Abstract
Following the coordinated efforts of five established scientific organizations, this report describes the “novel cellular therapy” activity (i.e., cellular treatments excluding hematopoietic stem cells [HSC] for the reconstitution of hematopoiesis) in Europe for the year 2011. Two hundred forty-six teams from 35 countries responded to the cellular therapy survey, 126 teams from 24 countries provided data on 1759 patients using a dedicated survey and 120 teams reported no activity. Indications were musculoskeletal/rheumatological disorders (46%; 99% autologous), cardiovascular disorders (22%; 100% autologous), hematology/oncology, predominantly including the prevention or treatment of graft-versus-host disease (18%; 2% autologous), neurological disorders (2%; 83% autologous), gastrointestinal (1%; 68% autologous), and other indications (12%; 77% autologous). Autologous cells were used predominantly for musculoskeletal/rheumatological (58%) and cardiovascular (27%) disorders, whereas allogeneic cells were used mainly for hematology/oncology (84%). The reported cell types were mesenchymal stem/stromal cells (56%), HSC (23%), chondrocytes (12%), dermal fibroblasts (3%), keratinocytes (2%), and others (4%). In 40% of the grafts, cells were delivered following ex vivo expansion, whereas cells were transduced or sorted, respectively, in 3% and 10% of the reported cases. Cells were delivered intraorgan (42%), intravenously (26%), on a membrane or gel (16%), or using 3D scaffolds (16%). Compared to last year, the number of teams participating in the dedicated survey doubled and, for the first time, all European Group for Blood and Marrow Transplantation teams reporting information on cellular therapies completed the extended questionnaire. The data are compared with those collected since 2008 to identify trends in the field. This year's edition specifically focuses on cardiac cell therapy.
Introduction
The clinical use of the so-called “novel cellular therapies,” namely those not aimed at the reconstitution of the hematopoietic system, is not only a challenging target for the scientific community, but also the subject of intense public debate.1,2 The landscape includes not only the scientific and clinical community together with the patients, their families, and the lay public, but also health regulators, national health services/health insurance companies, and service providers. Despite the direct interest by a broad set of involved parties, transparent access to accurate data on clinical use of cell therapies is extremely limited and confined within specific sectors.
In 2008, the European sections of the Tissue Engineering and Regenerative Medicine International Society-Europe (TERMIS-EU), of the International Society of Cellular Therapy (ISCT), and of the International Cartilage Repair Society (ICRS), in a joint initiative with the European group for Blood and Marrow Transplantation (EBMT) and the European League Against Rheumatism (EULAR), established a survey on novel cellular therapies. This has allowed the number of patients treated in Europe with cells or engineered tissues to be collected and to be sorted by specific therapeutic indications, cell types used, and cell processing/delivery modes.3–5 The survey aims to offer a transparent and unbiased update on the constructive work carried out, thanks to the coordinated efforts of the different stakeholders, including scientists, clinicians, and their patients, in compliance with the required authorizations.
Here, we report the results of the fourth survey for the activity in 2011, with a comparison to the previously identified trends and a specific discussion on cell-based treatments in the field of cardiovascular therapy. The information presented is complementary to that available in published studies and public databases (e.g., www.clinicaltrials.gov), as it does not include safety/efficacy data and specifies the conducted as opposed to planned numbers of treatments.
Patients and Methods
Definitions
For the purpose of this survey, novel cellular therapies include the use of cells other than hematopoietic stem cells (HSC) or of HSC for uses other than reconstitution of the hematopoietic system. The term HSC, which is often ambiguously used in the field of novel cellular therapies, here indicates a mixture of stem and progenitor cells predominantly of the hematopoietic lineage. Donor lymphocyte infusions often used in relapsing patients after HSC transplantation are considered to be an integral part of the HSC transplant procedure and are excluded.
Data collection and validation
Participating teams were requested to report their data for 2011 by indication, cell type and source, donor type, processing method, and delivery mode. The survey followed the traditional principles of the EBMT, concentrating on numbers of patients with a first cellular therapy. EBMT teams from 49 countries (39 European and 10 affiliated countries) were contacted for the 2011 report (EBMT survey), as were members of the 4 participating societies, teams who had reported activity to previous surveys, together with 118 additional contacts identified either through the clinicaltrials.gov database or literature search. The non-European countries affiliated with the EBMT were Algeria, Azerbaijan, Iran, Israel, Jordan, Lebanon, Nigeria, Saudi Arabia, South Africa, and Tunisia. Extended questionnaires, in the format displayed in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/tea), were received in paper form or electronically. Quality control measures, for EBMT members only, included several established independent systems: confirmation of validity of the entered data by the reporting team, selective comparison of the survey data with MED-A data sets in the EBMT ProMISE data system, cross-checking with the National Registries, and onsite visits of selected teams. No quality control system could be applied to the non-EBMT reporting teams as yet.
For this survey, a number of changes in the data collection sheet were introduced (i) to better capture and group the disease indications and (ii) to distinguish between automated and manual cell processing. In the accompanying guidelines, automated cell processing was described as being appropriate when the cell isolation or culture was performed using an automated device.
Transplant rates
Transplant rates, defined as the reported numbers of patients receiving cellular therapies or the number of teams reporting treatments per 10 million inhabitants, were computed for each country, without adjustments for patients who crossed borders or received treatment in a foreign country. Population numbers were obtained from the 2011 US census office database (www.census.gov).
Results
Participating teams
Two hundred forty-six teams in 35 countries (29 European and 6 EBMT affiliated countries) responded to the novel cellular therapy survey, 126 teams (24 countries) reported performing novel cellular therapies providing detailed information on indication, cell source and type, donor type, processing, and delivery mode, whereas 120 teams reported no activity. In previous years, a number of teams have reported using the standard EBMT transplant activity survey sheet, allowing the inclusion of limited information. This year, for the first time, all EBMT teams reporting information on cellular therapies completed the extended questionnaire. Teams that responded to the activity are listed in Appendix in alphabetical order by country, city, EBMT CIC code (if applicable), along with the total numbers of reported novel cellular therapies.
Number of novel cellular therapies and disease indications
According to the received reports, 1759 patients were treated with novel cellular therapies, 373 (21%) with allogeneic, and 1386 (79%) with autologous cells (Table 1). Indications were musculoskeletal/rheumatological disorders (46%; 99% autologous), cardiovascular disorders (22%; 100% autologous), HSC graft enhancement/prevention or treatment of graft-versus-host disease (GvHD), herewith grouped using the term “hematology/oncology” (18%; 2% autologous), neurological disorders (2%; 83% autologous), gastrointestinal (1%; 68% autologous), and other indications (12%; 77% autologous).
Table 1.
