Abstract
We report on a biomimetic scaffold as a model system to evaluate smooth muscle cell (SMC) migration in three dimensions. To accomplish this, bio-inert poly (ethylene glycol) (PEG)-based hydrogels were designed as the scaffold substrate. To mimic properties of the extracellular matrix, cell-adhesive peptide (GRGDSP) derived from fibronectin and collagenase-sensitive peptide (GPQGIAGQ) derived from collagen type I were incorporated into the PEG macromer chain. Copolymerization of the biomimetic macromers results in the formation of bioactive PEG hydrogels with cell adhesivity and biodegradability. By utilizing these biomimetic scaffolds, we studied the effect of adhesive ligand concentration, proteolysis, and network cross-linking density on cell migration. Our results showed that three-dimensional SMC migration has a biphasic dependence on adhesive ligand density, and both adhesive and collagenase-sensitive peptides were required for cell migration to occur. Furthermore, network cross-linking density was shown to dramatically influence the behavior of cell migration in the hydrogels.
Introduction
Smooth muscle cell (SMC) migration plays a key role in a variety of physiological and pathological situations, ranging from vascular development to intimal hyperplasia after vascular injury.1–3 During vascular development, migration of pericytes and smooth muscle precursor cells occurs after the formation of an endothelial cell tube, assisting in the development of vessel wall construction and biomechanical functionality of the blood vessels.2,3 In response to vascular injury, SMCs up-regulate the secretion of matrix metalloproteinases (MMPs) and increase their rate of cell migration, which is required for wound healing and vascular repair.3 The development of materials that facilitate SMC migration has been a critical strategy in vascular tissue engineering because of the essential role of cell migration in vascular remodeling.4–6 However, excessive SMC migration, followed by SMC proliferation, if uncontrolled, will induce pathogenic vascular remodeling, which is a key step in the development of intimal hyperplasia.1,3 Therefore, understanding the mechanisms involved in SMC migration and the development of strategies to regulate this process have become emerging areas of research.
Published studies of SMC migration on two-dimensional (2D) surfaces have suggested that cell migration is largely governed by the balance between attachment and detachment, presenting a biphasic dependence on cell-substratum adhesiveness.7 However, conditions for cell migration in vivo are more complex. Besides providing a variety of biochemical cues to guide cell function, the extracellular matrix (ECM) also imposes biophysical resistance to cell movement.8–10
Naturally derived materials, such as collagen gel and fibrin gel,11–13 have been utilized to investigate cell migration in three dimensions, because they possess many critical biological functions such as cell adhesion and biodegradability.14,15 However, biological materials used in vitro have some deficiencies, including relatively poor mechanical properties, batch-to-batch variability, and limited design flexibility, which restrict their potential to become an ideal model.14–16
Synthetic poly (ethylene glycol) (PEG) hydrogels have been widely used in tissue engineering, because of their adjustable mechanical properties, design flexibility, and intrinsic resistance to protein adsorption and cell adhesion.17–19 The bio-inert PEG hydrogels can function as a blank slate to incorporate bioactive factors in a controlled manner, which makes it possible to engineer the PEG gels with desired bioactivities and examine their effects on cell responses.19 For example, PEG hydrogels can be rendered cell adhesive by the incorporation of a cell-adhesive peptide (e.g., Arg-Gly-Asp [RGD]) to the polymeric network.20–24 To tune the degradation rate of PEG hydrogels systematically, enzyme-sensitive peptides or α-hydroxy acids, such as lactic acid, have been conjugated to the macromer backbone.25–34 Growth factors or other bioactive molecules also have been incorporated in PEG gels to study their effect on cell functions.35–37 Further, network properties of PEG hydrogels can be tuned by simply varying the molecular weight (MW) and/or concentration of PEG.38–40
The objective of this work is to engineer a defined, synthetic poly (ethylene glycol) (PEG) hydrogel to facilitate detailed studies of SMC migration in three dimension. To mimic properties of the ECM, cell-adhesive peptide, GRGDSP derived from fibronectin (FN),41 and collagenase-ensitive peptide, GPQGIAGQ derived from collagen type I,42 are incorporated into the PEG chain. Copolymerization of these biomimetic macromers results in the formation of bioactive PEG hydrogels with cell adhesivity and biodegradability. There are many studies on biomimetic hydrogels by incorporating various enzyme-sensitive peptides, such as GPQGIWGQ, GGLGPAGG, and VPMSMRGG,26–33 but there are a few concerning GPQGIAGQ, a native sequence derived from collagen type I. Engineering biomimetic hydrogels using this native sequence mimics the cellular environment more closely, and also provides greater insights into cell/material interactions. In addition, this sequence is less sensitive to MMPs compared with other sequences (e.g., GPQGIWGQ) from the peptide library.26,32 Thus, our biomimetc PEG hydrogels incorporating a less MMP-sensitive sequence may have the advantage of regulating excessive SMC migration and, therefore, reduce the development of intimal hyperplasia. By utilizing this biomimetic hydrogel, we study the effect of adhesive peptide concentration, proteolysis, and network cross-linking density on three-dimensional (3D) SMC migration.
Materials and Methods
Materials
All reagents were obtained from Sigma–Aldrich (St. Louis, MO) and used as received unless otherwise stated.
Synthesis of biomimetic macromers
The cell-adhesive peptide GRGDSP (RGD) and diaminopropionic acid (Dap)-capped collagenase-sensitive peptide GPQGIAGQ-Dap (GIA-Dap) were synthesized on an amide (Knorr) resin using standard Fmoc chemistry on an Applied Biosystems peptide synthesizer (Model 433A, Foster City, CA). The peptides were cleaved from the resin using trifluoroacetic acid and purified by reverse-phase high-performance liquid chromatography (Waters 2690 Alliance system).
The biomimetic macromers, RGD-PEGMA and GIA-PEGDA, were synthesized by conjugating peptides with Acrylate-PEG-Succinimidyl Valerate (Acr-PEG-SVA, MW ∼3400; Laysan Bio, Arab, AL). For the synthesis of RGD-PEGMA, Acr-PEG-SVA (added dropwise, 30–40 mg/mL) was reacted with GRGDSP (15% molar excess) in aqueous sodium bicarbonate solution (50 mM, pH 8.5) at room temperature for at least 4 h. Then, the mixture was dialyzed against water with membranes of molecular weight cut off (MWCO) 2000 for 48 h, to remove salts and unreacted peptides. The purified product was lyophilized and stored at −20°C. GIA-PEGDA was synthesized by the same method as RGD-PEGMA using Acr-PEG-SVA with GIA peptide in a molar ratio of 2:1. The final product was dialyzed against water with membranes of MWCO 5000 for 48 h. The synthesis of biomimetic macromers was confirmed by matrix-assisted laser desorption/ionization mass spectroscopy (MALDI-MS).
