Abstract
miR-30d has been observed to be significantly down-regulated in human anaplastic thyroid carcinoma (ATC), and is believed to be an important event in thyroid cell transformation. In this study, we found that miR-30d has a critical role in modulating sensitivity of ATC cells to cisplatin, a commonly used chemotherapeutic drug for treatment of this neoplasm. Using a mimic of miR-30d, we demonstrated that miR-30d could negatively regulate the expression of beclin 1, a key autophagy gene, leading to suppression of the cisplatin-activated autophagic response that protects ATC cells from apoptosis. A reporter gene assay demonstrated that the binding sequences of miR-30d in the beclin 1-3′ UTR was the region required for the inhibition of beclin 1 expression by this miRNA. We further showed that inhibition of the beclin 1-mediated autophagy by the miR-30d mimic sensitized ATC cells to cisplatin both in vitro (cell culture) and in vivo (animal xenograft model). These results suggest that dysregulation of miR-30d in ATC cells is responsible for the insensitivity to cisplatin by promoting autophagic survival. Thus, miR-30d may be exploited as a potential target for therapeutic intervention in the treatment of ATC.
Keywords: miR-30d, autophagy, apoptosis, Beclin1, cisplatin, Anaplastic thyroid cancer
1. Introduction
Anaplastic thyroid carcinoma (ATC) is the most aggressive type among thyroid malignancies. Although ATC represents less than 2% of all thyroid tumors, this neoplasm causes up to 14%–39% of thyroid cancer-related deaths [1]. The current treatments for patients with ATC include surgery, radiotherapy and chemotherapy. Nevertheless, these therapies rarely succeed in improving the prognosis for patients with ATC. One of the causes for treatment failure is therapeutic resistance. For instance, resistance to chemotherapy occurs in most patients with ATC [2]. Yet, the underlying molecular mechanisms of therapeutic resistance remain incompletely understood.
MicroRNAs (miRNAs) are a class of endogenous, 19–25 nucleotides non-coding RNA molecules that are highly conserved in eukaryotic organisms. miRNAs can bind to the 3′-untranslated regions (3′-UTR) of the target genes, playing important roles in post-transcriptional regulation of gene expression through induction mRNA degradation or translational repression [3]. Because miRNAs have the ability to target numerous mRNAs, these small RNA molecules operate highly complex regulatory networks and impact the expression of genes in many pathways that are associated with tumor initiation, development and progression. [4; 5; 6; 7]. miR-30d is one of the members of the miR-30 family that locate in human chromosome 8q24.22, and has been reported to be up-regulated in several types of human cancers [8; 9]. On the other hand, this miRNA is significantly down-regulated in ATC and chronic lymphocytic leukemia [10; 11; 12], and the down-regulation of miR-30d was shown to contribute to the development and progression of ATC [13]. Here we report that the down-regulation of miR-30d in ATC may account for insensitivity of this type of malignancy to cisplatin, a platinum-containing chemotherapeutic drug commonly used in treatment of patients with advanced or metastatic ATC, and that the effect of miR-30d on cisplatin sensitivity is mediated through the beclin 1-regulated autophagy, a catabolic process of self-digestion of organelles and macromolecules [14]. We found that miR-30d can negatively regulate the expression of beclin 1, a key autophagy-promoting gene, and can suppress autophagy and promote apoptosis in the cisplatin-treated tumor cells. Our study demonstrates that miR-30d is a novel regulator of cisplatin sensitivity in ATC, and may be exploited as a potential new therapeutic target for reinforcement of the efficacy of this chemotherapy.
2. Materials and Methods
2.1. Cell lines and cell culture
The human anaplastic thyroid carcinoma cell lines, SW1736 and 8305C, were purchased from American Type Culture Collection (Manassas, VA). SW1736 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum; 8305C cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. Human breast cancer cell line MDA-MB-468, lung cancer cell line H1299, and glioma cell line T98G were purchased from American Type Culture Collection (Manassas, VA). H1299 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum; MDA-MB-468 cells were cultured in Dulbecco’s modied Eagle’s medium supplemented with 10% fetal bovine serum. T98G cells was maintained in Ham’s F-10/DMEM (10:1) medium. All of the cell culture media contain 100 U/ml penicillin and 100 mg/ml streptomycin. Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2/95% air.
2.2. Reagents and antibodies
Cisplatin, chloroquine diphosphate, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Bafilomycin A1 and 3-methyl adenine were purchased from Sigma (St Louis, MO). Western blot reagents were obtained from Pierce Biotechnology (Rockford, IL). The antibodies to LC3, β-actin, PARP, caspase-3 and beclin 1 were purchased from Cell Signaling Technology (Danvers, MA). The mimic and antagomir of miR-30d, and a control miRNA, were purchased from Invitrogen (Carlsbad, CA). All of the cell culture media and other reagents were purchased from Invitrogen.