Number of Reported Novel Cellular Therapy Treatments in Europe in 2011 Sorted by Indication, Cell Source, and Donor Type
| Cell type and source | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Autologous | Allogeneic | |||||||||||
| Indication | HSC | MSC | Chondrocyte | Dermal fibroblast | Other | HSC | MSC | Keratinocyte | Other | Autologous | Allogeneic | Total |
| Cardiovascular | ||||||||||||
| Peripheral artery disease | 70 | 6 | 76 | 0 | 76 | |||||||
| Cardiomyopathy | 49 | 4 | 53 | 0 | 53 | |||||||
| Heart failure | 51 | 51 | 0 | 51 | ||||||||
| Myocardial ischemia | 89 | 1 | 5 | 95 | 0 | 95 | ||||||
| Bypass graft | 9 | 9 | 0 | 9 | ||||||||
| Decubitus and leg ulcers | 58 | 58 | 0 | 58 | ||||||||
| Other/unspecified | 25 | 4 | 6 | 35 | 0 | 35 | ||||||
| Musculoskeletal/rheumatological | ||||||||||||
| Bone repair (maxillofacial) | 19 | 5 | 24 | 0 | 24 | |||||||
| Bone repair (orthopedics) | 24 | 36 | 60 | 0 | 60 | |||||||
| Osteogenesis imperfecta | 2 | 2 | 2 | 2 | 4 | |||||||
| Cartilage repair (orthopedics) | 40 | 207 | 247 | 0 | 247 | |||||||
| Muscle repair | 9 | 9 | 0 | 9 | ||||||||
| Tendon/ligament | 8 | 8 | 0 | 8 | ||||||||
| Reconstructive surgery/tissue enhancement | 382 | 7 | 3 | 392 | 0 | 392 | ||||||
| Scleroderma | 4 | 3 | 7 | 0 | 7 | |||||||
| Arthritis | 38 | 38 | 0 | 38 | ||||||||
| Other/unspecified | 12 | 1 | 13 | 0 | 13 | |||||||
| Neurological | ||||||||||||
| Multiple sclerosis | 2 | 8 | 1 | 2 | 10 | 3 | 13 | |||||
| Parkinson's | 1 | 1 | 0 | 1 | ||||||||
| Other/unspecified | 19 | 4 | 4 | 23 | 4 | 27 | ||||||
| Gastrointestinal | ||||||||||||
| Crohn's disease | 13 | 2 | 13 | 2 | 15 | |||||||
| Liver insufficiency | 4 | 0 | 4 | 4 | ||||||||
| Hematology/oncology | ||||||||||||
| GvHD prevention or treatment | 13 | 252 | 0 | 265 | 265 | |||||||
| HSC graft enhancement | 7 | 1 | 47 | 8 | 47 | 55 | ||||||
| Miscellaneous | ||||||||||||
| Skin reconstruction | 29 | 36 | 0 | 31 | 65 | 31 | 96 | |||||
| Cornea repair | 4 | 4 | 0 | 4 | ||||||||
| Diabetes | 4 | 0 | 4 | 4 | ||||||||
| Solid tumor | 10 | 28 | 38 | 0 | 38 | |||||||
| Other | 9 | 5 | 12 | 21 | 11 | 47 | 11 | 58 | ||||
| Total | 397 | 659 | 214 | 49 | 67 | 14 | 320 | 31 | 8 | 1386 | 373 | 1759 |
HSC, hematopoietic stem cells; MSC, mesenchymal stromal/stem cells; GvHD, graft-versus-host disease.
Among the musculoskeletal/rheumatological disorders, the reconstructive surgery/tissue enhancement was the most frequently reported indication, followed by cartilage and bone repair. Among the cardiovascular disorders, myocardial ischemia and peripheral artery disease were the main reasons for a cellular therapy, followed by cardiomyopathy and heart failure. The number of patients treated for neurological and gastrointestinal indications was rather limited and mostly confined to multiple sclerosis (neurological) and Crohn's disease (gastrointestinal). Among the remaining indications, most patients were treated for skin reconstruction or for solid tumor (Table 1).
Cell type, source, and donor type
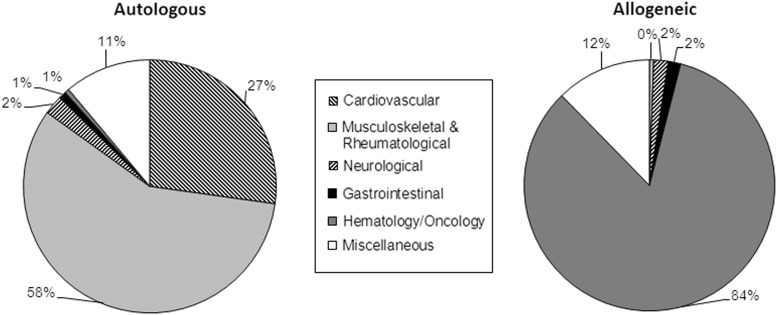
The reported cell types were mesenchymal stem/stromal cells (MSC) (56%), HSC (23%), chondrocytes (12%), dermal fibroblasts (3%), keratinocytes (2%), and others (4%). Of the 411 HSC treatments, 97% were autologous transplants and 74% of these were for cardiovascular diseases (Table 1). All 214 chondrocyte and 49 dermal fibroblast transplants were autologous, whereas all 31 keratinocytes transplants were allogeneic. From 979 MSC-based therapies, 67% were autologous. The donor type was associated with the disease indication: autologous cells were used predominantly for musculoskeletal/rheumatological (58%) and cardiovascular (27%) disorders, whereas the main use of allogeneic cells was for hematology/oncology (84%) (Fig. 1). MSC were mainly obtained from adipose tissue (50%) or bone marrow (49%) and mostly used for the reconstructive surgery/tissue enhancement within the area of musculoskeletal/rheumatological disorders (54%) or for hematology/oncology (31%). For the HSC treatments, cells were derived from bone marrow (65%) or peripheral blood (35%).
FIG. 1.
Percentage of indications for novel cellular therapies in Europe in 2011, sorted by donor type.
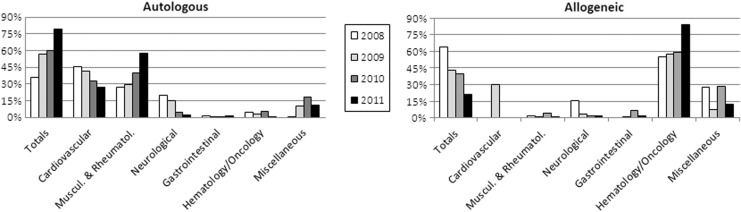
The percentage of treatments using autologous versus allogeneic cells steadily increased from 36% in 2008 to 79% in 2011. This was reflected in the trends for the various therapy areas, with the exception of hematology/oncology (Fig. 2).
