Abstract
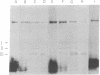
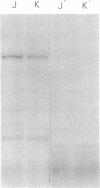
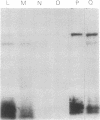
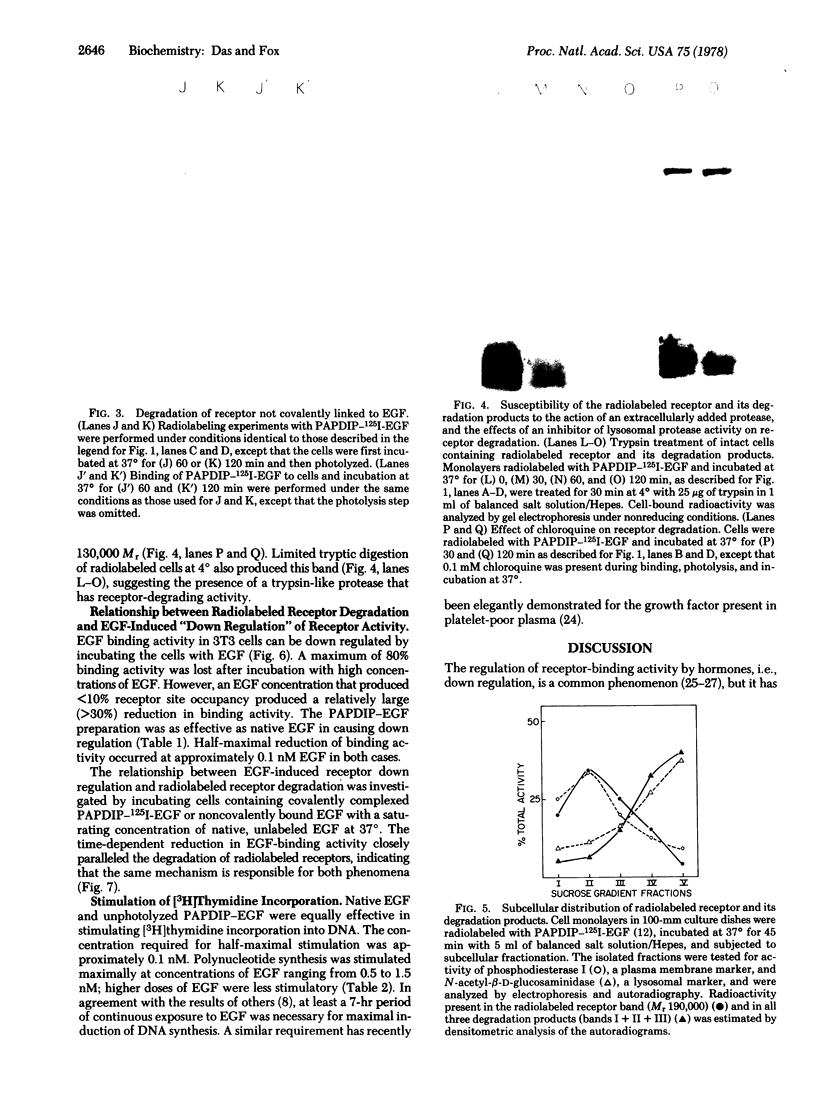
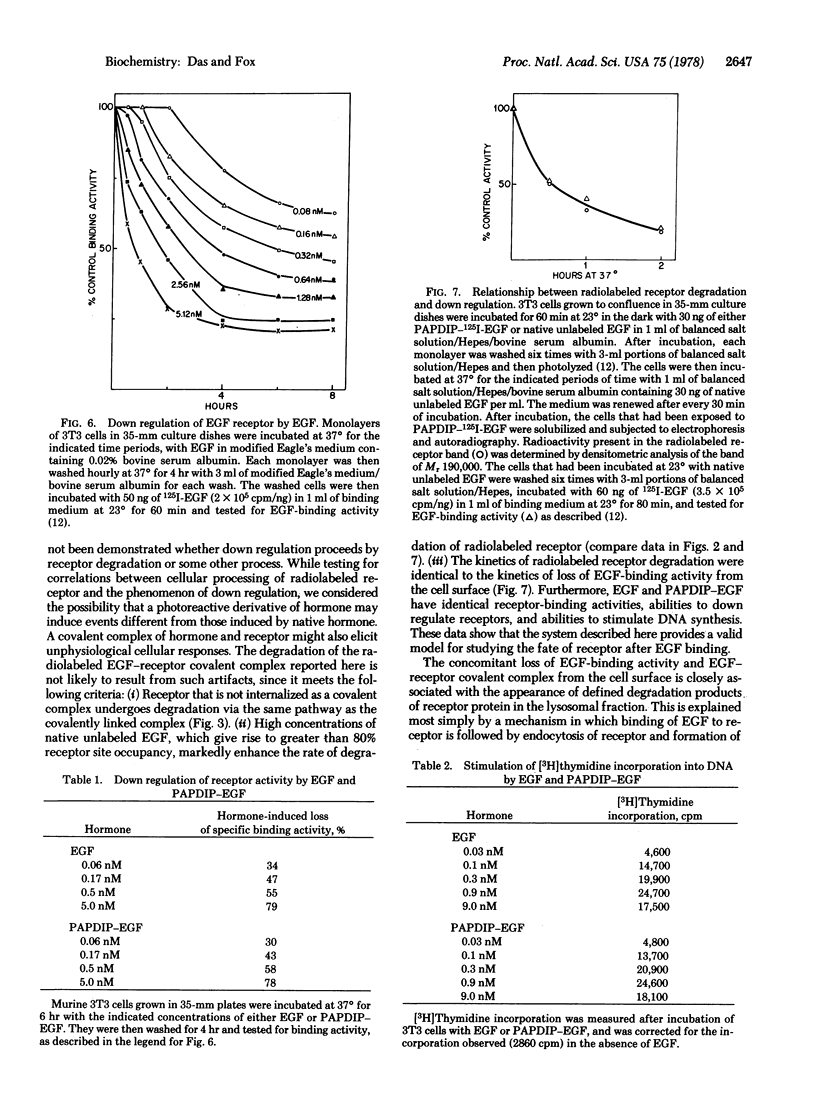
An affinity labeling technique used previously for identification of a membrane receptor for epidermal growth factor (EGF) was exploited to investigate the physiological fate of receptor after binding of EGF. Incubation of affinity-labeled cells at 37° resulted in a time-dependent loss of radioactivity from the EGF-receptor covalent complex (Mr 190,000). Ninety percent of the radioactivity lost from the band of Mr 190,000 during a 1-hr incubation at 37° appeared in three bands of Mr 62,000, 47,000, and 37,000. The crosslinked EGF-receptor complex (Mr 190,000) on intact cells was accessible to the action of trypsin at 4° and cofractionated with the plasmalemmal fraction. The proteolytic processing products of receptor were inaccessible to trypsin and banded with the lysosomal fraction upon subcellular fractionation. The rate of internalization and proteolytic processing of radiolabeled receptor was the same as the rate of reduction of binding activity induced by EGF. A study of the relationship between EGF-induced receptor internalization and processing, and stimulation of DNA synthesis, showed that both these processes were half-maximally stimulated at approximately 0.1 nM EGF, a concentration at which only 10% of the receptor sites are occupied. These data indicate that at concentrations of EGF subsaturating for binding but optimal for biological activity, there is a slow, continuous process of receptor internalization and degradation which could be limiting for EGF-induced mitogenesis.