2.3. miRNAs transfection
Cells in exponential phase of growth were plated in 60-mm tissue culture plates at 1 × 106 cells/plate and cultured overnight, and then transfected with a mimic or antagomir of miR-30d, or a control miRNA (100 nM), using Lipofectamine 2000 and OPTI-MEM I reduced serum medium (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocol.
2.4. Plasmid transfection
The pcDNA3.1-FLAG-beclin1 plasmid was synthesized by QIAGEN (Valencia, CA). Transfection of the plasmid was performed according to the manufacturer’s protocol. Briefly, cells in exponential phase of growth were plated in six-well cell culture plates at 1 × 105 cells/well, grown for 24h, and then transfected with the plasmid using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
2.5. Western blot analysis
Cells were lysed in M-PER mammalian protein extraction reagent (Pierce Biotechnology, Rockford, IL) supplemented with a protease inhibitor cocktail (Roche, Indianapolis, IN), followed by centrifugation at 12 000 × g for 10min. At the end of centrifugation, cell lysates were collected and protein concentration of the cell lysates were measured. Proteins (10–20 μg) were resolved by SDS–polyacrylamide gel electrophoresis and transferred to PVDF membranes (Bio-Rad, Hercules, CA). The membranes were then incubated with primary antibodies in 3% bovine serum albumin/Tris-buffered saline/Tween-20 at 4°C overnight, followed by incubation with secondary antibodies at room temperature for 1 h. The protein signals were detected by ECL method.
2.6. Real-time RT-PCR analysis of miRNA
TaqMan® miRNA assays were performed to measure the endogenous mature miRNA expression. Briefly, miRNAs were converted to cDNA using TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA), followed by TaqMan® based quantitate PCR on the Stratagene 3005P Real-Time PCR system using the miRNA-specific primers from Applied Biosystems. Small nuclear RNA (RNU66) was used as an internal control. The expression level of miRNA was calculated using MxPro software (Version 4.00, Stratagene).
2.7. Dual luciferase reporter assay
The 1,574 bp fragment of the beclin1 3′-UTR containing the miR-30d targeting sequence (GTTTACA) was cloned into the psiCHECK™ dual Luciferase reporter plasmid at the 3′ end of the coding sequence of R. reniformis luciferase, as described previously [15]. For the reporter assays, cells were cultured to approximately 80% confluence in a 6-well plate, and then co-transfected with either psiCHECKTM2-WT-BECN-3′-UTR (wild type) or psiCHECKTM2-MT-BECN-3′-UTR (mutant) vector and the miR-30d mimic for 48 hours. Firefly and Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay system (Promega, Madison, WI), and the Renilla luciferase activity was normalized to firefly luciferase activity.
2.8. qRT-PCR
Total RNAs were extracted from cells using Trizol Reagent (Invitrogen) according to the manufacturer’s protocol. qRT-PCR was performed as described previously [15] using the following primers: the human beclin1: forward: 5′-CAA GAT CCT GGA CCG TGT CA-3′, reverse: 5′-TGG CAC TTT CTG TGG ACA TCA-3′; the β-actin: forward: 5′-GCC AAC ACA GTG CTG TCT GG-3′, reverse 5′-GCT CAG GAG GAG CAA TGA TCT TG-3′. After 40 cycles, data were collected and analyzed using MxPro software (Stratagene, La Jolla, CA).
2.9. Autophagy assays
Autophagy was measured by using following methods: (1) Western blot analysis of LC3; (2) microscopic observation of GFP-LC3 puncta. These methods were described previously [16; 17].
2.10. Apoptosis assays
Apoptosis was determined by: (1) flow cytometric analysis of Annexin V and 7-aminoactinomycin D staining. Briefly, 100 μl Guava Nexin reagent from Millipore (Bedford, MA) was added to 1×105 cells (in 100 μl) and the cells were incubated with the reagent for 20 min at room temperature in the dark. At the end of incubation, the cells were analyzed by a Guava EasyCyte Plus FlowCytometry System (Millipore); (2) Western blot analysis of the cleaved PARP and caspase-3.
2.11. Cell viability assay
Cellular viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Briefly, cells were plated at a density of 5×103 cells/well in 96-well tissue culture plates and subjected to different treatment. Following a 48h incubation at 37 °C in a humidified atmosphere containing 5% CO2/95% air, the cells were incubated for another 4 h with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reagent. The formazan product was dissolved in dimethyl sulfoxide and read at 570nm on a Victor3 Multi Label plate reader (PerkinElmer, Boston, MA).
