle and length of each microvessel was quantified using a custom MATLAB code. An anisotropy index for each image (n=6 per sample) was calculated by decomposing each microvessel length into components parallel and perpendicular to the alignment direction, and then taking their ratio such that a higher value indicated stronger alignment. The average angle of microvessel alignment was taken to be the alignment direction because it was not possible to keep the section orientation the same for every image. Visual assessment of the sections and their microvessels indicated that the average microvessel angle coincided with fibril alignment.
Additional longitudinal sections were stained with HQ Silver (Nanoprobes), which deposited silver particles on the GNP, enlarging them to be visible under transmitted light.
Microvessel permeability assessment
Microvessel permeability was assessed by exposing constructs to GNP at the time of harvest and measuring the ability of GNP to enter microvessel lumens. GNP were used here instead of fluorescent dextrans to be consistent with the above use of GNP to track fluid flow. The 15-nm GNP diameter is slightly larger than the measured hydrodynamic diameter for 70 kDa dextran.27 Day 8 and 11 control constructs, and day 11 low flow constructs, were assessed. Constructs were embedded in agarose, and a GNP solution pumped into the constructs at a flow rate of 1 μL/min (22 μm/s) for 30 min. Delivery of GNP via interstitial flow was required due to the presence of a cell monolayer on the surface of day 11 control constructs that prevented diffusion of GNP into the constructs from the surrounding medium. Although nonideal due to the potential effects of acute shear stress, all constructs experienced a large step increase in shear stress. Constructs were then fixed, sectioned longitudinally, and stained with HQ Silver as above. Because the process was not at steady-state, only lumens (n=8 across three samples) at a defined distance from the inlet were analyzed.
Fibril orientation
Fibrin fibril orientation was determined by polarized light imaging.28 A custom MATLAB script determined the average birefringence (a measure of fibril alignment) of each construct. Optical density was also measured from these images by averaging the pixel intensity of the region of interest.
Cross-sectional area measurements
The thickness of tissue constructs was measured using a linear variable differential transformer, and three measurements were averaged for each construct. The width of each construct at the locations of the thickness measurements was measured with a caliper.
Results
Figures 2 and 3 detail the effects of compaction and interstitial flow on microvessel characteristics. Representative images of longitudinal and cross sections (from the center of the constructs) stained for CD31 are shown in Figures 2C–J and 3A–H, respectively.
FIG. 3.
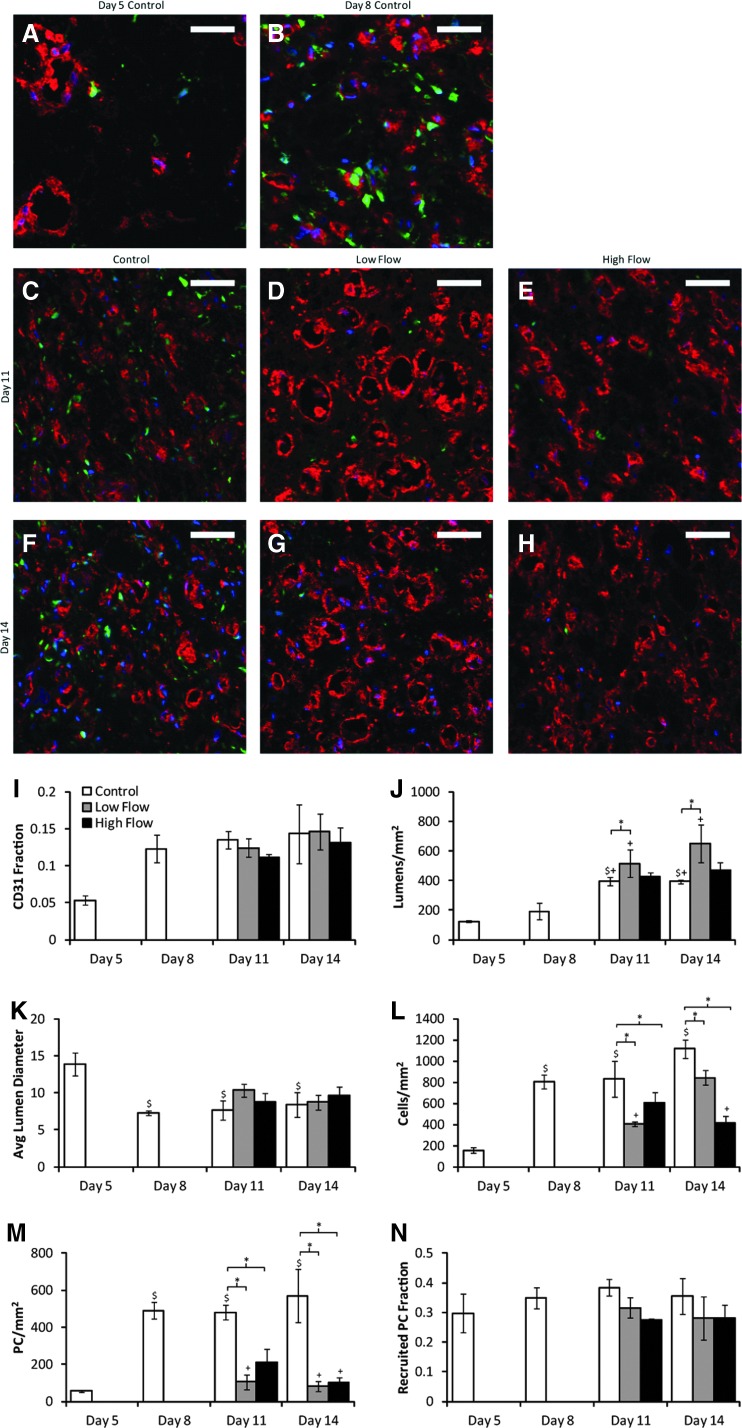
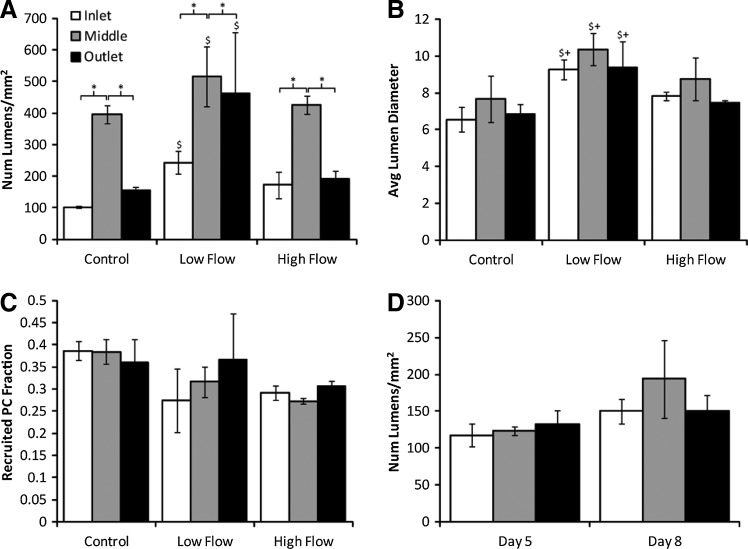