Hydrogel preparation
RGD-PEGMA-co-GIA-PEGDA hydrogels were prepared at various compositions (RGD-PEGMA: 0–5 mM, GIA-PEGDA: 5–10% [w/w] in phosphate-buffered saline [PBS, pH 7.4]) using 0.1% (w/v) of Irgacure 2959 (4-[2-hydroxyethoxy]-phenyl-[2-hydroxy-2-propyl]-ketone; Ciba Specialty Chemicals, Tarry-town, NY) as photoinitiator. The precursor solution was then dispensed into a stainless steel mold (D=6 mm, H=1.2 mm), covered with a cover slip, and exposed to a UV light (365 nm, 5–10 mW/cm2) for 10 min.
Swelling ratio measurement
The hydrogels were prepared as previously described, and allowed to swell in excess distilled water at room temperature for 48 h. The swollen gel was weighed and lyophilized. The mass swelling ratio, q, was determined by the following equation:
 |
where ms is the mass of swollen hydrogel, and mp is the mass of the hydrogel after lyophilization.
In vitro degradation of hydrogel
The degradation profile of GIA-PEGDA (5%, w/w) hydrogels was determined in the presence of collagenase. Briefly, gel disks were incubated in Hank's-buffered saline solution (HBSS) for 48 h to allow pre-equilibration. The swollen gels were weighed and incubated in collagenase solution over the concentration range of 0–0.5 μg/mL in HBSS at 37°C. At predetermined time intervals, gels were weighed after the incubation medium had been completely removed. After weighing, the fresh collagenase solution was replenished. The weight percentage was determined by dividing the mass of gel sample at each time interval by the mass of initial swollen hydrogel before collagenase incubation.
Cell culture
Human coronary artery SMCs (HCASMCs; Lonza, Walkersville, MD) were maintained at 37°C, 5% CO2 in SmGM-2 (Lonza) growth medium, which contains 5% fetal bovine serum (FBS) and proprietary amounts of basic fibroblast growth factor, epidermal growth factor, and insulin. Supplied antimicrobials were not added. For all experiments, HCASMCs at passages P8-P11 were used.
Cell attachment and spreading on the hydrogel
To examine cell adhesivity, GIA-PEGDA (5%, w/w) hydrogels with various concentrations of RGD-PEGMA (0–5 mM) were prepared. Briefly, hydrogel precursor solution was sterilized by filtration (0.22 μm pore), and then dispensed into a sterilized stainless steel mold. The gel disks (D=10 mm, H=1.2 mm) were formed as previously described. Hydrogels were then transferred to a 24-well culture plate and incubated in PBS to swell overnight. Before seeding, gels were rinsed thrice with PBS and secured with a ring of silicone tubing. HCASMCs were seeded at a density of 3.0×104 cells/cm2 on hydrogels in serum-free medium (SFM: insulin-trasferrin-selenium supplement [ITS-X, 1×; Invitrogen, Carlsbad, CA], taurine [5 mM], and bovine serum albumin [1 mg/mL] in Dulbecco's-modified Eagle's medium [DMEM; Invitrogen]) for 6 h. Then, the medium was changed to SmGM-2 growth medium. Human FN (1 μg/cm2) was coated on tissue culture polystyrene that served as positive controls. GIA-PEGDA hydrogels with 5 mM of RDG-PEGMA (synthesized by the same method as RGD-PEGMA using the reaction of Acr-PEG-SVA with the scrambled peptide: GRDGSP) were prepared as negative controls (underline indicates the scrambled peptide). After incubation for 24 h after seeding, cells were imaged on a Nikon Diaphot 200 Inverted Phase-Contrast Microscope (10×). The silicone tubing rings were removed, and the samples were washed gently with PBS and frozen at −80°C. Frozen cells were lysed at room temperature using CyQUANT lysis buffer (Invitrogen) containing PicoGreen (Invitrogen). DNA content of the lysate was measured using a fluorescent microplate reader (BioTek, Winooski, VT) at the emission wavelength λem of 520 nm (excitation at λex of 480 nm).
Gelatin zymography
To detect enzyme secretion from HCASMCs, cells were seeded at various densities (0–6×104 cells/cm2) in a 48-well culture plate in SmGM-2 growth medium. After 24 h, media were collected and analyzed for enzyme activity by gelatin zymography. One part of conditioned media was mixed with two parts of zymography sample buffer (1×; Bio-Rad, Hercules, CA) and incubated at room temperature for 10 min. The resulting mixed samples were loaded onto a 10% precast polyacrylamide gel with gelatin (Bio-Rad). Recombinant human MMP-2 (10 ng; R&D, Minneapolis, MN) was used as a standard. Gel electrophoresis was performed in the Tris-Glycine sodium dodecyl sulfate (SDS) Running Buffer at 125V until the dye front reached the bottom of the gel. After electrophoresis, the gels were incubated in zymography renaturing buffer (Triton-X-100, 2.5% [v/v] in water) with gentle agitation for 60 min to remove SDS. After decanting the renaturing buffer, gels were incubated in zymography development buffer (Bio-Rad) at 37°C overnight. The gels were then stained with 0.1% (w/v) Coomassie Brilliant Blue R-250 solution and finally destained with destaining solution (methanol:acetic acid:water=50:5:45). Enzymatic activity was visualized by clear bands against a dark blue background. Gel samples were imaged, and band intensity of each sample was quantified by Image J analysis.
3D cell invasion
A modified 3D cell invasion method was used to evaluate cell migration in 3D.13,26 Generally, a sandwich-like gel was prepared by embedding a cell pellet-loaded gel between two layers of gel (70 μL for each layer) with the same composition. The bottom layer of hydrogel was formed in a stainless steel mold (D=10 mm). To prepare the middle layer of cell pellet-loaded gel, 5 μL of cell suspension (1.5×107 cells/mL matrigel) was applied onto a separate glass plate mold (a glass plate covered with a silicone sheet with punched circles). After 5 min of gelation, hydrogel precursor solution (50 μL) was added into the glass plate mold, covered with a cover slip, and polymerized for 5 min under UV light. Then, the cell pellet-loaded gel was removed from the glass plate mold and placed on top of the hydrogel layer in the stainless steel mold. Excess precursor solution was spread evenly on the top of the cell pellet-loaded gel, and placed under a UV light for 10 min to form the top layer of hydrogel. Once completely polymerized, gel samples (H=1.2 mm) were then transferred to a 12-well culture plate and cultured in SmGM-2 growth medium for approximately 14 days. The medium was changed every 2 days. At predetermined culture times, cells were imaged by inverted phase-contrast microscopy (10×). Digital images were assembled together in Adobe Photoshop for quantification.13 The assembled images were aligned with a grid evenly divided into 36 intervals. The radius of the sample was determined by averaging the distance between the center and the furthest point on each grid line with an invading chain of cells. The migration distance was obtained by subtracting the radius of the initial cell pellet from the radius of the sample.
Statistics
Statistical analysis was done using Origin 8.0 and Minitab 1.6. Data are represented as mean±standard deviation of at least triplicate samples. Single comparisons were made using an un-paired Student's t-test. Analysis of variance (ANOVA) followed by Turkey's post hoc test was used for data sets with multiple comparisons. A value of p<0.05 was considered statistically significant.