2.12. Animal experiments
SW1736 tumor cells transfected with a miR-30d-lentiviral vector or a control lentiviral vector were inoculated subcutaneously into 5-week-old BALB/c mice (1×106 cells/mouse). Twenty days later, the tumor-bearing mice were divided into groups (5 mice/group) and treated with vehicle or cisplatin (3 mg/kg, q.2d, every other day, i.p.). Tumor volumes were determined by measuring the length (L) and the width (W) of the tumors and calculating using the formula: . At the end of the experiment (34 days later), the mice were euthanized and tumors were surgically dissected. The tumor specimens were either processed for Western blot analysis or fixed in 4% paraformaldehyde for histopathologic examination. Animal maintenance and experimental procedures were approved by the Institutional Animal Care and Use Committee of Soochow University.
2.13. Statistical analysis
Student t-test was used to analyze the significance of differences. Results with p < 0.05 were considered statistically significant.
3. Results
3.1. miR-30d acts as a negative regulator of beclin 1 expression
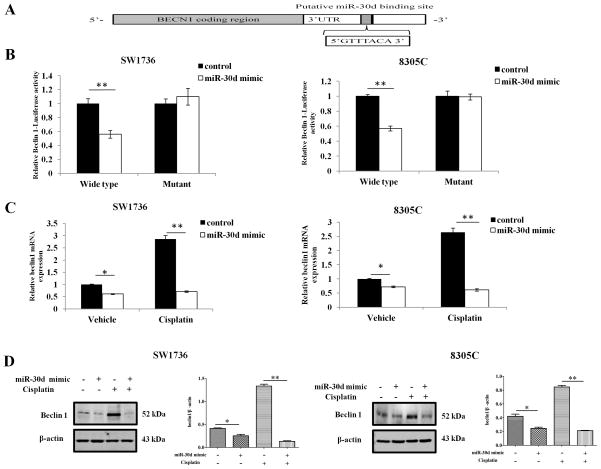
A recent study showed that miR-30d could impair autophagic process by targeting multiple genes in the autophagy pathway [18]. Consistently, in our study of the roles of miRNAs in regulation of autophagy, we observed that there were 13 miRNAs showing differential expressions during activation of autophagy under stressful conditions [15], and among these miRNAs, the expression of miR-30d had a 19~26% decrease (data not shown). Nevertheless, the precise mechanism by which miR-30d affects autophagic process remains unclear. Because the mature sequences are highly conserved between miR-30d and miR-30a, and miR-30a was discovered by our group to act as a negative regulator of autophagy through altering the expression of the key autophagy-promoting gene beclin 1 [15], we thus tested whether miR-30d could also target beclin 1 expression. As miR-30a and miR-30d are predicted to have the same consensus sequences within the 3′UTR of beclin1 (http://pictar.bio.nyu.edu, Figure 1A), we took advantage of the beclin 1 dual luciferase reporter system that we generated for the study of the role of miR-30a in regulation of beclin 1 expression [15]. As shown in Figure 1B, co-transfection of the human ATC cell lines, SW1736 and 8305C, with a miR-30d mimic (100 nM) and the reporter gene expression vector containing the wild-type targeting sequences (psiCHECKTM2-WT-BECN-3′-UTR) led to a significant reduction of the reporter gene activity, in comparison with the co-transfection with a control miRNA. By contrast, no reduction of the reporter gene activity was observed with co-transfection with the reporter gene vector containing the deletion mutant (psiCHECKTM2-MTBECN-3′-UTR) (Figure 1B). Identical experiments were performed in T98G glioma, MDA-MB-468 breast caner and H1299 lung cancer cell lines, and similar results were obtained (data not shown), which further support the role of miR-30d in regulating beclin 1 expression. These data suggest that similar to miR-30a, the binding sequences of miR-30d in the beclin 1-3′ UTR is the region required for the miR-30d-mediated inhibition of beclin 1 expression.
Figure 1. miR-30d down-regulates beclin 1 expression by directly targeting its 3′-UTR.
(A) Base pairing complement suggested the putative miR-30d binding position at 3′-UTR of beclin 1. (B) Luciferase reporter assays. Cells were co-transfected with either psiCHECKTM2-WT-BECN-3′-UTR or psiCHECKTM2-MT-BECN-3′-UTR vector and a miR-30d mimic or a control RNA. Firefly and Renilla luciferase activities were measured using the Dual-luciferase Reporter Assay system (Promega), and Renilla luciferase activity was normalized to firefly luciferase activity. Results shown are the mean ± SD of triplicate determinations from one of three identical experiments. **P<0.01, t-test. (C and D) SW1736 and 8305C cells transfected with a mimic of miR-30d (100 nM) or a control RNA (100 nM) were treated with the indicated concentration of cisplatin (30 μg/ml) for 24 h. At the end of treatment, (C) beclin 1 mRNA expression was measured by real-time PCR and calculated by 2−ΔCt method; (D) beclin 1 protein from above cells was detected by Western blotting, β-actin was used as a loading control. Data shown are the representative of three identical experiments. *P<0.05 and **P<0.01, t-test.