(A–H) Representative cross sections stained for CD31 (red) from the middle region of constructs from all days and flow conditions studied. PCs were GFP-labeled (green), and nuclei were stained with Hoechst 33342 (blue). Scale bars=50 μm. (I–N) Quantification of images. (I) The fraction of the section stained positively for CD31 remained constant across all compacted conditions. (J) The number of lumens per square millimeter was increased by low flow relative to time-matched controls but not by high flow. (K) The mean diameter of lumens decreased with compaction but was independent of day or flow condition. (L) The cell number per square millimeter, based on Hoechst 33342 staining, was somewhat variable between conditions, but no trends emerged. (M) The number of PCs per square millimeter was reduced with exposure to either flow rate. (N) The fraction of PCs that were recruited to CD31+ microvessels was the same across all conditions. *p<0.05. $p<0.05 in comparison with day 5 control. +p<0.05 in comparison with day 8 control. Color images available online at www.liebertpub.com/tea
Cell-induced gel compaction results in aligned fibrils and aligned microvessels and increases lumen density
Uncompacted constructs demonstrated weak fiber alignment as observed via alignment mapping, whereas strong fiber alignment in the longitudinal direction was observed in compacted constructs (Fig. 2A, B, K). Longitudinal sections (Fig. 2C–E, H, L) showed that the BOEC microvessels were also highly aligned longitudinally in compacted constructs. Cross sections (Fig. 3A–C, F) revealed a decrease in lumen size with compaction. Assuming circular lumens, the mean lumen diameter was 14 μm for uncompacted constructs and 7 μm for compacted constructs.
The lumen density of day 8 constructs (after 3 days of compaction) was not different from that of day 5 constructs (prior to gel compaction; Fig. 3J), despite the fact that the density of CD31+ structures was increased (data not shown). After further culture, at days 11 and 14, the lumen density of control constructs was increased over both days 5 and 8 controls. Using the measured construct cross-sectional areas and assuming all of the lumens present in the day 5 constructs would be present in the day 8 constructs, the lumen density of compacted constructs was predicted to be 1285 lumens/mm2 in contrast to the measured value of 194.
Low interstitial flow increases microvessel lumen density while maintaining other microvessel properties
Lumen density, as measured in cross sections from the center of constructs stained for CD31, increased with low interstitial flow over control at both days 11 and 14 (Fig. 3J). This increase was not seen with high interstitial flow. Lumen density remained constant within conditions between days 11 and 14, and lumen diameter was the same between compacted constructs (Fig. 3K).
Total cell number was decreased in flow conditions compared to controls, but no differences were observed between the two flow rates (Fig. 3L). PC density was substantially reduced in both flow conditions (Fig. 3M), but the fraction of the section stained positively for CD31 (indicative of EC density) did not vary between the conditions yielding compacted constructs, which includes all of the day 8, 11, and 14 conditions (Fig. 3I). PC recruitment remained constant at 30%–35% among all conditions (Fig. 3N).
Both fibril and microvessel alignment remained constant among all conditions yielding compacted constructs, regardless of flow condition or time point (Fig. 2K–L). Birefringence values were near 15×10−5, and anisotropy index values were near 5, both indicating high levels of alignment. Among constructs exposed to low flow for 3 days (day 11), lumens extending greater than 200 μm in the plane of the section were frequently observed.