FIG. 2.
Comparative analysis of indications for novel cellular therapies in Europe from 2008 to 2011, sorted by donor type. Data used for this chart were derived from the current study and the three previous reports.3–5
Cell processing and delivery mode
Of all the grafted products, 65% required cell expansion, 3% were transduced cells, and 10% were sorted (Table 2). Nonexpanded cells were used to treat 95% of cardiovascular, 70% of musculoskeletal/rheumatological, and 63% of neurological indications, while gastrointestinal indications were exlusively treated with expanded cells. Expanded cells were also used for 97% of hematology/oncology treatments. Cell sorting was applied predominantly for musculoskeletal/rheumatological (10%) and cardiovascular (10%) disorders. Transplanted cells were genetically transduced for 58% of solid tumor cases, 21% of gastrointestinal (all liver insufficiency), and 6% of cardiovascular diseases. Twenty-four percent of cells were reported to be processed using an automated device. These cells were mostly used to treat cardiovascular (39%), musculoskeletal/rheumatological (25%), or gastrointestinal (21%) diseases.
Table 2.
Number of Reported Novel Cellular Therapy Treatments in Europe in 2011 Sorted by Cell Processing Mode
| Cell processing | ||||||||
|---|---|---|---|---|---|---|---|---|
| Indications | Nonexpanded | Expanded | Untransduced | Transduced | Unsorted | Sorted | Automated | Manual |
| Cardiovascular | ||||||||
| Peripheral artery disease | 70 | 6 | 52 | 24 | 62 | 14 | 43 | 33 |
| Cardiomyopathy | 53 | 53 | 52 | 1 | 1 | 52 | ||
| Heart failure | 51 | 51 | 46 | 5 | 13 | 38 | ||
| Myocardial ischemia | 90 | 5 | 95 | 89 | 6 | 3 | 92 | |
| Bypass graft | 9 | 9 | 9 | 9 | ||||
| Valve replacement | ||||||||
| Decubitus+leg ulcers | 58 | 58 | 58 | 58 | ||||
| Other | 29 | 6 | 35 | 31 | 4 | 20 | 15 | |
| Musculoskeletal/rheumatological | ||||||||
| Bone repair (maxillofacial) | 24 | 24 | 24 | 5 | 19 | |||
| Bone repair (orthopedics) | 51 | 9 | 60 | 51 | 9 | 31 | 29 | |
| Osteogenesis imperfecta | 4 | 4 | 3 | 1 | 1 | 3 | ||
| Cartilage repair (orthopedics) | 86 | 161 | 247 | 219 | 28 | 21 | 226 | |
| Muscle repair | 9 | 9 | 9 | 9 | ||||
| Tendon/ligament | 8 | 8 | 8 | 8 | ||||
| Reconstructive surgery/tissue enhancement | 377 | 15 | 392 | 387 | 5 | 126 | 266 | |
| Scleroderma | 4 | 3 | 7 | 4 | 3 | 1 | 6 | |
| Arthritis | 38 | 38 | 38 | 38 | ||||
| Other | 13 | 13 | 13 | 13 | ||||
| Neurological | ||||||||
| Multiple sclerosis | 2 | 11 | 13 | 13 | 1 | 12 | ||
| Parkinson's | 1 | 1 | 1 | 1 | ||||
| Other | 23 | 4 | 27 | 27 | 27 | |||
| Gastrointestinal | ||||||||
| Crohn's disease | 15 | 15 | 15 | 0 | 15 | |||
| Liver insufficiency | 4 | 4 | 4 | 4 | ||||
| Hematology/oncology | ||||||||
| GvHD prevention or treatment | 11 | 254 | 264 | 1 | 263 | 2 | 9 | 256 |
| HSC graft enhancement | 55 | 55 | 54 | 1 | 55 | |||
| Miscellaneous | ||||||||
| Skin reconstruction | 37 | 59 | 96 | 70 | 26 | 8 | 88 | |
| Cornea repair | 4 | 4 | 4 | 4 | ||||
| Diabetes | 4 | 4 | 4 | 4 | ||||
| Solid tumor | 32 | 6 | 16 | 22 | 14 | 24 | 2 | 36 |
| Other | 21 | 37 | 58 | 58 | 9 | 49 | ||
| Total | 1050 | 709 | 1708 | 51 | 1578 | 181 | 382 | 1377 |
Just under one half (42%) of the cell grafts was delivered intra-organ, 26% intravenously, 16% on a membrane or gel, and 16% using a 3D scaffold (Table 3). Cells were delivered intra-organ for 66% of cardiovascular, 58% of neurological, and 50% of musculoskeletal/rheumatological disorders. Intravenous delivery was reported for all hematology/oncology treatments and about half (47%) for gastrointestinal disorders. The use of a membrane or a gel for cell delivery was reported almost exclusively for musculoskeletal/rheumatological (33%) treatments. A 3D scaffold was used for musculoskeletal/rheumatological indications (16%), in particular for cartilage or bone repair (42%) and for cardiovascular (21%) disorders—within this mainly for decubitus and leg ulcers (74%).
Table 3.
Number of Reported Novel Cellular Therapy Treatments in Europe in 2011 Sorted by Delivery Mode
| Cell delivery mode | ||||
|---|---|---|---|---|
| Indications | Intravenous | Intra-organ | Membrane/gel | 3D scaffold |
| Cardiovascular | ||||
| Peripheral artery disease | 7 | 69 | ||
| Cardiomyopathy | 1 | 52 | ||
| Heart failure | 20 | 31 | ||
| Myocardial ischemia | 11 | 84 | ||
| Decubitus+leg ulcers | 58 | |||
| Other | 10 | 5 | 20 | |
| Musculoskeletal/rheumatological | ||||
| Bone repair (maxillofacial) | 24 | |||
| Bone repair (orthopedics) | 14 | 34 | 12 | |
| Osteogenesis imperfecta | 2 | 2 | ||
| Cartilage repair (orthopedics) | 47 | 120 | 80 | |
| Muscle repair | 9 | |||
| Tendon/ligament | 8 | |||
| Reconstructive surgery/tissue enhancement | 268 | 118 | 6 | |
| Scleroderma | 3 | 3 | 1 | |
| Arthritis | 38 | |||
| Other | 13 | |||
| Neurological | ||||
| Multiple sclerosis | 13 | |||
| Parkinson's | 1 | |||
| Peripheral nerve regeneration (trauma) | ||||
| Other | 4 | 23 | ||
| Gastrointestinal | ||||
| Crohn's disease | 7 | 8 | ||
| Liver insufficiency | 3 | 1 | ||
| Hematology/oncology | ||||
| GvHD prevention or treatment | 265 | |||
| HSC graft enhancement | 55 | |||
| Miscellaneous | ||||
| Skin reconstruction | 29 | 67 | ||
| Cornea repair | 4 | |||
| Diabetes | 4 | |||
| Solid tumor | 14 | 24 | ||
| Other | 25 | 21 | 12 | |
| Total | 452 | 729 | 287 | 282 |
3D, three-dimensional.