To further demonstrate the effect of miR-30d on beclin 1 expression, we transfected SW1736 and 8305C cells with a mimic of miR-30d or a control miRNA, and then examined the expression of beclin 1 in the cells treated with avehicle or cisplatin, a chemotherapeutic agent commonly used in treatment of solid tumors including ATC and known to induce autophagy [19; 20]. Figure 1C shows that introduction of the miR-30d mimic decreased the expression of beclin 1 mRNA in both of the cell lines treated or untreated with cisplatin. Expression of Beclin 1 protein was also inhibited by the miR-30d mimic (Figure 1D). Similar effects of miR-30d on beclin 1 mRNA and protein expression were observed in T98G glioma, MDA-MB-468 breast caner and H1299 lung cancer cell lines (data not shown), validating the role of miR-30d in regulation of beclin 1 expression. These results demonstrate that beclin 1 is a putative target for miR-30d, and that miR-30d has a negative role in the regulation of beclin 1 expression.
3.2. Cisplatin-induced autophagy is blunted by a miR-30d mimic in ATC cells
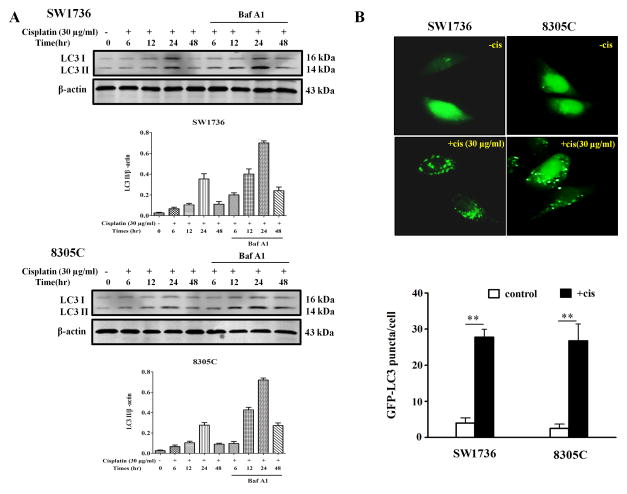
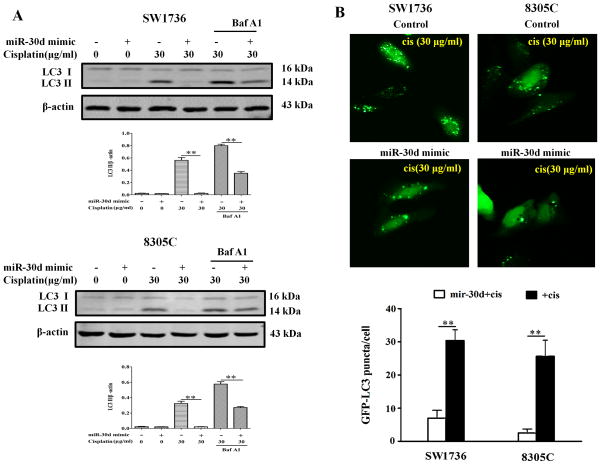
As miR-30d is frequently down-regulated in ATC and cisplatin treatment activates autophagy, we next determined whether the down-regulation of miR-30d in ATC likely plays a role in modulating the efficacy of cisplatin through altering autophagy. We examined the effect of miR-30d on autophagy in the ATC cells treated with this drug. Figure 2A shows that treatment of SW1736 and 8305C cell lines with cisplatin (30μg/ml) caused a robust activation of autophagy, as indicated by the increases in the amount of LC3 II (Figure 2A) and in the number of GFP-LC3 puncta (Figure 2B). At 24 hr after cisplatin treatment, autophagy activity reached a greatest level (Figure 2A). LC3 II levels were further elevated in the presence of bafilomycin A1 (Figure 2A), a late-phase autophagy inhibitor that prevents autophagosome–lysosome fusion and LC3 II degradation, indicating an increase of autophagic flux in the cisplatin-treated ATC cells. Notably, transfection of SW1736 and 8305C cells with the miR-30d mimic blunted their autophagic response to cisplatin, as compared to the transfection with a control miRNA (Figure 3). These experiments indicate that miR-30d can suppress the autophagic response induced by cisplatin treatment.