High levels of basement membrane were produced by the cells
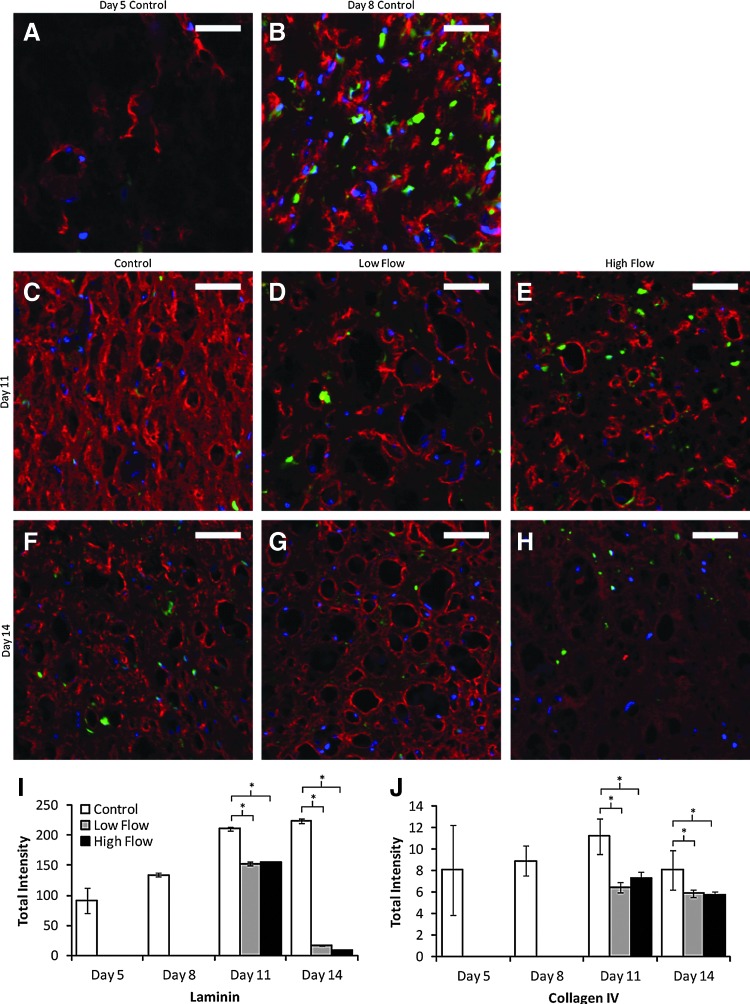
Figure 4A–H show representative images of laminin staining of cross sections from construct centers. The total fluorescent intensity per nonlumen area was quantified from images of both laminin and collagen IV staining (Fig. 4I–J). For both basement membrane proteins, staining intensity was decreased with interstitial flow (for both flow rates) in comparison to time-matched controls. However, visual inspection of the images suggests that the higher basement membrane levels in the controls are due to increased levels of laminin and collagen IV in the interstitium, rather than within the perivascular region.
FIG. 4.
(A–H) Representative cross sections from the middle region of constructs in all day and flow conditions studied, stained for laminin (red). PCs were GFP-labeled (green), and nuclei were stained with Hoechst 33342 (blue). Scale bars=50 μm. Collagen IV staining was similar and therefore is not shown. (I, J) Quantification of the total staining intensity per nonlumen area for laminin (I) and collagen IV (J). The increased staining in control constructs appeared to be due to additional protein in the interstitial space rather than the perivascular region. *p<0.05 for main effect. Color images available online at www.liebertpub.com/tea
Microvessel properties varied along the length of the construct
Variations in some microvessel properties were observed in cross sections taken from the inlet, middle, and outlet regions of the constructs. At day 11, the lumen density was substantially higher in the middle region than in either the inlet or outlet region for all conditions (Fig. 5A), but the average lumen diameter did not depend on position (Fig. 5B). At day 14, both lumen density and lumen diameter were increased in the middle region (data not shown). Despite the variation in lumen density across tissue regions, low interstitial flow increased lumen density within each region. In addition, when data from all regions were combined for flow conditions and their time-matched controls, an increase in lumen density was still observed with low interstitial flow. Interestingly, at days 5 and 8, no differences in lumen density (Fig. 5C) or lumen diameter (data not shown) were observed between regions. PC recruitment, matrix alignment, and tissue optical density did not depend on position at any time for all conditions.
FIG. 5.
Quantification of CD31 stained cross sections from various tissue regions. (A) At day 11, lumen density was increased in the middle region over the inlet and outlet regions. However, even when all of the data were combined, the increase in lumen density with low flow remained. (B) Average lumen diameter at day 11 did not vary by tissue region, but was larger in constructs exposed to low flow. (C) No differences in PC recruitment occurred between regions or flow conditions at day 11. (D) At days 5 and 8, no differences in lumen density occurred across tissue regions. *p<0.05 for main effects. $p<0.05 for main effects between control and low flow. +p<0.05 for main effects between low and high flow.