Results
Synthesis and characterization of biomimetic macromers
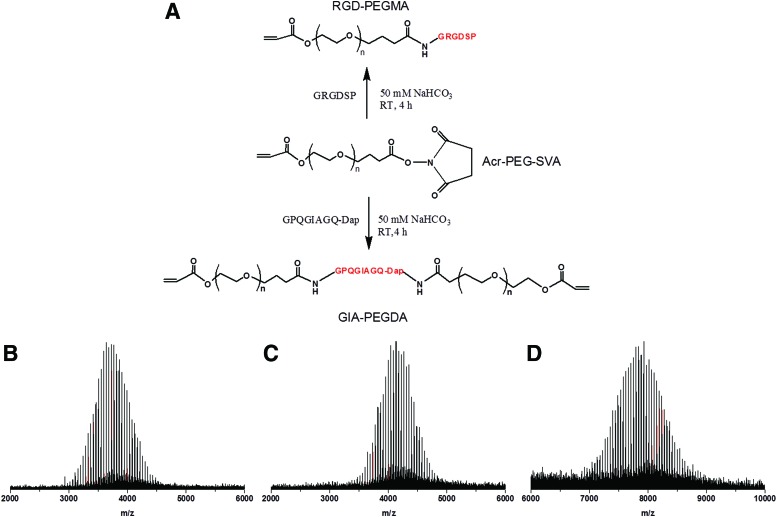
The preparation of biomimetic macromers was performed by utilizing the reaction between the free amino group on the peptide and the N-hydroxysuccinimide (NHS) group on Acr-PEG-SVA (Fig. 1A). Figure 1B shows the MALDI spectrum of unmodified Acr-PEG-SVA with a maximum peak at 3683. After conjugation with RGD peptide in a ratio of 1:1, the maximum peak of RGD-PEGMA was shifted to 4077 (Fig. 1C), indicating successful conjugation of Acr-PEG-SVA with RGD peptide. To conjugate one molecule of GIA peptide with two molecules of Acr-PEG-SVA, the C-terminus of GIA was capped with diaminopropionic acid to provide an additional free amine.43 The MALDI spectrum of GIA-PEGDA with a maximum peak at 7882 confirmed successful conjugation of one molecule of GIA peptide with two molecules of Acr-PEG-SVA (Fig. 1D).
FIG. 1.
Synthesis and characterization of biomimetic macromers. (A) Synthesis scheme of RGD-PEGMA and GIA-PEGDA macromers. (B) MALDI mass spectrum of Acr-PEG-SVA with a maximum peak at 3683. (C) MALDI mass spectrum of RGD-PEGMA with a maximum peak at 4077. (D) MALDI mass spectrum of GIA-PEGDA with a maximum peak at 7882. PEG, poly (ethylene glycol). MALDI, matrix assisted laser desorption/ionization. Color images available online at www.liebertpub.com/tea
Mass swelling ratios of hydrogels
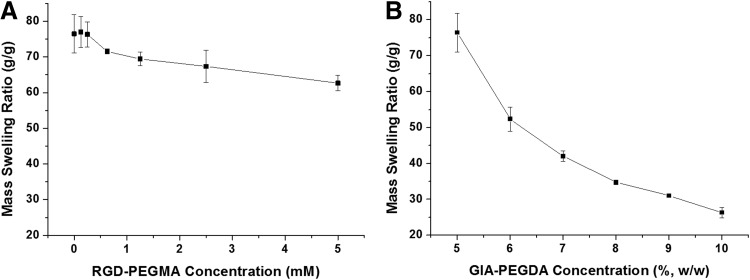
Hydrogels with various compositions (RGD-PEGMA: 0–5 mM, GIA-PEGDA: 5%–10% [w/w]) were prepared, and the mass swelling ratio was determined gravimetrically (Fig. 2). For hydrogels with a fixed concentration of GIA-PEGDA (5%, w/w), increasing the concentration of RGD-PEGMA did not change, or slightly decreased, the swelling ratio of hydrogels, when the RGD-PEGMA concentration was relatively low (0–2.5 mM). Compared with hydrogels formed from GIA-PEGDA alone (5%, w/w), the addition of RGD-PEGMA resulted in a significant decrease of swelling ratio only when the concentration of RGD-PEGMA reached 5 mM (Fig. 2A).
FIG. 2.
The effect of gel compositions on mass swelling ratio of hydrogels. (A) Mass swelling ratio of hydrogels (GIA-PEGDA: 5%, w/w) as a function of RGD-PEGMA (0–5 mM). (B) Mass swelling ratio of hydrogels as a function of GIA-PEGDA (5–10%, w/w).
To study the effect of GIA-PEGDA concentration on the hydrogel network, the mass swelling ratio of gels formed from 5%–10% (w/w) of GIA-PEGDA without RGD-PEGMA was determined (Fig. 2B). When the concentration of GIA-PEGDA was increased from 5% to 10%, the swelling ratio of hydrogels decreased significantly from 76.4 to 26.3. To estimate the network structure of these hydrogels, the mass swelling ratio was further used to calculate the volume swelling ratio.44 Then, the number-average molecular weight between cross-links (Mc) and the average mesh size were determined by Flory-Rehner calculations.44–46 The increase in GIA-PEGDA concentration results in a decreased Mc and mesh size (Table 1), which indicates an increased network cross-linking density.
Table 1.
The Effect of GIA-PEGDA Concentration on the Network Property of Hydrogel
| GIA-PEGDA concentration (%, w/w) | Mc (g/mol) | Mesh size (Å) |
|---|---|---|
| 5 | 3625±39 | 172.5±5.0 |
| 6 | 3416±55 | 147.6±4.3 |
| 7 | 3270±38 | 134.2±2.3 |
| 8 | 3114±25 | 122.9±1.3 |
| 9 | 3032±14 | 116.8±0.6 |
| 10 | 2853±92 | 107.2±3.7 |
In vitro degradation of hydrogels
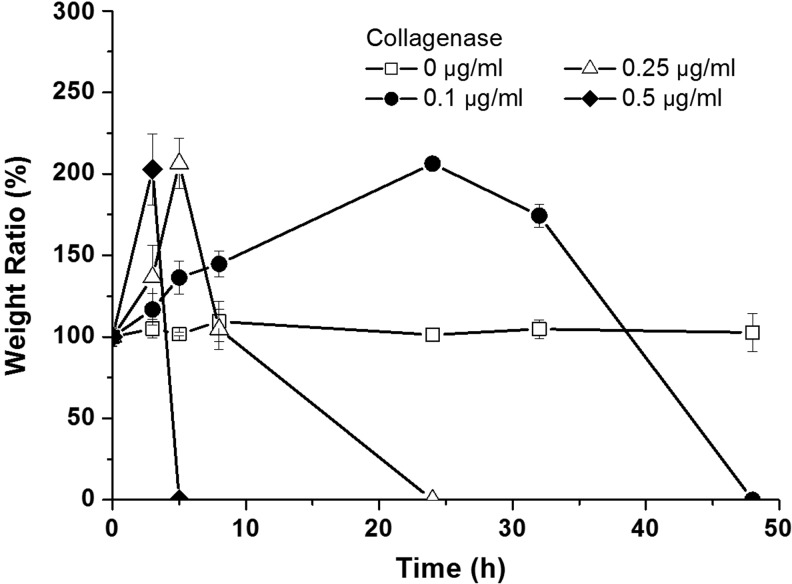
To test the degradability of GIA-modified PEG hydrogels, GIA-PEGDA (5%, w/w) hydrogels were incubated in collagenase solution (0–0.5 μg/mL), and the mass change was measured during degradation (Fig. 3). For gels incubated in the buffer solution without collagenase, the mass of gels did not change over the incubation period, indicating that no degradation occurs in the absence of collagenase. By adding collagenase into the buffer solution, GIA-PEGDA hydrogels gained mass during the early period. This indicates increased water diffusion into the hydrogel network, after a decrease in cross-linking density caused by enzymatic degradation. Through further incubation in collagenase solution, the mass of gels increased to a maximum at a critical time point, and then decreased to zero. This is attributed to the substantial decrease of cross-linking density, resulting in the dissolution of the network into soluble product. Gels were degraded completely within 48 h in 0.1 μg/mL collagenase solution. When the collagenase concentration was increased to 0.5 μg/mL, gels were degraded within 5 h. The rate of degradation of GIA-PEGDA hydrogels is dependent on the concentration of collagenase.