Figure 2. Cisplatin induces autophagy in anaplastic thyroid cancer (ATC) cells.
(A) SW1736 and 8305C cells were treated with 30 μg/ml of cisplatin for 6h, 12h, 24 h and 48h in the absence or presence of 10 nM of bafilomycin A1. At the end of treatment, cell lysates were prepared, resolved by SDS–polyacrylamide gel electrophoresis and subjected to western blot analysis using anti-LC3 or β-actin antibodies, respectively. β-actin was used as a loading control. Data shown are the representative of three identical experiments. (B) SW1736 and 8305C cells were transfected with a GFP-LC3 plasmid, followed by treatment with the indicated concentration of cisplatin for 24 h. At the end of treatment, the cells were inspected under a fluorescence microscope. Quantitation of the GFP-LC3 puncta was performed by counting 20 cells for each sample, and average numbers of puncta per cell were shown. The bars are the mean±s.d. of triplicate determinations; results shown are the representative of three identical experiments. **P<0.01, t-test, cisplatin versus vehicle.
Figure 3. MiR-30d mimic blunts autophagy in ATC cells treated with cisplatin.
(A) SW1736 and 8305C transfected with a mimic of miR-30d (100 nM) or a control RNA (100 nM) were treated with the indicated concentration of cisplatin for 24 h in the absence or presence of 10 nM of bafilomycin A1. At the end of treatment, cell lysates were prepared, resolved by SDS–polyacrylamide gel electrophoresis and subjected to western blot analysis of LC3 and β-actin, respectively. β-actin was used as a loading control. Data shown are the representative of three identical experiments. **P<0.01, t-test. (B) SW1736 and 8305C with or without a mimic of miR-30d (100 nM) were transfected with a GFP-LC3 plasmid, followed by treatment with the indicated concentration of cisplatin for 24 h. Quantitation of GFP-LC3 puncta was performed as described in Figure 1. The bars are the mean±s.d. of triplicate; results shown are the representative of three identical experiments. **P<0.01, t-test.
3.3. The miR-30d mimic can sensitize ATC cells to cisplatin
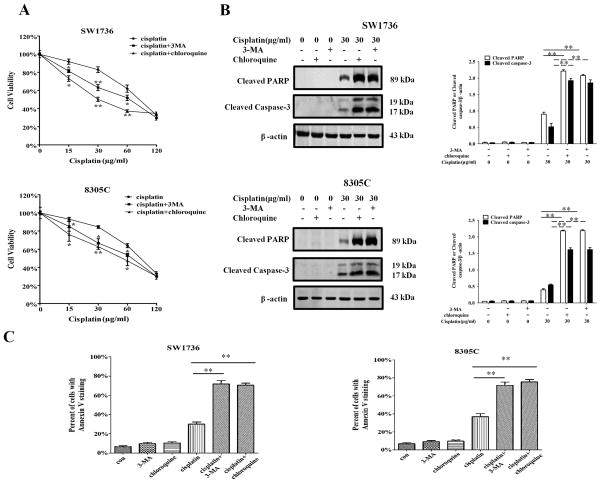
We previous demonstrated that the cisplatin-induced autophagy was cyto-protective in ovarian cancer and some other types of cancer cells [17]. Here, we sought to understand how autophagy activated by cisplatin affects the survival of ATC cells and how suppression of the cisplatin-activated autophagy by miR-30d affects their sensitivity to this drug. Figure 4A shows that co-treatment of ATC cell lines with cisplatin and 3-methyl adenine (3-MA) or chloroquine, the inhibitors of autophagy, caused a greater cytotoxicity in the treated cells, as compared with the treatment with cisplatin alone. The increased cytotoxicity was accompanied by enhanced apoptosis, as evidenced by the increases in the levels of the cleaved PARP, cleaved caspase-3, and Annexin V staining (Figure 4B and Figure 4C). These results indicate that autophagy has a cyto-protective role in ATC cells subjected to cisplatin treatment.
Figure 4. Inhibition of autophagy by 3- methyl adenine (3-MA) and chloroquine enhances sensitivity of ATC cells to cisplatin.
(A) SW1736 and 8305C cells were treated with the indicated concentrations of cisplatin for 48 h in the presence or absence of 3-MA (2 mM) or chloroquine (2.5 mM). At the end of treatment, cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay; (B, C) SW1736 and 8305C cells were treated with the indicated concentration of cisplatin for 24 h in the presence or absence of 3-MA (2 mM) or chloroquine (2.5 mM). Apoptosis was determined by: (B) western blot analysis of cleaved PARP and cleaved caspase-3. Data shown are the representative of three identical experiments. **P<0.01, t-test. (C) flow cytometric analysis of Annexin V staining. The bars are the mean±s.d. of triplicate determinations; results shown are the representative of three identical experiments. *P<0.05 and **P<0.01, versus control, t-test.