Transplant rates and active teams
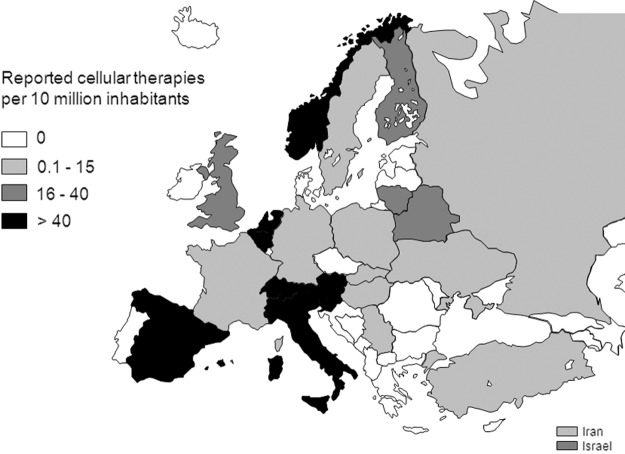
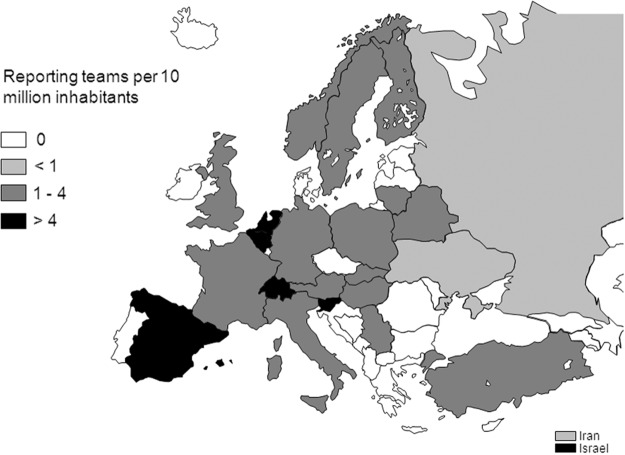
Reported cellular therapies were performed in a limited number of countries and with different intensity. Figure 3 displays the reported cellular therapy transplants per 10 million inhabitants in the different European- and EBMT-associated countries. High transplant rates (i.e., >100 per 10 million population) were reported in Italy and Slovenia. The number of teams reporting novel cellular therapies was also mapped in the different European- and EBMT-associated countries after normalization to the inhabitant numbers (Fig. 4). The number of reporting teams per 10 million inhabitants was higher than four in Belgium, Israel, The Netherlands, Slovenia, Spain, and Switzerland.
FIG. 3.
Number of novel cellular therapies per 10 million inhabitants reported in Europe in 2011.
FIG. 4.
Number of teams per 10 million inhabitants reporting novel cellular therapies in Europe in 2011.
Discussion
The data collected in the fourth edition of the novel cellular therapy survey indicate a further increase compared to the previous year in the number of reporting teams (+19%), of total treatments reported (+39%), and of total treatments reported using the dedicated form (+74%). These results indicate that, thanks to the networks of the involved societies and the introduced strategy of head-hunting for known active teams, the program is receiving a growing recognition as a reference platform to collect and disseminate information that is not available in public databases or scientific publications. Moreover, the comparative analysis of data generated in the four surveys3–5 allows the identification of some established features and developing trends.
The steady increase in the percentage of treatments using autologous versus allogeneic cells is possibly due to a combination of cultural, regulatory, and/or commercial issues. Due to the oft claimed “minimal manipulation” and “homologous use” of the cells, the use of nonexpanded autologous MSC for reconstructive surgery/tissue enhancement is considered by some as “tissue transplantation” rather than “biological drug,” and therefore not subject to the same rigorous regulatory framework as are expanded MSC. This distinction may become somewhat artificial, as shown recently in the Celltex case.6 The number of treatments for GvHD prevention or treatment remained relatively stable throughout the 4 years (from 240 in 2008 to 265 in 2011), possibly due to the combination of increasing encouraging phase I/II data,7 but there is a lack of conclusive data from adequately powered, prospective randomized controlled (PRC) trials.
The most obvious changes in the indications addressed were in the important introduction of nonexpanded MSC (predominantly freshly harvested from autologous adipose tissue) for plastic and reconstructive surgery, as well as for decubitus and leg ulcers (total of 440 treatments). In this regard, evidence of efficacy is still limited to case reports and small series at this stage.8,9 It is thus vital that PRC clinical trials are carried out in the next years to demonstrate a statistical superiority of outcome for the cell-based versus cell-free treatments. The trend in the cell delivery mode was rather stable: the exception is of a fourfold increase in the percentage of use of 3D scaffolds, mostly associated with cartilage/bone repair treatments, skin reconstruction, and ulcers.
This year, for the first time, the data collection for cell processing included a query of whether cell graft manufacturing included a step performed with an automated system. Although the definition of “automation” could be interpreted differently by the responding teams, and respondents did not specify whether the system was closed or streamlined with the rest of the manufacturing line, it is nonetheless remarkable that 22% of the total treatments were claimed to involve an automated process. A closer analysis of the associated cell sources indicates that an automated device was introduced predominantly for the direct implantation of freshly harvested, autologous cells. The collected data are consistent with the commercial availability of systems for the device-assisted isolation or concentration of HSC (e.g., Sepax; Biosafe SA, www.biosafe.ch), of adipose-derived cells (e.g., Celution®; Cytori, www.cytori.com) or of bone marrow-derived MSC (e.g., Reamer Irrigator Aspirator; Synthes, www.synthes.com).