FIG. 3.
Degradation of GIA-PEGDA (5%, w/w) hydrogels in collagenase solution (0–0.5 μg/mL) at 37°C.
Cell attachment and spreading on the hydrogel
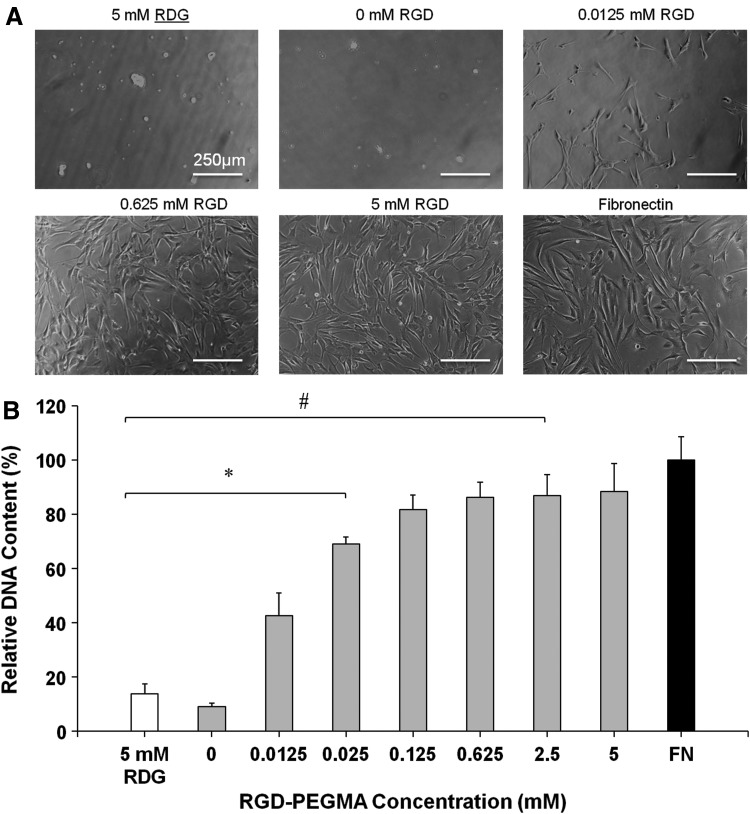
The effect of adhesive ligand concentration on HCASMC attachment and spreading was assessed by utilizing GIA-PEGDA (5%, w/w) hydrogels with varying concentrations of RGD-PEGMA (0–5 mM) (Fig. 4). A few cells adhered, and cell spreading was not observed after 24 h on hydrogels without RGD modification (Fig. 4A). In contrast, by including RGD-PEGMA into the hydrogels, cell attachment and spreading was supported (Fig. 4A). SMCs on PEG hydrogels with 5 mM of RGD-PEGMA were well spread, similar to SMCs on the FN-coated surfaces (Fig. 4A). DNA quantification showed that cell attachment increased and reached a plateau, as the RGD-PEGMA concentration was increased approximately 0.125 mM (Fig. 4B). However, SMCs did not adhere to hydrogels with a high concentration of the scrambled peptide GRDGSP (5 mM), which was similar to the result for plain PEGDA hydrogels.
FIG. 4.
SMC attachment and spreading (after a 24 h seeding period) as a function of RGD concentration (RGD-PEGMA: 0–5 mM) on GIA-PEGDA (5%, w/w) hydrogels. (A) Phase-contrast micrographs (10×) of cell morphology on RGD modified hydrogels. Hydrogels with 5 mM RDG (RDG-PEGMA) served as the negative control. Human FN (1 μg/cm2)-coated tissue culture polystyrene served as the positive control. (B) Cell attachment on RGD-modified hydrogels. Attachment was quantified by measuring DNA content reported relative to the positive control: FN. *p<0.05 with regard to hydrogels with 5 mM RGD; #p<0.05 with regard to FN. SMC, smooth muscle cell; FN, fibronectin.
Enzyme detection
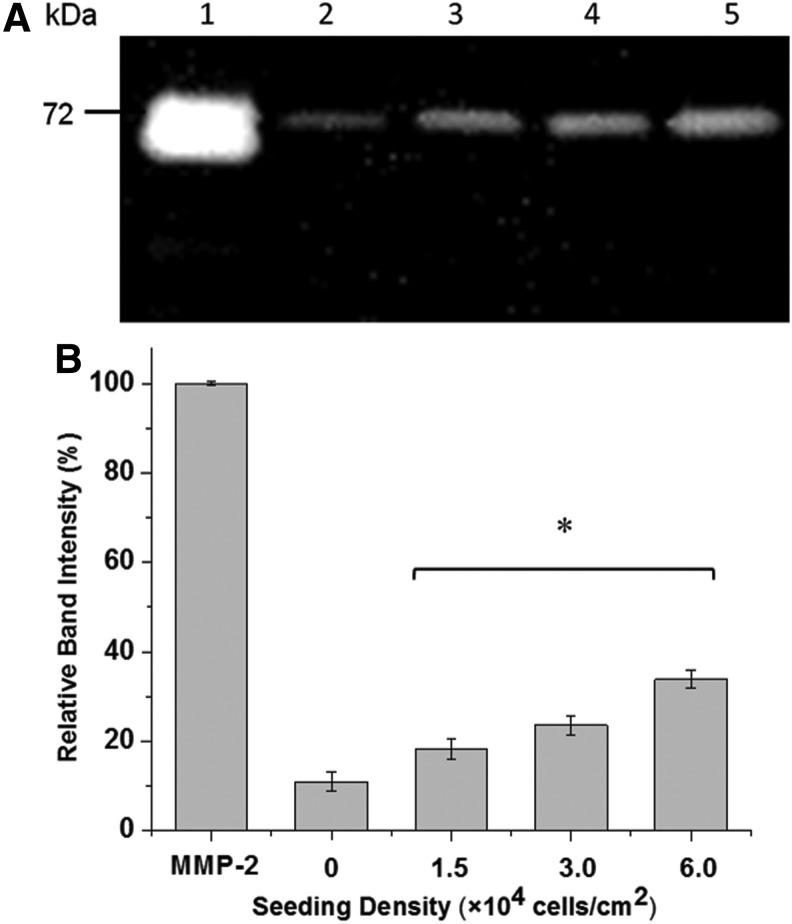
To assess the ability of SMCs to degrade the hydrogels, the presence of enzyme in the conditioned medium from cell culture was determined by gelatin zymography (Fig. 5). By a comparison of sample bands with a molecular weight marker and an MMP-2 standard, it was confirmed that the SMCs expressed MMP-2 (Fig. 5A) and the amount of MMP-2 expression increased with increasing cell number (Fig. 5B). Further, in vitro degradation experiments showed that GIA-PEGDA hydrogels (5%, w/w) were degraded completely by 5 μg/mL MMP-2 solution in 2 h (data not shown).