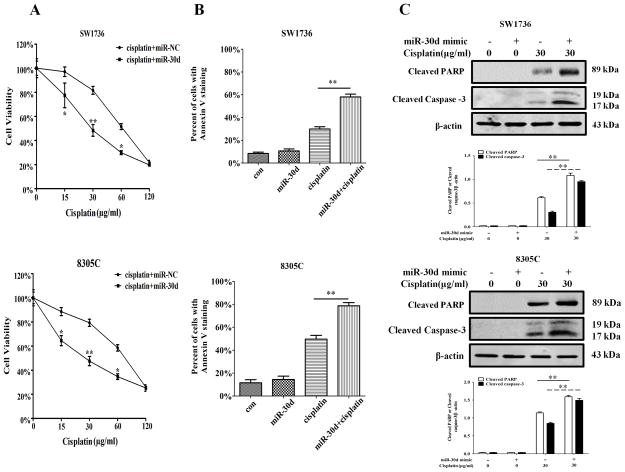
Because the cisplatin-activated autophagy could be blunted by the miR-30d mimic (Figure 3), we next tested the effect of miR-30d mimic on sensitivity of ATC cells to cisplatin. SW3716 and 8305C cells were transfected with a mimic of miR-30d or a control miRNA, and then were tested for their sensitivity to cisplatin. Figure 5A shows that the cells transfected with the miR-30d mimic had a significantly greater sensitivity to cisplatin than the cells transfected with a control miRNA. The miR-30d mimic also significantly augmented the cisplatin-induced apoptosis in the ATC cells, as shown by the increases in Annexin V staining and in the amounts of the cleaved caspase-3 and cleaved PARP (Figure 5B and Figure 5C). These results indicate that miR-30d can sensitize ATC cells to cisplatin by suppressing the drug-induced autophagic response.
Figure 5. MiR-30d mimic increases sensitivity of ATC cells to cisplatin.
SW1736 and 8305C cells transfected with a mimic of miR-30d (100 nM) or a control RNA (100 nM) were treated with the indicated concentrations of cisplatin for 48 h. At the end of treatment, (A) cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Each point represents mean±s.d. of triplicate determinations; results shown are the representative of three identical experiments. *P<0.05 and **P<0.01; (B, C) apoptosis was determined by flow cytometric analysis of Annexin V staining (B) and by western blot analysis of cleaved PARP and cleaved caspase-3 (C). β-actin was used as a loading control. Data shown are the representative of three identical experiments. **P<0.01, t-test.
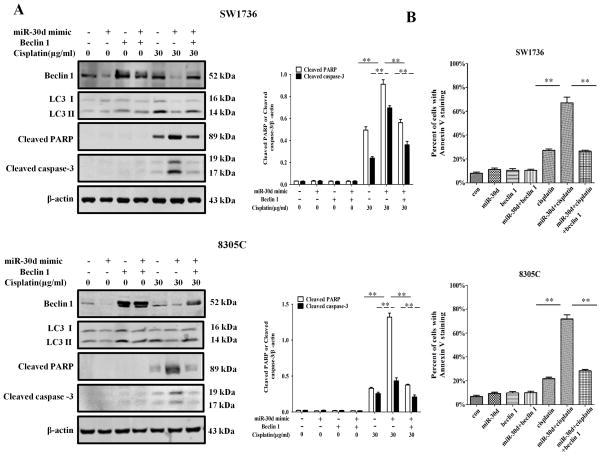
3.4. Forced expression of beclin 1 can rescue the down-regulation of autophagy caused by the miR-30d mimic
To further prove the role of miR-30d in suppressing the beclin 1-mediated autophagy, we co-transfected SW3716 and 8305C cells with the miR-30d mimic and a beclin 1 expression plasmid, and then measured the protein levels of beclin 1 and LC3 II. Figure 6A shows that ectopic expression of beclin 1 rescued the down-regulation of autophagy in the cells transfected with the miR-30d mimic, as reflected in the level of LC3 II, a marker for autophagy. Moreover, in the ATC cells transfected with the miR-30d mimic, ectopic expression of beclin 1 also decreased the cisplatin-induced apoptosis, as evidenced by the decreases in the amounts of the cleaved PARP and cleaved caspase-3 (Figure 6A), and in Annexin V staining (Figure 6B). These results confirmed that the effect of miR-30d on autophagy is mediated through modulating the expression of beclin 1, thereby affecting cellular sensitivity to cisplatin.