Keywords: radiolabeled hormone receptors, down regulation of hormone receptors, receptor internalization, DNA synthesis, mitogenic hormone
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A. Pituitary extracts and steroid hormones in the control of 3T3 cell growth. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2702–2706. doi: 10.1073/pnas.70.9.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J., Roy C., Rajerison R., Jard S. Specific binding of (3H) lysine-vasopressin to pig kidney plasma membranes. Relationship of receptor occupancy to adenylate cyclase activation. J Biol Chem. 1973 Sep 10;248(17):5922–5931. [PubMed] [Google Scholar]
- COHEN S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962 May;237:1555–1562. [PubMed] [Google Scholar]
- Carney D. H., Cunningham D. D. Initiation of check cell division by trypsin action at the cell surface. Nature. 1977 Aug 18;268(5621):602–606. doi: 10.1038/268602a0. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J Cell Physiol. 1976 Jun;88(2):227–237. doi: 10.1002/jcp.1040880212. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Dufau M. L. Spare gonadotrophin receptors in rat testis. Nat New Biol. 1973 Aug 15;244(137):219–221. doi: 10.1038/newbio244219a0. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Gudor R. C., Sun T. T., Chen A. B., Mosesson M. W. Control of a cell surface major glycoprotein by epidermal growth factor. Science. 1977 Aug 19;197(4305):776–778. doi: 10.1126/science.302030. [DOI] [PubMed] [Google Scholar]
- Cohen S. Epidermal growth factor. J Invest Dermatol. 1972 Jul;59(1):13–16. doi: 10.1111/1523-1747.ep12625690. [DOI] [PubMed] [Google Scholar]
- Darfler F. J., Marinetti G. V. Synthesis of a photoaffinity probe for the beta-adrenergic receptor. Biochem Biophys Res Commun. 1977 Nov 7;79(1):1–7. doi: 10.1016/0006-291x(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Das M., Miyakawa T., Fox C. F., Pruss R. M., Aharonov A., Herschman H. R. Specific radiolabeling of a cell surface receptor for epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2790–2794. doi: 10.1073/pnas.74.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Hinkle P. M., Tashjian A. H., Jr Thyrotropin-releasing hormone regulates the number of its own receptors in the GH3 strain of pituitary cells in culture. Biochemistry. 1975 Aug 26;14(17):3845–3851. doi: 10.1021/bi00688a017. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Insulin and epidermal growth factor. Human fibroblast receptors related to deoxyribonucleic acid synthesis and amino acid uptake. J Biol Chem. 1975 May 25;250(10):3845–3853. [PubMed] [Google Scholar]
- Kiehm D. J., Ji T. H. Photochemical cross-linking of cell membranes. A test for natural and random collisional cross-links by millisecond cross-linking. J Biol Chem. 1977 Dec 10;252(23):8524–8531. [PubMed] [Google Scholar]
- Kono T., Barham F. W. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J Biol Chem. 1971 Oct 25;246(20):6210–6216. [PubMed] [Google Scholar]
- Lefkowitz R. J., Mukherjee C., Limbird L. E., Caron M. G., Williams L. T., Alexander R. W., Mickey J. V., Tate R. Regulation of adenylate cyclase coupled beta-adrenergic receptors. Recent Prog Horm Res. 1976;32:597–632. doi: 10.1016/b978-0-12-571132-6.50033-6. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S. P., Pruss R. M., Herschman H. R. Initiation of 3T3 fibroblast cell division by epidermal growth factor. J Cell Physiol. 1975 Dec;86 (Suppl 2)(3 Pt 2):593–598. doi: 10.1002/jcp.1040860504. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Savage C. R., Jr, Inagami T., Cohen S. The primary structure of epidermal growth factor. J Biol Chem. 1972 Dec 10;247(23):7612–7621. [PubMed] [Google Scholar]
- Schimmel S. D., Kent C., Bischoff R., Vagelos P. R. Plasma membranes from cultured muscle cells: isolation procedure and separation of putative plasma-membrane marker enzymes. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3195–3199. doi: 10.1073/pnas.70.11.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffer R. L. Angiotensin-converting enzyme and the regulation of vasoactive peptides. Annu Rev Biochem. 1976;45:73–94. doi: 10.1146/annurev.bi.45.070176.000445. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Cohen S. Transformation by murine and feline sarcoma viruses specifically blocks binding of epidermal growth factor to cells. Nature. 1976 Nov 4;264(5581):26–31. doi: 10.1038/264026a0. [DOI] [PubMed] [Google Scholar]
- Touster O., Aronson N. N., Jr, Dulaney J. T., Hendrickson H. Isolation of rat liver plasma membranes. Use of nucleotide pyrophosphatase and phosphodiesterase I as marker enzymes. J Cell Biol. 1970 Dec;47(3):604–618. doi: 10.1083/jcb.47.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittenberger B., Glaser L. Inhibition of DNA synthesis in cultures of 3T3 cells by isolated surface membranes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2251–2255. doi: 10.1073/pnas.74.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]