This year's edition offers a perspective on cardiac cell therapy, in an attempt to complement the collected data with those available from other sources. Cardiovascular disease represents a leading disease worldwide associated with a high morbidity and mortality.10 Current therapeutic options for patients suffering from heart failure due to myocardial infarction or other cardiomyopathies comprise medical treatment, ventricular assist devices, and heart transplantation. However, although heart transplantation has emerged as the standard of care and the only curative treatment for these patients, the key problem of organ shortage—while patient numbers continually rise—remains. In line with regenerative strategies in other medical fields, the concept of cardiac stem cell therapy has created substantial hope for the treatment of heart failure due to myocardial infarction and other cardiomyopathies.11 Therefore, numerous experimental and preclinical animal studies have been performed in the past decade12 utilizing a wide range of different adult stem cells. These include different subpopulations of bone marrow-derived progenitors, skeletal myoblasts, adipose-tissue derived MSC, prenatal progenitors, blood-derived progenitors as well as the recently discovered cardiac-resident stem cells.13–19 In addition, embryonic stem cells or induced pluripotent cells have been shown to be able to differentiate into functional cardiomyocytes and are in the focus as a potential cell source.20 Based on promising preclinical data, various cell types have already progressed into use in clinical pilot studies primarily aiming at demonstrating feasibility and safety in patients suffering from acute myocardial infarction, chronic heart disease/refractory angina, as well as ischemic cardiomyopathy.18,21–25
Bone marrow-derived progenitors represent the most frequently used cell source. Historically, Prof. Bodo Strauer from the University of Düsseldorf was, in 2001, the first to treat patients with acute myocardial infarction with intracoronary-infused bone marrow mononuclear cells (BMMC).26 Based on this study, numerous cohort studies and randomized trials such as the BOOST study and the REPAIR-AMI trial have been performed, testing intracoronary infusion of bone marrow-derived progenitor cells for cardiac cell therapy.21,25,27 While all study groups reported a sufficient safety profile for these cells, the results with regard to efficacy (i.e., improvement of ventricular function) are still under controversial discussion. A recent meta-analysis of 50 studies (with a total of 2625 patients) comprising mixed results of cohort studies and PRC trials demonstrated a significant, but still relatively limited (∼4%) improvement of left ventricular ejection fraction (LVEF) after BMMC therapy when compared to control patients.28
In line with the collected data (Table 1), the application of one subpopulation of BMMC, namely MSC, has been reported to be a valid and safe option for cardiac cell therapy. In contrast to BMMC, the use of MSC in smaller cohort studies has demonstrated a more pronounced effect on LVEF; this, however, needs to be confirmed in current PRC trials such as the TAC-HFT (TAC-HFT/ClinicalTrials.gov Identifier: NCT00768066). In parallel, the utilization of adipose tissue-derived MSC has been investigated in several preclinical studies and has lead to the initiation of first clinical trials such as the APOLLO study (APOLLO/ClinicalTrials.gov Identifier: NCT00442806).
An interesting concept to enhance the efficacy and the cardiogenic potential of bone marrow-derived MSC is the concomitant application of a cardiopoietic cocktail. This has been shown to be feasible in a preclinical study29 and has recently led to a pilot clinical study, the C-Cure trial, where the safety and importantly beneficial effect of cardiopoietic MSC could be demonstrated (C-Cure/ClinicalTrials.gov Identifier: NCT00810238) and was the basis for a randomized multicenter trial to be initiated.
Besides the utilization of BMMC or subpopulations thereof, the use of skeletal myoblasts has also been proposed as a potential cell source for myocardial repair and has advanced into numerous clinical studies such as the MAGIC trial30 and others (MYOHEART/ClinicalTrials.gov Identifier: NCT00054678). However, while preclinical and initial clinical data showed promising results, the major problem with these cells is the lack of electrical coupling with the hosting myocardium. Due to a lack of gap junctions and connexins, the skeletal myoblast therapy led to severe arrhythmia in some individuals and raised significant safety concerns. However, to ensure patient safety, systematic antiarrhythmic medical therapy or implantable cardioverter-defibrillator implantation represent valid tools to treat affected patients.31
Following the compelling evidence on the preclinical use of cardiac resident stem cells, two pilot clinical trials employing cardiac progenitors were recently initiated22,24 (CADUCEUS/ClinicalTrials.gov Identifier: NCT00893360; SCIPIO/ClinicalTrials.gov Identifier: NCT00474461). The promising preliminary results will need to be confirmed, in both the longer term and larger patient series.
Ten years after the first intracoronary clinical application of BMMC was reported,32 the concept of cardiac cell therapy has continuously evolved over time utilizing different cell types and application routes (intracoronary vs. intramyocardial) in different clinical scenarios. While most of these studies have focused on feasibility and safety, more recently initiated trials are targeting assessment of the efficacy of cardiac cell therapy concepts. Although the body of clinical experience is continuously growing, several key issues and questions are pending. These include not only the definition of appropriate product release criteria and clinical endpoints but also of a suitable cell type, route, and time of application. In this regard, the reported data here offer the unique opportunity to capture trends and monitor changes in the field, in a way which reflects the conducted (as opposed to planned) number of treatments and which can be communicated before a full report is published and publicly available.
Supplementary Material