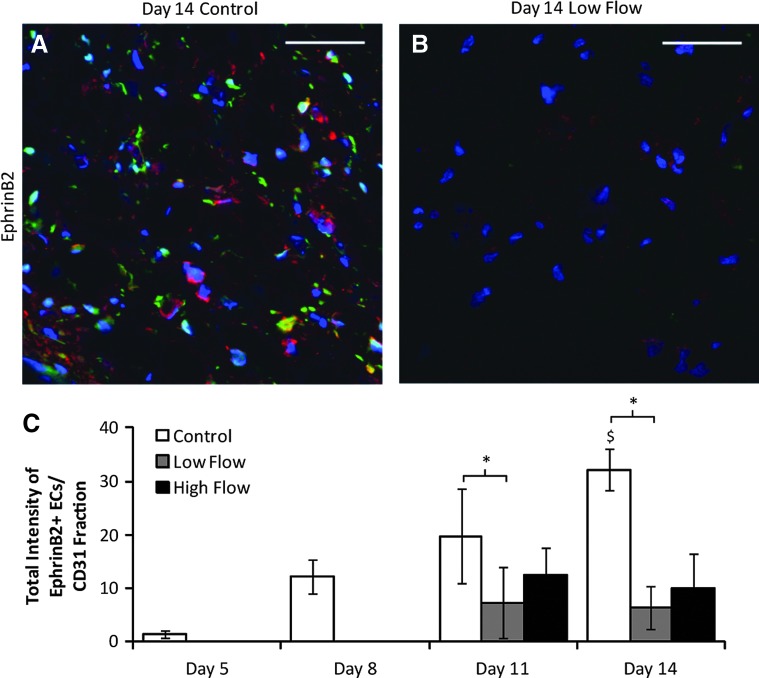
EphrinB2 expression increases over time in the absence of flow and is reduced in the presence of flow
Images of ephrinB2 expression are shown in Figure 6A, B, and the ephrinB2 expression of BOECs (obtained by normalizing BOEC ephrinB2 intensity to CD31+ area fraction data shown in Fig. 6I) is quantified in Figure 6C. The data show that ephrinB2 expression in BOECs increased over time in control constructs and that the application of low interstitial flow reduced BOEC ephrinB2 expression. EphB4 was expressed by BOECs in monolayers (Supplementary Fig. S2) but not in either the static or flow-conditioned constructs at any of the days.
FIG. 6.
(A, B) Representative images of sections of control (A) and low flow (B) constructs from day 14 stained for ephrinB2 (red). PCs are green and nuclei are blue. Scale bars=50 μm. (C) Quantification of blood outgrowth endothelial cell ephrinB2 staining, normalized to values of CD31 fraction obtained from staining of nearby sections (data shown in Fig. 3I). *p<0.05 for main effects. $p<0.05 in comparison with drugs control. Color images available online at www.liebertpub.com/tea
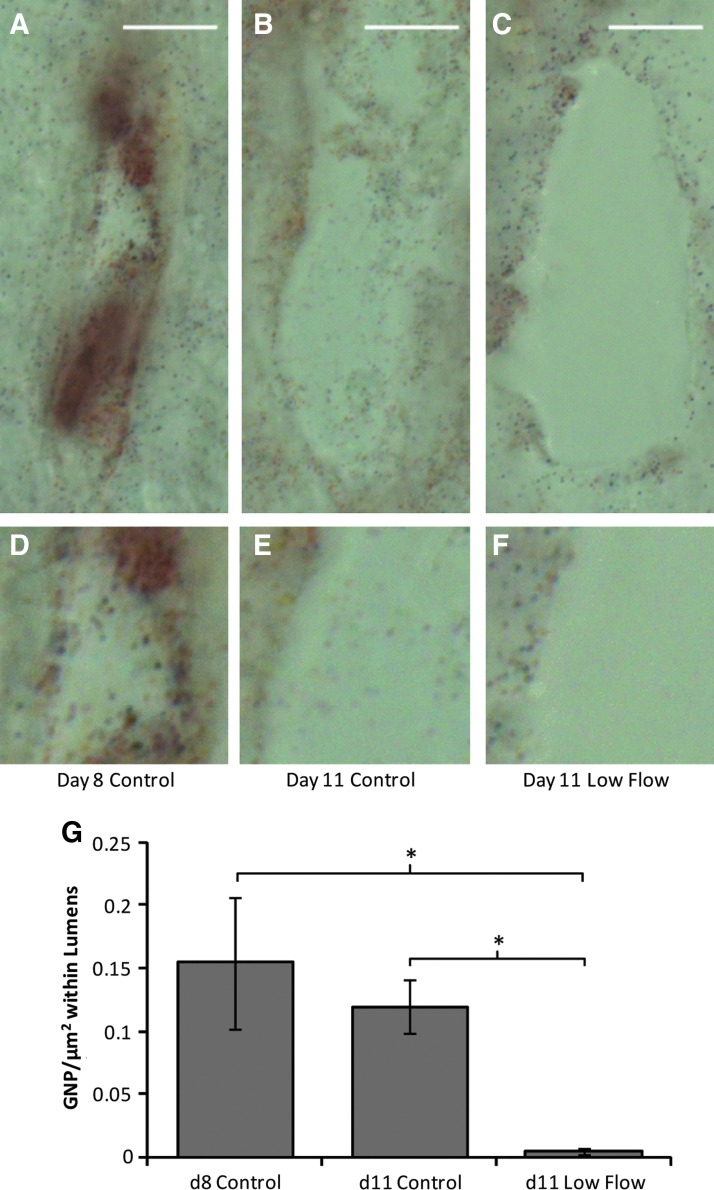
Low interstitial flow increased endothelial barrier function
Fluid flow through the constructs was tracked using GNP added to the perfusate during interstitial flow. GNP were observed throughout the interstitium in longitudinal sections, indicating that fluid was traveling through the constructs rather than channeling between the constructs and the agarose gel. However, GNP were rarely observed within lumens. This observation prompted an experiment to test the endothelial barrier function after flow exposure. Constructs exposed to low flow for 3 days and controls at days 8 and 11 were exposed to a solution containing GNP, and the number of GNP present within lumens at the same distance from the construct inlet (the input location of the GNP solution) was counted. Lumens within both control constructs contained substantially more GNP than those within constructs exposed to low flow, which rarely contained GNP (Fig. 7).
FIG. 7.