FIG. 5.
Enzyme detection in conditioned medium (after a 24 h seeding period in SmGM-2 growth medium) by zymography. (A) Zymography image: Lane 1: MMP-2 standard (10 ng); lane 2–5: conditioned medium with a series of seeding density (lane 2: 0×104 cells/cm2; lane 3: 1.5×104 cells/cm2; lane 4: 3.0×104 cells/cm2; lane 5: 6.0×104 cells/cm2) (B) Quantification of enzyme secretion by measuring band intensity reported relative to a MMP-2 standard (10 ng). *p<0.05 with respect to lane 2: 0×104 cells/cm2. MMP, matrix metalloproteinases.
Cell migration in the hydrogel
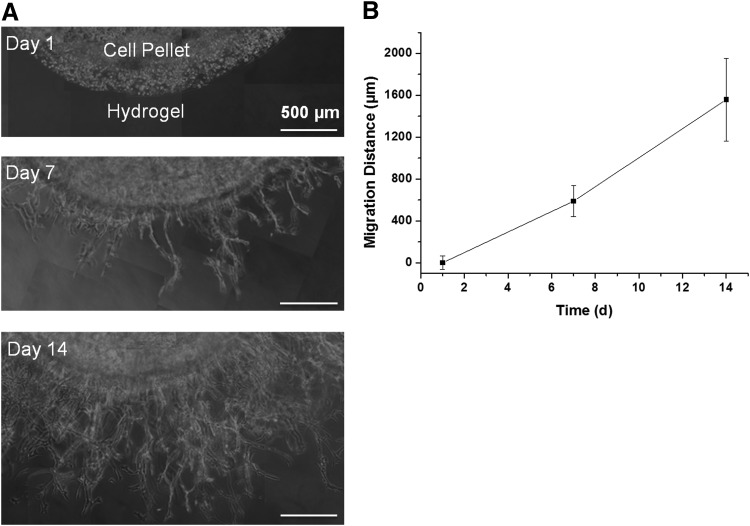
A modified 3D cell invasion method was used to investigate SMC migration behavior in 3D biomimetic PEG hydrogels.13,26 SMCs migrated in the GIA-PEGDA (5%, w/w) hydrogels with 5 mM of RGD-PEGMA (Fig. 6). Initially, the SMC pellet was embedded in the hydrogel disks (Fig. 6A). Then, SMCs migrated from the pellet into surrounding hydrogels (Fig. 6A). The migration distance increased significantly with culture time (Fig. 6B).
FIG. 6.
SMC migration in 3D hydrogels (GIA-PEGDA: 5%, w/w; RGD-PEGMA: 5 mM). (A) Phase-contrast micrographs of cell migration in 3D gels on day 1, 7, and 14. (B) Quantification of migration distance by Image J. 3D, three-dimensional.
The effect of RGD concentration on cell migration
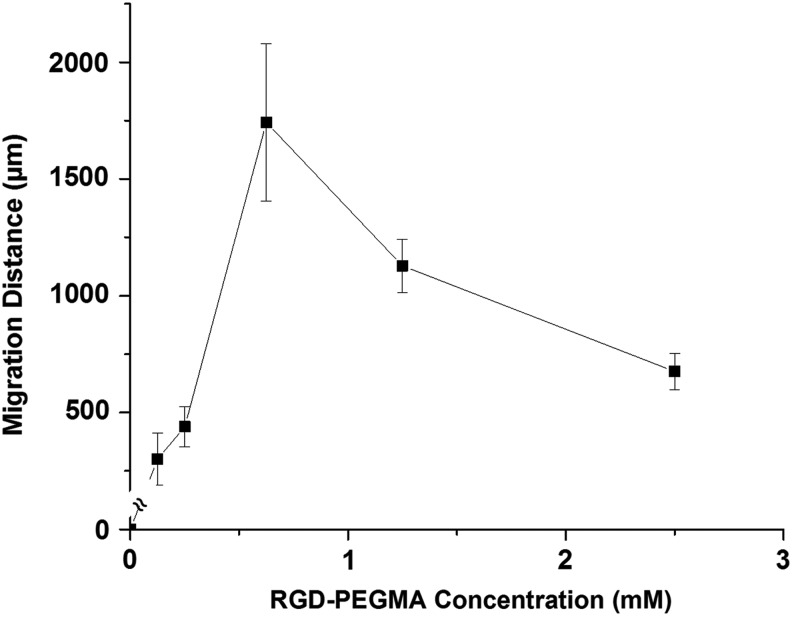
The effect of cell adhesive ligand concentration on cell migration was investigated by comparing the migration distance of SMCs after 7 days in GIA-PEGDA (5%, w/w) hydrogels as a function of RGD-PEGMA concentration (0–2.5 mM in the precursor solution). Figure 7 shows the RGD ligand concentration-mediated SMC migration in a biphasic manner. SMC migration was significantly enhanced in 3D gels with an RGD concentration of 0.625 mM, compared with other tested concentrations. For hydrogels without RGD incorporation or with low adhesive ligand concentration (≤0.025 mM), cell migration was absent. Cell migration in GIA-PEGDA (5%, w/w) hydrogels with various concentrations of RDG-PEGMA (0–2.5 mM) was also determined. Again, no migration was observed in RDG-modified hydrogels (Data not shown).
FIG. 7.
The effect of cell-adhesive ligand (RGD) concentration on SMC migration (on day 7) in 3D hydrogels (GIA-PEGDA: 5%, w/w; RGD-PEGMA: 0–2.5 mM).