Figure 6. Forced expression of beclin 1 diminishes the sensitizing effect of miR-30d mimic on cisplatin-induced apoptosis in ATC cells.
SW1736 and 8305C cells with miR-30d mimic were transfected with an empty control vector or beclin 1-expressing plasmids for 24 h, followed by treatment with the indicated concentration of cisplatin for another 24 h. At the end of treatment, cell lysates were prepared and analyzed for protein levels of beclin 1, cleaved PARP, and cleaved caspase-3 by Western blot analysis (A). Data shown are the representative of three identical experiments. **P<0.01, t-test. Apoptosis was determined by flow cytometric analysis of Annexin V staining (B). Each point represents mean ± SD of triplicate determinations; results shown are the representative of three identical experiments. **P < 0.01, t-test.
3.5. The miR-30d mimic enhances the antitumor efficacy of cisplatin in an ATC xenograft mouse model
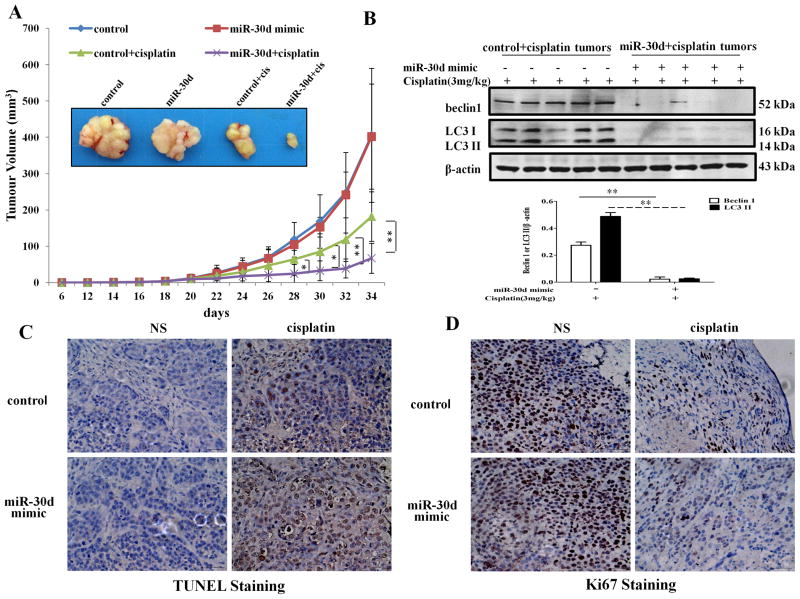
To determine whether the observed role of miR-30d in modulating the sensitivity of ATC to cisplatin could be recapitulated in vivo, we evaluated the antitumor efficacy of cisplatin in mice inoculated with the SW1736 cells transfected with either a miR-30d-lentiviral plasmid (SW1736/miR-30d) or a control plasmid (SW1736/control). In these experiments, tumor-bearing mice were treated with cisplatin i.p., every other day for 2 weeks, and the tumor volumes were monitored daily. Figure 7A shows that the tumor-inhibitory action of cisplatin was significantly greater in the mice bearing SW1736/miR-30d tumors, as compared with that in the mice bearing SW1736/control tumors. In the absence of cisplatin treatment, there were no differences in the tumor growth between the SW1736/control and SW1736/miR-30d tumors. Western blot analyses of the tumor specimens showed that the SW1736/miR-30d xenografts had decreased levels of beclin 1 expression and autophagy (as indicated by the amount of LC3 II) after cisplatin treatment, as compared with the SW1736/control xenografts (Fig 7B). TUNEL staining displayed that the numbers of the tumor cells positive for TUNEL staining were higher in the SW1736/miR-30d tumors than that in the SW1736/tumors following treatment with cisplatin (Figure 7C). By contrast, immunohistochemistry staining for Ki67, a marker of cell proliferation, was higher in the SW1736/control tumors than that in the SW1736/miR-30d tumors (Fig. 7D). These observations provide further evidence for the role of miR-30d in determining the ATC sensitivity to cisplatin.
Figure 7. Mimic expression of miR-30d enhances the efficiency of cisplatin in augmenting apoptosis and blocking proliferation of implanted tumor cells.
(A) Tumor sizes in mice treated with or without various reagents including 3 mg/kg body weight cisplatin or 3 mg/kg body weight cisplatin plus lenti-miR-30d or lenti-vector. Tumor size was measured every two days after cisplatin treatment. Each point represents mean ± SD; results shown are the representative of 3 identical experiments. *, P < 0.05; **, P < 0.01, t test. (B) Western blot analysis of LC3-I, LC3-II, and beclin 1 in implanted tumors. Data shown are the representative of three identical experiments. **P<0.01, t-test. (C) TUNEL staining in paraffin sections of the tumors. (D) Expression of the cell proliferation marker, Ki67, in paraffin sections of the tumors. Scale bar, 50 μm.