Endothelial barrier function assay. (A–C) Representative images of lumens within control constructs at days 8 (A) and 11 (B), and constructs exposed to low flow for 3 days (from day 8 to 11; C). Scale bar=10 μm. (D–F) Enlarged portions of the lumens showing in (A–C), for easier visualization of the gold nanoparticles (GNP). (G) Quantification of the GNP present within the lumens (mean±SEM); low levels of GNP within lumens of constructs exposed to low flow indicated strong EC barrier function. *p<0.05. Color images available online at www.liebertpub.com/tea
Cells experienced low levels of shear stress during interstitial flow
The measured viscosity of the BOEC medium was 890 Pa·s (0.89 cP). Construct hydraulic conductivity was measured to be 2.44×10−3 cm2·Pa/s (1.83×10−5 cm2·mmHg/s) and converted to a hydraulic permeability for use in Equation (1) by multiplying by the viscosity. The final volume of each construct was calculated by measuring the thickness and width of each construct after fixation, and assuming the construct length was 1.5 cm based on the chamber dimensions. The average thickness of compacted constructs was 627 μm, and the average width was 1.26 mm, yielding a cross-sectional area of 0.0075 cm2, and a volume of 0.0112 cm3. These values corresponded to a cross-sectional area after compaction that was 10% of the original value.
To obtain the volume fraction of cells needed for parameter B of Equation (1), each cell was assumed to take up the volume of a sphere 10 μm in diameter. Using the initial cell concentration, 2.4 million/mL, the initial construct volume (400 μL), and the final measured volume of the construct, 0.0112 cm3, the cell volume fraction was calculated to be 0.045, yielding B=1.274. The above parameter values were used along with the volumetric flow rates in Equation (1) to estimate shear stresses on the cells. For low flow (0.015 μL/min), shear stress was 0.0033 (±0.0016) Pa [0.033 (±0.016) dyne/cm2], and for high flow (0.105 μL/min), shear stress was 0.023 (±0.012) Pa [0.23 (±0.12) dyne/cm2].
The COMSOL model of flow around a cylinder embedded in porous medium predicted that the shear stress was 0.0012 (±5.7×10−5) Pa [0.012 (±5.7×10−4) dyne/cm2] for low flow and 0.0056 (±4.1×10−4) Pa [0.056 (±4.1×10−3) dyne/cm2] for high flow. These values are threefold lower than those estimated above; the true shear stress experienced by the cells is likely between the estimated values.
Discussion
A major finding of this work is that cell-induced compaction can be used to align microvessels in a vasculogenic model (previous work had shown this to be possible in an angiogenic model6). Strong microvessel alignment not only mimics native microvasculature (e.g., myocardium4), but also provides natural inlet and outlet sides for flow. In addition to producing aligned microvessels, construct compaction provided a number of other improvements to the BOEC microvessels. Compaction caused a reduction in average lumen size, placing the diameter within the typical coronary capillary range of 5–8 μm.29 In addition, both the CD31+ structure density and the lumen density increased with compaction. Although the CD31+ structure density increased quickly, present at all time points after compaction, lumen density increase was delayed, only present at days 11 and 14. These results suggest that initially compaction caused some lumens to collapse, but once compaction had subsided, some lumens were re-established. Only one-sixth of the lumens were maintained following compaction, based on the predicted lumen density. Previous studies suggested that flow can induce alignment;16 however, increased alignment with flow was not observed here. This may merely indicate that compaction and associated fibrin fibril alignment provided a much stronger alignment effect via contact guidance, and that the additional alignment produced by flow was either present but negligible or was not present due to the fact that the fibrils and cells were highly aligned prior to the onset of flow. The fact that the microvessel anisotropy index was above one for isotropic samples is likely due to the fact that the index was necessarily defined relative to the average angle of microvessels in each image, which could be skewed by one long microvessel in isotropic samples.
A second major finding of this work is that interstitial flow can modulate the properties of existing microvessels. Previous work demonstrated the ability of interstitial flow to increase microvessel formation when the onset of flow was at the time of casting rather than after initial microvessel formation.16,17 The results presented here also indicate that the flow rate affects the results. Low flow produced an increase in lumen density over statically cultured controls, whereas high flow did not. However, similar to previous reports,16 the effects of shear stress and flow-induced morphogen gradients in producing the improvements could not be distinguished.
The lumen density observed in these constructs (both control and flow conditions) is substantially higher than previously reported for in vitro engineered tissues. At day 14, constructs exposed to low flow achieved a lumen density of nearly 650 lumens/mm2 in the middle region, more than six times higher than any previous report,9–12 and equivalent to or greater than most reports of engineered microvessels postimplantation.2,13,14 It is still less than the 2000-lumens/mm2 of native adult human myocardium,8 but is greater than that of adult human skeletal muscle (200–300 lumens/mm2)30,31 In addition, lumen density has been reported to increase dramatically postimplantation (14-fold or more10,12), suggesting that these constructs would exceed 2000 lumens/mm2 postimplantation, presumably still with strong alignment.
An unexpected result was that lumen density was greater in the middle region of constructs. This was true for both control and flow conditions at days 11 and 14, indicating that it is not an effect of flow. Additionally, this effect is not present in controls at days 5 or 8, nor is there any difference in birefringence (indicating differential levels of matrix alignment) or optical density (indicating differential levels of matrix density) between construct regions at any time point. However, the means for most inlet and outlet regions at day 11 fall within the 100–200-lumens/mm2 range, as do means for all regions at days 5 and 8. This suggests that the increases in lumen density observed both with additional days of compaction in static culture (after day 8) and with low flow occurred substantially in the middle region.