Appendix: List of Reporting Novel Cellular Therapy Centres in Europe in 2011
Format: City, Hospital, Department, Centre Identification Code (EBMT teams), Physicians (Total treatments; allogenic/autologous)
CIC, Centre Identification Code (as used for the standard EBMT survey)
Austria
Krems, University Krems, Regenerative Medicine and Orthopedics, S. Nehrer (18; 0/18)
Linz, AO Krankenhaus, 3. Medizinische Abteilung, M. A. Fridrik (11; 0/11)
Vienna, Medical University Hospital, Traumatology, S. Marlovits, Ch. Albrecht (7; 0/7)
Belarus
Minsk, Belorussian Center, CIC 591, O. Aleinikova (18; 18/0)
Minsk, Hospital No. 9, Belorussian Transplant Centre, N. Milanovich (9; 6/3)
Belgium
Antwerp, University Antwerpen, Haematology, CIC 996, W. Schroyens (50; 0/50)
Bruges, AZ Sint Jan, CIC 506, D. Selleslag, T. Lodewyck, A.v. Hoof, J.v. Droogenbroeck, K.v. Eygen (2; 2/0)
Brussels, Clinique Universitaire St. Luc, CIC 234, X. Poiré, C. Vermylen (1; 1/0)
Brussels, Institut Jules Bordet, Children's Hospital, CIC 215, D. Bron, C. Devalck, A. Ferster (1; 0/1)
Brussels, Military Hospital Queen Astrid, Burn Wound Centre, G. Verbeken (31; 31/0)
Brussels, U.L.B. Hôpital Erasme, Haematology, CIC 596, B. Bailly, A. Kentos, M. Lambermont (1; 0/1)
Brussels, University Hospital, Oncology, CIC 630, R. Schots, F. Trullemans (1; 1/0)
Leuven, University Hospital Gasthuisberg, CIC 209, G. Verhoef, M. Delforge, J. Maertens (1; 1/0)
Liège, University Hospital Sart-Tilman, CIC 726, Y. Béguin, B de Prijck (8; 8/0)
Finland
Helsinki, HUCH Jorvi Hospital, Orthopaedics, Traumatology, T. Paatela (18; 0/18)
France
Clermont Ferrand, CRCTCP, CHU Estaing, CIC 273, J.-O. Bay, F. Deméocq, P. Travade (15; 1/14)
Grenoble, CHU de Grenoble, Pathologie Neurovasculaire, O. Detante (4; 0/4)
Grenoble, Hospitalier A. Michallon, CIC 270, J.Y. Cahn, F. Garban, P. Drillat, C. Bulabois (12; 5/7)
Lyon, Institue d'Hématologie et d'Oncologylogie Pédiatrique, CIC 806, Y. Bertrand, V. Mialou (1; 1/0)
Paris, Hôpital Robert Debré, Haematology-Immunology, CIC 631, A. Baruchel, J.-H. Dalle, G. Cotten (2; 2/0)
Poitiers, CHU de Poitiers, Hôpital La Miletrie, Haematology, CIC 264, M. Maillard, C. Giraud (1; 1/0)
Toulouse, University Hospital of Rangueil, Cardiology, J. Roncalli (4; 0/4)
Germany
Dresden, Universitätsklinikum Carl Gustav Carus, CIC 808, G. Ehninger, M. Bornhäuser, M. Gahr (27; 27/0)
Düsseldorf, Universitätsklinikum, Paediatrics–Haematology, Oncology, CIC 651, A. Borkhardt, R. Meisel, F. Schuster (1; 1/0)
Frankfurt, J. W. Goethe Universität, Kinderheilkunde lll, CIC 138, T. Klingebiel, P. Bader (5; 5/0)
Frankfurt, Klinikum Frankfurt Oder, Innere Medizin, CIC 190, M. Kiehl (9; 9/0)
Giessen, Universitätsklinikum, Paediatrics–Haematology, Oncology, CIC 326, A. Reiter, W. Wössmann (1; 1/0)
Halle, BG-Clinic Bergmannstrost, Neurosurgery, E. Herrmann (16; 0/16)
Halle, Universitätsklinikum, KinderKlinikum, CIC 654, D. Körholz, C. Mauz-Körholz (1; 1/0)
Hannover, Medizinische Hochschule, Haematology, Oncology, CIC 295, A. Ganser, J. Krauter (14; 0/14)
Regensburg, Universitätsklinikum, Haematology, Oncology, CIC 787, R. Andreesen, S. Corbacioglu (1; 1/0)
Tübingen, Universitätsklinikum, Paediatrics, CIC 535, R. Handgretinger, P. Lang (16; 13/3)
Hungary
Debrecen, University of Debrecen, Dept of Immunology, CIC 648, Z. Boda, E. Rajnavolgyi (2; 0/2)
Iran, Islamic Rep.
Shiraz, Nemazee Hospital, Haematology, Oncology, CIC 188, M. Ramzi (1; 0/1)
Teheran, Shariati Hospital, Haematology, Oncology, CIC 633, M. Jahani (3; 3/0)
Israel
Haifa, Rambam Medical Centre, Haematology, CIC 345, J.M. Rowe (1; 1/0)
Jerusalem, Hadassah University Hospital, CIC 258, R. Or, S. Slavin (16; 16/0)
Petach-Tikva, Beilinson Hospital, Adult Haematology, CIC 409, M. Yeshurun (1; 1/0)
Petach-Tikva, Childrens Medical Centre, Paediatrics, CIC 755, J. Stein (3; 3/0)
Italy
Bergamo, Ospedale Riuniti, CIC 658, A. Rambaldi (5; 5/0)
Bologna, 6th div, Istituto Ortopedico Rizzoli (IOR), RIT-Cell Factory, L. Roseti (14; 0/14)
Bologna, Istituto Ortopedico Rizzoli (IOR), Orthopaedic Pathology, Osteoarticular TR, D. Donati (28; 0/28)
Bolzano, Ospedale S. Maurizio, CIC 299, S. Cortelazzo, M. Casini, I. Cavattoni (1; 1/0)
Florence, Policlinico di Careggi, CIC 304, A. Bosi, S. Guidi (10; 4/6)
Genoa, Istituto Giannina Gaslini, Haematology, Oncology, CIC 274, G. Dini, E. Lanino (1; 1/0)
Genoa, Ospedale Villa Scassi, Plastic Surgery, F. Casabona (251; 0/251)
Milan, Orthopaedic Arthroscopic Surgery International Bioresearch Foundation, Gobbi NPO, A. Gobbi, G. Karnatzikos (14; 0/14)
Monza, L'Università di Milano-Bicocca, Ospedale San Gerardo dei Tintori, CIC 544, E. Pogliani, P. Pioltelli, M. Parma (1; 1/0)
Monza, Ospedale San Gerardo, CTMO–Clinica Pediatrica, CIC 279, A. Rovelli (3; 3/0)
Padua, Centro Leucemie Infantili, CIC 285, C. Messina, M. Pillon, E. Calore (2; 2/0)
Rome, Rome Transplant Network, CIC 756, W. Arcese, P. De Fabritiis (3; 3/0)
Rome, Università “La Sapienza,” Plastic Surgery, A. Conversi, N. Scuderi (21; 0/21)
Rome, Università degli Studi di Roma “Tor Vergata,” Reconstructive Surgery, V. Cervelli, D.J. Bottini, B. De Angelis (258; 0/258)
Lithuania
Kaunas, LUHS Hospital Kaunas Clinics, Sports Trauma, R. Gudas (14; 0/14)
Netherlands
Amsterdam, Antoni Van Leeuwenhoek Cancer Institute, Oncology, CIC 976, S. Rodenhuis, J. Baars (8; 0/8)
Amsterdam, University Medical Center VUMC, Orthopaedic Surgery, M. Helder (5; 0/5)
Groningen, University Hospital, Haematology, CIC 546, G. van Imhoff (1; 1/0)
Leiden, University Hospital, CIC 203, J.H. Veelken, M. Egeler (76; 19/57)
Utrecht, UMC, Orthopaedic Surgery, D. Saris (55; 0/55)
Utrecht, UMCU/WKZ, Paediatrics, CIC 239.2, M. Bierings, N.M. Wullffraat (5; 5/0)
Utrecht, University Hospital UMCU, CIC 239, E. Petersen (7; 7/0)
Norway
Oslo, University Hospital Rikshospitalet, Ex vivo cell lab, CIC 235, J. Brinchmann (19; 0/19)
Poland
Bydgoszcz, Nicolaus Copernicus University, Paediatrics, Haematology, Oncology, CIC 764, M. Wysocki, J. Styczynski (4; 0/4)
Cracow, University Children's Hospital JUMC, CIC 507, J. Gozdzik (1; 1/0)
Wroclaw, Lower Silesian Centre for Cellular Transplantation with National Bone Marrow Donor Registry, CIC 538, A. Lange (9; 0/9)
Wroclaw, University of Medicine, Paediatrics, CIC 817, A. Chybicka, J. Owoc-Lempach (1; 1/0)
Russian Fed.