The effect of proteolysis on cell migration
To determine the effect of proteolysis on cell migration, GIA-PEGDA and IGA-PEGDA (5%, w/w) hydrogels with a constant RGD ligand concentration (5 mM in the precursor solution) were utilized (Fig. 8). For hydrogels that were MMP sensitive, after 7 days, cells were able to migrate from the pellet into the surrounding gel (Fig. 8A). In contrast, little migration was observed in IGA-PEGDA hydrogels (Fig. 8B), which are not sensitive to collagenase degradation.
FIG. 8.
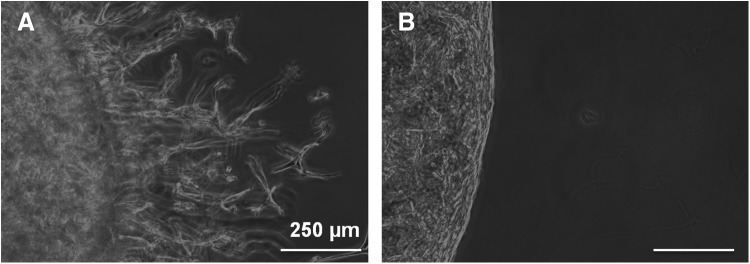
The effect of proteolysis on SMC migration (on day 7) in 3D hydrogels. (A) Cell migration in 5% GIA-PEGDA hydrogels with 5 mM RGD-PEGMA. (B) Cell migration in 5% IGA-PEGDA hydrogels with 5 mM RGD-PEGMA.
The effect of network cross-linking density on cell migration
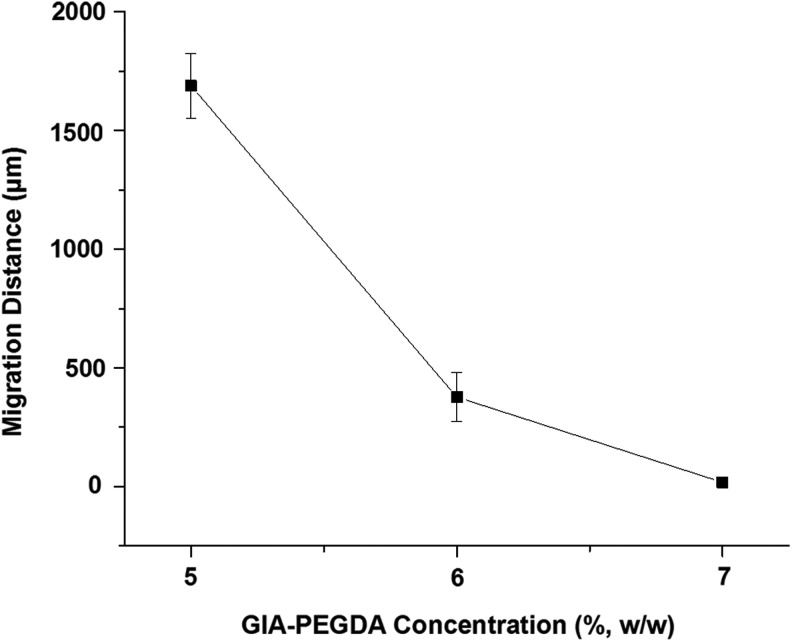
Cell migration into 3D gels with various concentrations of GIA-PEGDA (5%-7%, w/w) was evaluated at a constant RGD ligand concentration of 0.441 mM in the swollen gel (achieved by adjusting the RGD concentration in the precursor solution by correcting for the differences in swelling of the hydrogels) (Fig. 9). Increasing the GIA-PEGDA concentration from 5% to 6%, migration distances decreased significantly from 1688 to 378 μm. At higher concentrations of GIA-PEGDA (7%, w/w), cell migration was restricted completely.
FIG. 9.
The effect of network cross-linking density on SMC migration (on day 7) in 3D hydrogels (GIA-PEGDA: 5%–7%, RGD-PEGMA: 0.441 mM in all the swollen gels).
Discussion
The role of SMC migration as an essential process in physiological and pathological vessel wall remodeling makes the study of mechanisms involved in cell migration a major focus of research.1–3 In contrast to cell migration on 2D surfaces, 3D cell migration is more complex, because migration is mediated not only by biochemical factors (e.g., adhesive ligand concentration), but also by biophysical factors (e.g., network cross-linking density).8–10
Hydrogels have been utilized for 3D studies of cell functions, because they can provide a soft tissuelike environment for cell growth and allow diffusion of nutrients through the highly swollen 3D network.47,48 Synthetic PEG hydrogels were selected as the scaffold in this study, because PEG resists protein adsorption and cell attachment, providing a biological blank slate for controlled bioactive modifications.17–19 To render PEG hydrogels cell adhesive, GRGDSP derived from FN41 was grafted into PEG hydrogels via PEG-monoacrylates during copolymerization. Our experiments indicate that the incorporation of cell-adhesive peptides into the PEG network facilitates SMC attachment and spreading on hydrogels (Fig. 4), which is attributed to the specific binding of RGD ligands to the integrin receptors on SMCs.21 By varying the concentration of adhesive ligand, cell-matrix adhesiveness can be modulated.20–24 The results show that cell attachment increased with increasing RGD-PEGMA concentration (up to 0.125 mM), and was comparable to FN-coated surfaces at an RGD concentration of 2 mM (Fig. 4B). The concentration of adhesive ligand to reach optimal cell attachment and spreading varies in published reports.49,50 This is because multiple other factors, such as mechanics,49 seeding density,43 also affect cell adhesion. To mimic proteolytic degradation mechanisms presented in native ECM, various enzyme-sensitive peptides have been incorporated into PEG network for enzyme-sensitive degradation.25–33 The enzyme-sensitive peptide chosen for this study is GPQGI↓AGQ, which is derived from collagen type I and is responsible for proteolytic degradation of collagen by enzymes such as collagenase or other MMPs.42 The conjugation of GIA peptide into the PEG chain provides the mechanism for proteolytic degradation, and the degradation rate of GIA-PEGDA hydrogels depends on the amount of collagenase present (Fig. 3). Thus, by copolymerization of biomimetic macromers (RGD-PEGMA and GIA-PEGDA), desired biological functions (e.g., cell adhesion, proteolytic degradation) can be introduced into the PEG network in a controlled manner, and their effect on cell behavior can be examined systematically.
A modified 3D cell invasion method was used to investigate SMC migration behavior in 3D biomimetic PEG hydrogels.13,26 A pellet of SMCs was embedded in the hydrogel, and SMC outgrowth from the pellet to surrounding hydrogel was measured. SMC proliferation in the surrounding hydrogel may contribute to the cell number and migration distance. Techniques to separate migration from proliferation, such as mitomyosin C (MMC) to inhibit SMC proliferation in 3D cell invasion experiments, have been described.13 The results showed that MMC treatment attenuated the distance of SMC invasion compared with untreated controls. However, there are concerns that MMC may affect secretion of MMPs or inhibitors,51 and thereafter, affect SMC migration. The observations from our experiments (Fig. 6A) clearly show that SMCs migrated from the pellet into surrounding hydrogel. With culture time extended, SMCs migrated further (Fig. 6). This demonstrates the ability of SMCs to migrate in this biomimetic hydrogel.