Lumens greater than 200 μm in length were frequently observed in longitudinal sections of constructs exposed to low flow for 3 days (day 11). However, due to the potential tortuosity of the lumens and the possibility that sections were not taken exactly parallel to the alignment direction, it is unclear exactly how far the lumens extended. Attempts to image constructs under multiphoton confocal microscopy yielded data only to a depth of ∼200 μm, likely due to the construct density and opacity, providing no further information about lumen length.
The increase in ephrinB2 expression by BOECs over time in control constructs and loss of ephB4 expression relative to the pre-entrapment state suggests that they were maturing toward an arterial phenotype. Additionally, the fact that shear stress induced a reduction in ephrinB2 expression in BOECs indicates that the BOECs were more similar to a mature EC than an endothelial progenitor cell (EPC) when flow began.24 Given the substantial loss of PCs in the flow-conditioned samples, there are at least two alternative explanations. First, ephrinB2 expression decreased in the absence of PC contact. Second, reduction of ephrinB2-positive endothelial cells may lead to the reduction of PCs, since ephrinB2 is required for proper endothelial-PC assembly.32
Fluid tracking via GNP indicated that fluid was traveling through the construct interstitium, as expected. The fact that few GNP were found within lumens suggests that fluid was not entering and flowing through lumens, but rather was limited to the interstitium. This was consistent with time lapse analysis of fluorescent NP during flow (data not shown), suggesting that in these constructs the lumen ends were not open to flow. Mechanisms of achieving lumen perfusion will thus need to be explored. Interestingly, however, an increase in EC barrier function was observed after 3 days of low flow exposure, consistent with EC monolayers exposed to laminar shear flow.19,33,34 The shear stress in this study was applied ablumenally, evidently, and at a level not previously studied in monolayers (∼100-fold lower), suggesting that either the effect of chronic shear stress on EC barrier function is similar over a wide range of shear stresses and application methods, or that tight junction formation occurs differently in microvessels versus EC monolayers.
The data presented here demonstrate the success of using PCs as a support cell for BOEC microvessels formed in a remodeled fibrin gel. PCs were recruited to the microvessels, and abundant basement membrane was formed. Mural cell recruitment and basement membrane formation are the hallmarks of microvessel stability in vivo,1 and thus the PC recruitment and basement membrane formation observed here likely contributed to the microvessel stability over a 2-week period, even in statically cultured constructs.
The exposure of constructs to interstitial flow at either flow rate reduced the number of PCs in comparison with time-matched controls. Because the PC density was high at day 8, it is clear that this difference is the result of PC loss rather than reduced proliferation. The fact that the fraction of recruited PCs remained constant across conditions suggests that PCs were lost equally from recruited and unrecruited positions. The reason for this loss is not clear; however, it may be related to the relatively low levels of culture medium received by the constructs during flow exposure (greater than 100-fold lower than that of control constructs for low flow). The fact that the BOECs were not negatively affected under such conditions is remarkable. Regardless of the mechanism, it does not appear that a high density of PCs is required for microvessel stability in this system due to the deposition of basement membrane prior to flow exposure. In fact, assessment of laminin and collagen IV staining suggests that flow conditions had lower levels of staining in the interstitium, which may be related to the presence of fewer PCs (both PCs and ECs produce basement membrane proteins35).
Although the system described in this article has been successful, it is worth noting that others have generated microvessels in vitro with systems utilizing other endothelial cell types and stromal cells. Mature ECs from a variety of species and vessel locations, including human umbilical vein ECs, have been widely studied despite their potential clinical limitations as nonautologous cell types.3,36–49 Additionally, various stem cell, progenitor, and other blood-derived EC types have been investigated.2,6,13 Of note are BOECs, used in this study, and EPC-derived ECs (EPC-ECs), both of which can be isolated from the blood mononuclear fraction. EPC-ECs are isolated via CD31+ cell selection after plating,13 whereas BOECs are plated in endothelial growth medium and late outgrowth cells are selected.5,50,51 Support cells such as fibroblasts and mesenchymal stem cells have been shown to improve microvessel formation and display PC-like behavior in some cases.2,3,6 Some recent data indicate that the choice of support cell has a large influence on the resulting microvessel formation.52 For a recent review of in vitro models of endothelial assembly in fibrin gel, see Morin and Tranquillo.53
The system used in these studies is highly relevant for tissue engineering. The human BOECs are isolated from peripheral blood, and thereby represent a potentially autologous source of ECs. Although the human brain PCs used here are not an autologous source, PCs can be isolated from dermis.54 Fibrin is degraded by cells and stimulates them to produce ECM;55 therefore, a fully mature tissue-engineered construct formed from a fibrin gel could in fact be completely cell-produced.56 If residual fibrin proved immunogenic, autologous fibrinogen (isolated from peripheral blood) could be used to prepare the tissue. Finally, these remodeled-fibrin constructs have the potential to achieve physiological strength.56,57 The results presented herein thus make strides toward the production of vascularized tissue comprising aligned microvessels of a size and strength relevant to humans.