Moscow, Research Haematology Centre of RAS, CIC 930, V.G. Savtchenko (7; 7/0)
Moscow, Russian Children's Hospital, CIC 694, A. Maschan, E. Skorobogato, E. Pachanov (33; 33/0)
Novosibirsk, Research Institute for Clinical Immunolgy, CIC 376, I. Lisukov (7; 0/7)
St. Petersburg, Pavlov Medical University, Haematology, CIC 725, B.V. Afanasyev, L. Zubarovskaya (36; 0/36)
Serbia
Belgrade, Military Medical Academy, CIC 582, D. Stamatovic, S. Obradovic (5; 0/5)
Slovak Republic
Bratislava, National Cancer Institute, J. Lakota (3; 3/0)
Slovenia
Ljublijana, Educell d.o.o, N. Kregar-Velikonja (18; 0/18)
Ljublijana, UMC Ljubljana, Cardiology, B. Vrtovec (26; 0/26)
Ljublijana, University Medical Centre, Haematology, CIC 640, S. Zver, J. Pretnar (27; 0/27)
Spain
Barcelona, Hospital Clinic, CIC 214, M. Rovira (1; 1/0)
Barcelona, Institut de Teàpia Regenerativa Tissular, F. Soler (57; 0/57)
Barcelona, Santa Creu I Sant Pau, CIC 260, J. Sierra, S. Brunet (2; 0/2)
Cordoba, Hospital Reina Sofia, Haematology, CIC 238, A. Torres-Gomez (29; 0/29)
Granada, Hospital Virgen de la Nieves, Haematology, CIC 559, J.M. De Pablos Gallego, M. Jurado Chacon (3; 3/0)
Madrid, Hospital Materno-Infantil Gregorio Marañón, Paediatrics, Oncology, CIC 410, C. Belendez (1; 0/1)
Madrid, Hospital de la Princesa, Haematology, CIC 236, A. Figuera, A. Alegre (4; 2/2)
Madrid, Hospital General Universitario Gregorio Marañón, Servicio de Haematology-UTMO, CIC 819, J.L. Diez-Martin (4; 4/0)
Madrid, Hospital La Paz, CIC 734, R. Arrieta (3; 0/3)
Madrid, Hospital Universitario San Carlos, Haematology, J. Diaz-Mediavilla, L. Llorente, R. Martinez (4; 0/4)
Malaga, Carlos Haya Hospital, Haematology, CIC 576, M. Gonzalez, M. Pascual (3; 3/0)
Murcia, Hospital General Universitario Morales Meseguer, CIC 735, V. Vicente-Garcia, I. Heras (2; 0/2)
Murcia, Hospital Virgen de la Arrixaca, CIC 323, J.M. Moraleda, A. Morales Lazaro (32; 1/31)
Palma de Mallorca, Hospital Universitari Son Espases (Son Dureta), CIC 722, J. Besalduch, M. Canaro (5; 0/5)
Pamplona, Clinica Universidad de Navarra, Cell Therapy Area, F. Prosper Cardoso (54; 7/47)
Pamplona, Hospital de Navarra, Haematology, CIC 577, E. Olavarria (1; 1/0)
Salamanca, Complejo Hospital, Haematology, CIC 727, D. Caballero (14; 14/0)
Seville, Hospital Universitario Virgen del Rocio, Haematology, CIC 769, I. Espigado, F. Marquez (2; 2/0)
Valencia, Hospital Clinico Universitario, CIC 282, C. Solano (3; 3/0)
Sweden
Stockholm, Karolinska University Hospital, Huddinge, CIC 212, P. Ljungman (11; 11/0)
Switzerland
Basel, Bruderholzspital, Orthobiologie und Knorpelersatz, M. Arnold (2; 0/2)
Basel, University Hospital Basel, Reconstructive Surgery, D. Schäfer (3; 0/3)
Geneva, Concept Clinic, KU. Schlaudraff (11; 0/11)
Lucerne, Kantonsspital Luzern, Herzzentrum, P. Erne (4; 0/4)
Lugano, Cardiocentro Ticino, Cardiology, D. Sürder (15; 0/15)
Zürich, Schulthess Klinik, Orthobiologie und Knorpelregeneration, M. Steinwachs (26; 0/26)
Turkey
Adana, Baskent University Adana, Haematology, CIC 589, H. Ozdogu, C. Boga, S. Asma, S. Yuce (1; 1/0)
Ankara, Ankara Research and Education Hospital, Haematology, CIC 423, F. Altuntas, M. Yüksel (1; 1/0)
Ankara, Children's Hospital, Oncology, CIC 436, B. Tunc, D. Uckan-Cetinkaya, F. M. Azik (5; 5/0)
Ankara, Gazi University, Besevler, Haematology, CIC 169, G. Sucak (1; 1/0)
Ankara, University of Ankara, Paediatrics, CIC 620, E. Unal, M. Ertem (2; 2/0)
Antalya, Medical Park Hospitals, Haematology, Oncology, CIC 919, Y. Koc (2; 2/0)
Gaziantep, Gaziantep University Medical School, Haematology, CIC 402, M. Pehlivan (9; 9/0)
Istanbul, Cellest Plastic Surgery Clinic, T. Tiryaki (31; 0/31)
Istanbul, Medical Park Bahcelievler Hospital, Paediatrics, CIC 457, G. öztürk, F. Erbey (1; 1/0)
Istanbul, University of Istanbul, Haematology, CIC 760, D. Sargin, S. Kalayoglu-Besisik (1; 1/0)
Izmir, Dokuz Eylul University, Paediatrics, Haematology, H. ören (1; 1/0)
Izmir, Dokuz Eylul University, CIC 688, H. özsan (1; 1/0)
Kayseri, Erciyes University Hospital, Haematology, Oncology, CIC 627, A. Unal, M. Cetin (1; 1/0)
Ukraine
Kiev, Kiev City BMT Centre, E. Karamanesht, V. Khomenko, I. Korenkova (1; 1/0)
Odessa, National Medical University, I. Karpenko (5; 0/5)
United Kingdom
Bristol, Royal Hospital for Sick Children, CIC 386, J.M. Cornish, D. Marks (9; 0/9)
Glasgow, Southern General Hospital, Institute of Neurosciences, K. Muir (6; 0/6)
London, Great Ormond Street Hospital, CIC 243, P. Veys (11; 11/0)
London, Hammersmith Hospitals NHS Trust, Imperial College, CIC 205, J. Apperley, E. Olavarria, E. Kanfer, A. RaHaematologytulla, R. Szydlo (20; 20/0)
London, King's College Hospital, CIC 763, A. Pagliuca (1; 1/0)
London, St. Bartholomew's and the Royal London Hospital, CIC 768, J. Gribben, J. Cavenagh, S. Agrawal, T. Lister (27; 0/27)
Manchester, Royal Children's Hospital, CIC 521, R. Wynn (4; 4/0)
Manchester, School of Cancer and Enabling Sciences, CT Unit, R. Guest (1; 0/1)
Oswestry, Oswestry Orthopaedic Hospital, P. Harrison (28; 0/28)
Sheffield, Teaching Hospitals NHS Trust, Children's Hospital, CIC 778, J. Snowden, A. Vora (2; 1/1)
Acknowledgments
We greatly appreciate the cooperation of all participating teams and their staff (listed in Appendix) and the engagement of the different working groups and their highly committed representatives, namely TERMIS-EU (Sarah Wilburn), ISCT-Europe (Francesco Lanza), ICRS (Stephan Seiler), EBMT (Alejandro Madrigal), and EULAR. We are also grateful to Dietlinde John for her database support. The work was supported in part by the European Union's Seventh Framework Programme (FP7/2007–2013) (project “Bio-Comet”) under grant agreement no. 278807. EBMT is supported by grants from the corporate sponsors: Gentium SpA, Celgene International SARL, Gilead Sciences Europe Ltd., Astrellas Pharma Europe Ltd., Sonofi Oncology, Fresenius Biotech GmbH, TerumoBCT, Therakos Photopheresis, TEVA, Miltenyi Biotec GmbH, Clinigen Group Ltd., Sandoz Biopharmaceuticals, Medac Hematology. Macropharma, Pierre Fabre Médicament SAS, Takeda, Amgen Oncology Europe GmbH, Exem Consulting SA, and Chugai Sanofi Aventis.