Cell-matrix adhesion is a governing parameter of cell migration on 2D surfaces,7 consequently, it is reasonable to anticipate that cell-matrix adhesion also will play a key role in 3D cell migration. To explore the effect of a single parameter (e.g., adhesive ligand concentration) on cell migration in a 3D model, the interdependence of variables (e.g., adhesive ligand concentration vs. hydrogel network property) should be considered. To investigate the effect of adhesive ligand concentration on the hydrogel network, studies of mass swelling ratio as a function of RGD-PEGMA concentration were performed. The results indicated that the hydrogel network was not affected by the inclusion of RGD-PEGMA, while adhesive ligand concentration was in the range of 0–2.5 mM. Therefore, this concentration range (0–2.5 mM) was chosen to study the effect of adhesive ligand concentration on SMC migration. Similar to previous studies of cell migration on 2D surfaces7,52 and within 3D matrices,26,27,29 a biphasic relationship between migration distances and cell-matrix adhesiveness was found (Fig. 7). Since cell migration is a product of the net force between counteracting detachment and adhesion forces, it is hypothesized that at a low ligand concentration, weak cell-matrix adhesiveness results in a decrease in traction forces for forward movement, which subsequently slows cell migration. In contrast, in the presence of a high ligand concentration in the 3D network, strong cell-matrix adhesiveness inhibits cell detachment, which results in decreased migration.26,27
In a 3D matrix, cells should not only interact with matrix adhesive ligands for force generation, but also develop additional strategies to overcome the biophysical resistance of the scaffold.8–10 Depending on the type of cell and matrix, either mechanistic strategy of changing cell morphology and/or matrix degradation are utilized by cells to overcome ECM resistance.8–10 Since the mesh size of the synthetic 3D scaffold (Table 1) is much smaller than the dimensions of SMCs, we hypothesize that proteolysis is required for SMC migration to occur. To investigate this hypothesis, we evaluated the behavior of cell migration in hydrogels that are sensitive or nonsensitive to MMP degradation. For hydrogels that were sensitive to MMP degradation (GIA-PEGDA hydrogels), cell migration was observed and invading cells assumed a spindle-like shape (Fig. 8A). This is markedly different from the well-spread cell morphology found on the surfaces of GIA-PEGDA hydrogels with the same concentration of RGD-PEGMA (Fig. 4A). After the single substitution of MMP sensitive peptide to an MMP nonsensitive peptide (GPQIGAGQ) in the PEG backbone, little cell migration was observed (Fig. 8B). A similar inhibition was observed in degradable matrices when MMPs inhibitor (e.g., GM6001, TIMP-2) was added to the culture medium, which confirms that cell migration in 3D hydrogels depends critically on the action of proteolysis by cell-secreted MMPs.26,29,53 In addition, the incorporated degradable peptides with different proteinase sensitivity were reported to affect the RGD concentration for maximal cell migration.27 The synergistic effect of proteolysis and adhesive ligand concentration on cell migration remains to be further investigated.
The effect of the hydrogel network on cell migration in 3D gels was evaluated by varying the concentration of GIA-PEGDA in the hydrogels. Mass swelling ratio studies showed that a variation in GIA-PEGDA concentrations results in a significant change in mass swelling ratios of the hydrogels (Fig. 2B), which is an important parameter that is closely related to the biophysical properties of the hydrogel network (e.g., mesh size, cross-linking density).37–40 At a constant concentration of RGD-PEGMA (0.441 mM, the concentration that shows maximum migration in 5% GIA-PEGDA gels) in the swollen hydrogel, increasing GIA-PEGDA concentrations significantly decreases migration distances (Fig. 9). This observation is consistent with previous studies, in which the network properties of PEG hydrogels were tuned by PEG molecular weight.26 Therefore, along with cell-matrix adhesiveness and proteolysis, network cross-linking density plays a critical role in 3D cell migration.
Conclusions
Bio-inert PEG gels can be rendered cell adhesive and biodegradable by the incorporation of cell-adhesive peptide (GRGDSP) and collagenase-sensitive peptide (GPQGIAGQ) into the polymeric network. By utilizing this biomimetic scaffold, 3D SMC migration behavior was investigated in which RGD ligand concentration, MMP sensitivity, and network cross-linking density were varied systematically. Our results indicate that 3D SMC migration was critically dependent on cell-matrix adhesiveness and proteolysis, and cell adhesive ligand-mediated cell migration in a biphasic manner. Further, the network cross-linking density of 3D hydrogels also plays a key role in SMC migration in 3D. Besides cell migration, these biomimetic scaffolds offer a useful tool in the fundamental studies of SMC functions (e.g., cell proliferation) in three dimensions, which will facilitate the development of functional tissue-engineered blood vessels.
Acknowledgments
The project described was supported by Grant Number 5RC1EB010795 and Grant Number 1R01HL087843 for the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1.Willis A.I., Pierre-Paul D., Sumpio B.E., and Gahtan V.Vascular smooth muscle cell migration: current research and clinical implications. Vasc Endovascular Surg 38,11, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Gerthoffer W.T.Mechanisms of vascular smooth muscle cell migration. Circ Res 100,607, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Louis S.F., and Zahradka P.Vascular smooth muscle cell motility: from migration to invasion. Exp Clin Cardiol 15,e75, 2010 [PMC free article] [PubMed] [Google Scholar]
- 4.Mann B.K., Gobin A.S., Tsai A.T., Schmedlen R.H., and West J.L.Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials 22,3045, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Almany L., and Seliktar D.Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials 26,2467, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Liu Y., and Chan-Park M.B.A biomimetic hydrogel based on methacrylated dextran-graft-lysine and gelatin for 3D smooth muscle cell culture. Biomaterials 31,1158, 2010 [DOI] [PubMed] [Google Scholar]
- 7.DiMilla P.A., Stone J.A., Quinn J.A., Albelda S.M., and Lauffenburger D.A.Maximal mgration of smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J Cell Biol 122,729, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedl P., and Brocker E.B.The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci 57,41, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedl P., Zanker K.S., and Broker E.B.Cell migration strategies in 3-D extracellular matrix: differences in morphology, cell matrix interactions, and integrin function. Microsc Res Tech 43,369, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Even-Ram S., and Yamada K.M.Cell migration in 3D matrix. Curr Opin Cell Biol 17,524, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Li S., Moon. J.J., Miao H., Jin G., Chen B.P., Yuan S., Hu Y., Usami S., and Chien S.Signal transduction in matrix contraction and the migration of vascular smooth muscle cells in three-dimensional matrix. J Vasc Res 40,378, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Shi Z., Ji X., Berardi D.E., Qazi H., and Tarbell J.M.Interstitial flow induces MMP-1 expression and vascular SMC migration in collagen I gels via an ERK1/2-dependent and c-Jun-mediated mechanism. Am J Physiol Heart Circ Physiol 298,H127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ucuzian A.A., Brewster L.P., East A.T., Pang Y., Gassman A.A., and Greisler H.P.Characterization of the chemotactic and mitogenic response of SMCs to PDGF-BB and FGF-2 in fibrin hydrogels. J Biomed Mater Res A 94,988, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosso F., Marino C., Giordano A., Barbarisi M., Parmeggiani D., and Barbarisi A.Smart materials as scaffolds for tissue engineering. J Cell Physiol 8,839, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Chen R., and Hunt J.A.Biomimetic materials processing for tissue-engineering processes. J Mater Chem 17,3974, 2007 [Google Scholar]