Supplementary Material
Acknowledgments
The authors thank Pat Schaeffer for assistance with histology and Michelle Lenz for creating the COMSOL model. This work was supported by NIH R01 HL108670 (to R.T.T.) and American Heart Association predoctoral fellowship 11PRE7610056 (to K.T.M.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Stratman A.N., Malotte K.M., Mahan R.D., Davis M.J., and Davis G.E.Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 114,5091, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X., Aledia A.S., Ghajar C.M., Griffith C.K., Putnam A.J., Hughes C.C., and George S.C.Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A 15,1363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao R.R., Peterson A.W., Ceccarelli J., Putnam A.J., and Stegemann J.P.Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis 15,253, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caulfield J.B., and Janicki J.S.Structure and function of myocardial fibrillar collagen. Technol Health Care 5,95, 1997 [PubMed] [Google Scholar]
- 5.Lin Y., Weisdorf D.J., Solovey A., and Hebbel R.P.Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest 105,71, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morin K.T., and Tranquillo R.T.Guided sprouting from endothelial spheroids in fibrin gels aligned by magnetic fields and cell-induced gel compaction. Biomaterials 32,6111, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Rakusan K., Flanagan M.F., Geva T., Southern J., and Van Praagh R.Morphometry of human coronary capillaries during normal growth and the effect of age in left ventricular pressure-overload hypertrophy. Circulation 86,38, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Rakusan K.Quantitative morphology of capillaries of the heart. Number of capillaries in animal and human hearts under normal and pathological conditions. Methods Achiev Exp Pathol 5,272, 1971 [PubMed] [Google Scholar]
- 9.Chang C.C., Nunes S.S., Sibole S.C., Krishnan L., Williams S.K., Weiss J.A., and Hoying J.B.Angiogenesis in a microvascular construct for transplantation depends on the method of chamber circulation. Tissue Eng Part A 16,795, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghanaati S., Fuchs S., Webber M.J., Orth C., Barbeck M., Gomes M.E., Reis R.L., and Kirkpatrick C.J.Rapid vascularization of starch-poly(caprolactone) in vivo by outgrowth endothelial cells in co-culture with primary osteoblasts. J Tissue Eng Regen Med 5,e136, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Hudon V., Berthod F., Black A.F., Damour O., Germain L., and Auger F.A.A tissue-engineered endothelialized dermis to study the modulation of angiogenic and angiostatic molecules on capillary-like tube formation in vitro. Br J Dermatol 148,1094, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Shepherd B.R., Hoying J.B., and Williams S.K.Microvascular transplantation after acute myocardial infarction. Tissue Eng 13,2871, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Chen X., Aledia A.S., Popson S.A., Him L., Hughes C.C.W., and George S.C.Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts. Tissue Eng Part A 16,585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shepherd B.R., Chen H.Y., Smith C.M., Gruionu G., Williams S.K., and Hoying J.B.Rapid perfusion and network remodeling in a microvascular construct after implantation. Arterioscler Thromb Vasc Biol 24,898, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Swartz M.A., and Fleury M.E.Interstitial flow and its effects in soft tissues. Annu Rev Biomed Eng 9,229, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Helm C.L., Fleury M.E., Zisch A.H., Boschetti F., and Swartz M.A.Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc Natl Acad Sci USA 102,15779, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng C.P., Helm C.L., and Swartz M.A.Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvasc Res 68,258, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Seebach J., Donnert G., Kronstein R., Werth S., Wojciak-Stothard B., Falzarano D., Mrowietz C., Hell S.W., and Schnittler H.-J.Regulation of endothelial barrier function during flow-induced conversion to an arterial phenotype. Cardiovasc Res 75,596, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Warboys C.M., Eric Berson R., Mann G.E., Pearson J.D., and Weinberg P.D.Acute and chronic exposure to shear stress have opposite effects on endothelial permeability to macromolecules. Am J Physiol Heart Circ Physiol 298,H1850, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Othman-Hassan K., Patel K., Papoutsi M., Rodriguez-Niedenführ M., Christ B., and Wilting J.Arterial identity of endothelial cells is controlled by local cues. Dev Biol 237,398, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Korff T., Dandekar G., Pfaff D., Füller T., Goettsch W., Morawietz H., Schaffner F., and Augustin H.G.Endothelial ephrinB2 is controlled by microenvironmental determinants and associates context-dependently with CD31. Arterioscler Thromb Vasc Biol 26,468, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Shin D., Garcia-Cardena G., Hayashi S., Gerety S., Asahara T., Stavrakis G., Isner J., Folkman J., Gimbrone M.A., Jr., and Anderson D.J.Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev Biol 230,139, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Obi S., Yamamoto K., Shimizu N., Kumagaya S., Masumura T., Sokabe T., Asahara T., and Ando J.Fluid shear stress induces arterial differentiation of endothelial progenitor cells. J Appl Physiol 106,203, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Goettsch W., Augustin H.G., and Morawietz H.Down-regulation of endothelial ephrinB2 expression by laminar shear stress. Endothelium 11,259, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Wang D.M., and Tarbell J.M.Modeling interstitial flow in an artery wall allows estimation of wall shear stress on smooth muscle cells. J Biomech Eng 117,358, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Morin K.T., Lenz M.S., and Tranquillo R.T.A mathematical model for understanding fluid flow through engineered tissues containing microvessels. Submitted. [DOI] [PubMed]
- 27.Choi J.J., Wang S., Tung Y.-S., Morrison B., 3rd, and Konofagou E.E.Molecules of various pharmacologically-relevant sizes can cross the ultrasound-induced blood-brain barrier opening in vivo. Ultrasound Med Biol 36,58, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tower T.T., and Tranquillo R.T.Alignment maps of tissues: II. Fast harmonic analysis for imaging. Biophys J 81,2964, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherf L., Ben-Shaul Y., Lieberman Y., and Neufeld H.N.The human coronary microcirculation: an electron microscopic study. Am J Cardiol 39,599, 1977 [DOI] [PubMed] [Google Scholar]