Disclosure Statement
No competing financial interests exist.
References
- 1.Abbott A.Stem-cell ruling riles researchers. Nature 495,418, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Editorial Smoke and mirrors. Nature 496,269, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Martin I., Baldomero H., Tyndall A., Niederwieser D., and Gratwohl A.A survey on cellular and engineered tissue therapies in Europe in 2008. Tiss Eng Part A 16,2419, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Martin I., Baldomero H., Bocelli-Tyndall C., Slaper-Cortenbach I., Passweg J., and Tyndall A.The survey on cellular and engineered tissue therapies in Europe in 2009. Tiss Eng Part A 17,2221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin I., Baldomero H., Bocelli-Tyndall C., Passweg J., Saris D., and Tyndall A.The survey on cellular and engineered tissue therapies in Europe in 2010. Tiss Eng Part A 18,2268, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Cyranoski D.Cowboy culture. Nature 494,166, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M.E., Remberger M., Dini G., Egeler R.M., Bacigalupo A., Fibbe W., and Ringdén O.Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371,1579, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Cervelli V., Gentile P., De Angelis B., Calabrese C., Di Stefani A., Scioli M.G., Curcio B.C., Felici M., and Orlandi A.Application of enhanced stromal vascular fraction and fat grafting mixed with PRP in post-traumatic lower extremity ulcers. Stem Cell Res 6,103, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Doi K., Tanaka S., Iida H., Eto H., Kato H., Aoi N., Kuno S., Hirohi T., and Yoshimura K.Stromal vascular fraction isolated from lipo-aspirates using an automated processing system: bench and bed analysis. J Tissue Eng Regen Med. Doi: 10.1002/term.14782012 [DOI] [PubMed] [Google Scholar]
- 10.Weir R.A., McMurray J.J., and Velazquez E.J.Epidemiology of heart failure and left ventricular systolic dysfunction after acute myocardial infarction: prevalence, clinical characteristics, and prognostic importance. Am J Cardiol 97,13F, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Segers V.F., and Lee R.T.Stem-cell therapy for cardiac disease. Nature 451,937, 2008 [DOI] [PubMed] [Google Scholar]
- 12.van der Spoel T.I., Jansen of Lorkeers S.J., Agostoni P., van Belle E., Gyongyosi M., Sluijter J.P., et al. . Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc Res 91,649, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Asahara T., and Kawamoto A.Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol 287,C572, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Emmert M.Y., Emmert L.S., Martens A., Ismail I., Schmidt-Richter I., Gawol A., et al. . Higher frequencies of BCRP+cardiac resident cells in ischaemic human myocardium. Eur Heart J 34,2830, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Pfister O., Mouquet F., Jain M., Summer R., Helmes M., Fine A., et al. . CD31- but not CD31+cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res 97,52, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Menasche P.Skeletal myoblasts for cardiac repair: Act II? J Am Coll Cardiol 52,1881, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Valina C., Pinkernell K., Song Y.H., Bai X., Sadat S., Campeau R.J., et al. . Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J 28,2667, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Williams A.R., and Hare J.M.Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res 109,923, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messina E., De Angelis L., Frati G., Morrone S., Chimenti S., Fiordaliso F., et al. . Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 95,911, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Haase A., Olmer R., Schwanke K., Wunderlich S., Merkert S., Hess C., et al. . Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell 5,434, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Assmus B., Honold J., Schachinger V., Britten M.B., Fischer-Rasokat U., Lehmann R., et al. . Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med 355,1222, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Bolli R., Chugh A.R., D'Amario D., Loughran J.H., Stoddard M.F., Ikram S., et al. . Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378,1847, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Hare J.M., Traverse J.H., Henry T.D., Dib N., Strumpf R.K., Schulman S.P., et al. . A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 54,2277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makkar R.R., Smith R.R., Cheng K., Malliaras K., Thomson L.E., Berman D., et al. . Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379,895, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schachinger V., Erbs S., Elsasser A., Haberbosch W., Hambrecht R., Holschermann H., et al. . Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 355,1210, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Strauer B.E., Brehm M., Zeus T., Gattermann N., Hernandez A., Sorg R.V., et al. . [Intracoronary, human autologous stem cell transplantation for myocardial regeneration following myocardial infarction]. Dtsch Med Wochenschr 126,932, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Wollert K.C., Meyer G.P., Lotz J., Ringes-Lichtenberg S., Lippolt P., Breidenbach C., et al. . Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364,141, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Jeevanantham V., Butler M., Saad A., Abdel-Latif A., Zuba-Surma E.K., and Dawn B.Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation 126,551, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Behfar A., Yamada S., Crespo-Diaz R., Nesbitt J.J., Rowe L.A., Perez-Terzic C., et al. . Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol 56,721, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, et al. . The myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation 117,1189, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Menasche P.Stem cell therapy for heart failure: are arrhythmias a real safety concern? Circulation 119,2735, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Strauer B.E., and Steinhoff G.10 years of intracoronary and intramyocardial bone marrow stem cell therapy of the heart: from the methodological origin to clinical practice. J Am Coll Cardiol 58,1095, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.