- 16.Pampaloni F., Reynaud E.G., and Stelzer E.H.The third dimensional bridges the gap between cell culture and live tissues. Nat Rev Mol Cell Biol 8,839, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Merrill E.A., and Salzman E.W.Polyethylene oxide as a biomaterial. ASAIO J 6,60, 1983 [Google Scholar]
- 18.Gombotz W.R., Guanghui W., Horbett T.A., and Hoffman A.S.Protein adsorption to poly (ethylene oxide) surfaces. J Biomed Mater Res A 25,1547, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Zhu J.Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 31,4639, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hern D.L., and Hubbell J.A.Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res A 39,266, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Mann B.K., and West J.L.Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. J Biomed Mater Res A 60,86, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Zhu J., Beamish J.A., Tang C., Kottke-Marchant K., and Marchant R.E.Extracellular matrix-like cell adhesive hydrogels form RGD-containing poly (ethylene glycol) diacrylate. Macromolecules 39,1305, 2006 [Google Scholar]
- 23.Beamish J.A., Fu A.Y., Choi A., Haq-Siddiqi N.A., Kottke-Marchant K., and Marchant R.E.The influence of RGD-bearing hydrogels on the re-expression of contractile vascular smooth muscle cell phenotype. Biomaterials 30,4127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu J., Tang C., Kottke-Marchant K., and Marchant R.E.Design and synthesis of biomimetic hydrogel scaffolds with controlled organization of cyclic RGD peptides. Bioconjug Chem 20,333, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West J.L., and Hubbell J.A.Polymeric biomaterials with degradation sites for protease involved in cell migration. Macromolecules 32,241, 1999 [Google Scholar]
- 26.Lutolf M.P., Lauer-Fields J.L., Schmoekel H.G., Metters A.T., Weber F.E., Fields G.B., and Hubbell J.A.Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissure regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A 100,5413, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gobin A.S., and West J.L.Cell migration through defined, synthetic extracellular matrix analogues. FASEB J 16,7751, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Raeber G.P., Lutolf M.P., and Hubbell J.A.Molecularly engineered PEG hdyrogels: a novel model system for proteolytically mediated cell migration. Biophys J 89,1374, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raeber G.P., Lutolf M.P., and Hubbell J.A.Mechanisms of 3-D migration and matrix remodeling of fibroblasts within artificial ECMs. Acta Biomater 3,615, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Adelow C., Segura T., Hubbell J.A., and Frey P.The effect of enzymatically degradable poly (ethylene glycol) hydrogels on smooth muscle cell phenotype. Biomaterials 29,314, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Bott K., Upton Z., Schrobback K., Ehrbar M., Hubbell J.A., Lutolf M.P., and Rizzi S.C.The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials 31,8454, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Patterson J., and Hubbell J.A.Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials 31,7836, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Patterson J., and Hubbell J.A.SPARC-derived protease substrates to enhance the plasmin sensitivity of molecularly engineered PEG hydrogels. Biomaterials 32,1301, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Sawhney A., Pathak C., and Hubbell J.A.Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(alpha-hydroxy acid) diacrylate macromers. Macromolecules 26,581, 1993 [Google Scholar]
- 35.Mann B.K., Schmedlen R.H., and West J.L.Tethered-TGF-β increases extracellular matrix production of vascular smooth muscle cells. Biomaterials 22,439, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Saik J.E., Gould D.J., Watkins E.M., Dickinson M.E., and West J.L.Covalently immobilized platelet-derived growth factor-BB promotes angiogenesis in biomimetic poly (ethylene glycol) hydrogels. Acta Biomater 7,133, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beamish J.A., Geyer L.C., Haq-Siddiqi N.A., Kottke-Marchant K., and Marchant R.E.The effects of heparin releasing hydrogels on vascular smooth muscle cell phenotype. Biomaterials 30,6286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lutolf M.P., and Hubbell J.A.Synthesis and physicochemical characterization of end-linked poly (ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules 4,713, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Munoz-Pinto D.J., Bulick A.S., and Hahn M.S.Uncoupled investigation of scaffold modulus and mesh size on smooth muscle cell behavior. J Biomed Mater Res A 90,303, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Beamish J.A., Zhu J., Kottke-Marchant K., and Marchant R.E.The effects of monoacrylated poly (ethylene glycol) on the properties of poly (ethylene glycol) diacrylate hydrogels used for tissue engineering. J Biomed Mater Res A 92,441, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pierschbacher M.D., and Ruoslahti E.Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309,30, 1984 [DOI] [PubMed] [Google Scholar]
- 42.Aimes R.T., and Quigley J.P.Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem 270,5872, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Zhu J., He P., Lin L., Jones D.R., and Marchant R.E.Biomimetic poly (ethylene glycol)-based hydrogels as scaffolds for inducing endothelial adhesion and capillary-like network formation. Biomacromolecules 13,706, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zustiak S.P., and Leach J.B.Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomcromolecules 11,1348, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cruise G.M., Scharp D.S., and Hubbell J.A.Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials 19,1287, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Canal T., and Peppas N.A.Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J Biomed Mater Res A 23,1183, 1989 [DOI] [PubMed] [Google Scholar]
- 47.Cushing M.C., and Anseth K.S.Hydrogel cell cultures. Science 316,1133, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Zhu J., and Marchant R.E.Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices 8,607, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peyton S.R., Raub C.B., Keschrumrus V.P., and Putnam A.J.The use of poly (ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials 27,4881, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Li L., Wu J., and Gao C.Gradient immobilization of a cell adhesion RGD peptide on thermal responsive surface for regulating cell adhesion ad detachment. Colloids Surf B Bioterfaces 85,12, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Hamner M.A., Vernon R.B., Gooden M.D., Koike T., and Reed M.J.Elongation and secretion of tissue inhibitor of metalloproteinases 1 by human microvascular endothelial cells cultured in collagen gels is stimulated by mitomycin c. Endothelium 12,97, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Wu J., Mao Z., and Gao C.Controlling the migration behaviors of vascular smooth muscle cells by methoxy poly(ethylene glycol) brushes of different molecular weight and density. Biomaterials 33,810, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Cheng L., Mantile G., Pauly R., Nater C., Felici A., Monticone R., Bilato C., Gluzband Y.Z., Crow M.T., Stetler-Stevenson W., and Capogrossi M.C.Adenovirus-mediated gene transfer of the human tissue inhibitor of metalloproteinase-2 blocks vascular smooth muscle cell invasiveness in vitro and modulates neointimal development in vivo. Circulation 98,2195, 1998 [DOI] [PubMed] [Google Scholar]