- 30.Gavin T.P., Stallings H.W., 3rd, Zwetsloot K.A., Westerkamp L.M., Ryan N.A., Moore R.A., Pofahl W.E., and Hickner R.C.Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J Appl Physiol 98,315, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Duey W.J., Bassett D.R., Jr, Torok D.J., Howley E.T., Bond V., Mancuso P., and Trudell R.Skeletal muscle fibre type and capillary density in college-aged blacks and whites. Ann Hum Biol 24,323, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Salvucci O., Maric D., Economopoulou M., Sakakibara S., Merlin S., Follenzi A., and Tosato G.EphrinB reverse signaling contributes to endothelial and mural cell assembly into vascular structures. Blood 114,1707, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colgan O.C., Ferguson G., Collins N.T., Murphy R.P., Meade G., Cahill P.A., and Cummins P.M.Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am J Physiol Heart Circ Physiol 292,H3190, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Siddharthan V., Kim Y.V., Liu S., and Kim K.S.Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res 1147,39, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandarino L.J., Sundarraj N., Finlayson J., and Hassell H.R.Regulation of fibronectin and laminin synthesis by retinal capillary endothelial cells and pericytes in vitro. Exp Eye Res 57,609, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Koolwijk P., van Erck M.G., de Vree W.J., Vermeer M.A., Weich H.A., Hanemaaijer R., and van Hinsbergh V.W.Cooperative effect of TNFalpha, bFGF, and VEGF on the formation of tubular structures of human microvascular endothelial cells in a fibrin matrix. Role of urokinase activity. J Cell Biol 132,1177, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nehls V., and Drenckhahn D.A novel, microcarrier-based in vitro assay for rapid and reliable quantification of three-dimensional cell migration and angiogenesis. Microvasc Res 50,311, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Nehls V., and Herrmann R.The configuration of fibrin clots determines capillary morphogenesis and endothelial cell migration. Microvasc Res 51,347, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Nehls V., Herrmann R., Huhnken M., and Palmetshofer A.Contact-dependent inhibition of angiogenesis by cardiac fibroblasts in three-dimensional fibrin gels in vitro: implications for microvascular network remodeling and coronary collateral formation. Cell Tissue Res 293,479, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Korff T., and Augustin H.G.Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol 143,1341, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montaño I., Schiestl C., Schneider J., Pontiggia L., Luginbühl J., Biedermann T., Böttcher-Haberzeth S., Barziulis E., Meuli M., and Reichmann E.Formation of human capillaries in vitro: the engineering of prevascularized matrices. Tissue Eng Part A 16,269, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Lafleur M.A., Handsley M.M., Knauper V., Murphy G., and Edwards D.R.Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type-matrix metalloproteinases (MT-MMPs). J Cell Sci 115,3427, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Frerich B., Lindemann N., Kurtz-Hoffmann J., and Oertel K.In vitro model of a vascular stroma for the engineering of vascularized tissues. Int J Oral Maxillofac Surg 30,414, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Morin K.T., Smith A.O., Davis G.E., and Tranquillo R.T.Aligned human microvessels formed in 3D fibrin gel by constraint of gel contraction. Microvasc Res 2013. [Epub ahead of print]; 10.1016/j.mvr.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffith C.K., Miller C., Sainson R.C., Calvert J.W., Jeon N.L., Hughes C.C., and George S.C.Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng 11,257, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Griffith C.K., and George S.C.The effect of hypoxia on in vitro prevascularization of a thick soft tissue. Tissue Eng Part A 15,2423, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Chen Z., Htay A., Dos Santos W., Gillies G.T., Fillmore H.L., Sholley M.M., and Broaddus W.C.In vitro angiogenesis by human umbilical vein endothelial cells (HUVEC) induced by three-dimensional co-culture with glioblastoma cells. J Neurooncol 92,121, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Grainger S.J., and Putnam A.J.Assessing the permeability of engineered capillary networks in a 3D culture. PLoS One 6,e22086, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xue L., and Greisler H.P.Angiogenic effect of fibroblast growth factor-1 and vascular endothelial growth factor and their synergism in a novel in vitro quantitative fibrin-based 3-dimensional angiogenesis system. Surgery 132,259, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Murohara T.Cord blood-derived early outgrowth endothelial progenitor cells. Microvasc Res 79,174, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Medina R.J., O'Neill C.L., Humphreys M.W., Gardiner T.A., and Stitt A.W.Outgrowth endothelial cells: characterization and their potential for reversing ischemic retinopathy. Invest Ophthalmol Vis Sci 51,5906, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Grainger S.J., Carrion B., Ceccarelli J., and Putnam A.J.Stromal cell identity influences the in vivo functionality of engineered capillary networks formed by co-delivery of endothelial cells and stromal cells. Tissue Eng Part A 19,1209, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morin K.T., and Tranquillo R.T.In vitro models of angiogenesis and vasculogenesis in fibrin gel. Exp Cell Res 319,2409, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helmbold P., Nayak R.C., Marsch W.C., and Herman I.M.Isolation and in vitro characterization of human dermal microvascular pericytes. Microvasc Res 61,160, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Neidert M.R., Lee E.S., Oegema T.R., and Tranquillo R.T.Enhanced fibrin remodeling in vitro with TGF-beta1, insulin and plasmin for improved tissue-equivalents. Biomaterials 23,3717, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Syedain Z.H., Meier L.A., Bjork J.W., Lee A., and Tranquillo R.T.Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials 32,714, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Syedain Z.H., Lahti M., Johnson S.L., Robinson P.S., Ruth G.R., Bianco R.W., and Tranquillo R.T.Implantation of a tissue-engineered heart valve from human fibroblasts exhibiting short term function in the sheep pulmonary artery. Cardiovasc Eng Technol 2,101, 2011 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.