4. Discussion
The role of miRNAs in regulation of autophagy, an important cellular process that impacts cell fate, has been increasingly appreciated in recent years. For example, miR-375 was shown to inhibit autophagy by suppressing the expression of ATG7 thereby impairing viability of HCC cells under hypoxic conditions [21]. miR-101 was reported to act as a potent inhibitor of autophagy by targeting STMN1, RAB5A and ATG4D [22]. We previous reported that miR-30a suppresses stress-induced autophagy through inhibition of Beclin 1 expression [15]. In the current study, we demonstrate that miR-30d, a member of the miR-30 family, acts similarly to miR-30a in regulating autophagy. We show that miR-30d regulates autophagy by directly targeting the binding sequences in the beclin 1-3′ UTR, affecting the expression of this key autophagy-promoting protein (Fig. 1). These results provide a mechanistic support for the reported involvement of miR-30d in regulation of autophagy [18].
It has been reported that miR-30d is frequently down-regulated in ATC [10; 11]; yet the association of the under-expression of this miRNA with the development and progression of ATC is unclear. To explore the implication of the down-regulation of miR-30d in ATC, we determined the effects of miR-30d on sensitivity of ATC cells to cisplatin, a platinum compound known to induce autophagy [19; 20]and widely used for the treatment of many types of cancers including ATC. Our results showed that miR-30d mimic could blunt the cisplatin-activated autophagy (Fig. 2 and 3), and sensitize ATC cells to this chemotherapeutic agent both in vitro (Fig. 5) and in vivo (Fig. 7). Collectively, this study suggests that the miR-30d under-expression observed in ATC cells may contribute to cisplatin insensitivity, which is mediated by increased expression of Beclin 1 and induction of autophagy.
Multiple mechanisms are known to contribute to cisplatin resistance, including increased repair of DNA damage, decreased intracellular accumulation of the drug, and reduced apoptosis [23]. Activation of autophagy has recently been appreciated as a new determinant of resistance to cisplatin [17; 19; 24; 25]. Here, we demonstrate that the miRNA, miR-30d, plays an important role in modulating cisplatin sensitivity, and the effect of miR-30d on cisplatin sensitivity is mediated through autophagy regulation. We also show that suppression of autophagy by the miR-30d mimic enhances sensitivity of ATC cells to cisplatin and augments apoptosis induced by this drug (Fig. 6 and 7). It appears that there exists a cross-talk between autophagy and apoptosis in cancer cells subjected to cisplatin treatment; however, the precise mechanisms and pathways involved remain to be investigated. Additionally, miR-30d can affect cancer cell sensitivity to the histone deacetylase inhibitor, trichostatin A, via down-regulating GRP78 expression [26]; down-regulation of miR-30d was observed in drug resistant ehrlich ascites tumor cells, which contributes to the drug-resistant phenotype by targeting Fox gene family-Zeb1/Zeb2 [27]. Thus, miR-30d may act as a modulator of sensitivity to various drugs through different molecular pathways and mechanisms in addition to autophagy.
It has been known that the members of the miR-30 family show high sequence similarity and share common target gene(s), regulating a particular cellular process. For instance, miR-30 family members were reported to regulate mesenchymal-epithelial transition (EMT) through targeting SMAD2 and TGFBR1 [28] and to regulate hedgehog signaling pathway through targeting smoothened [29]. Also, it has been shown that both miR-30a and miR-30d play a role in regulation of adipogenesis by targeting the transcription factor RUNX2 [30]. In this and a previous study [15], we found that miR-30d and miR-30a can target the same region within the 3′UTR of beclin 1 gene, regulating beclin 1 expression. Thus, our results are consistent with others’ observations as mentioned above. Although these two miRNAs share the same target, in the current study we focused on the implication of miR-30d, which is frequent down-regulated in human anaplastic thyroid carcinoma, in regulating beclin 1 expression and autophagy activity. In addition to in vitro (cell culture) and in vivo (animal) experiments, we demonstrated the effect of miR-30d mimic on beclin 1 expression using a reporter gene system (Figure 1), which clearly showed the role of this miRNA in regulating beclin 1 transcription.
Taken together, our study not only displayed how miR-30d regulates autophagy activity and impacts sensitivity of ATC cells to cisplatin, but also revealed the potential clinical implication of the down-regulation of this miRNA in ATC, a thyroid neoplasm that often responds poorly to therapies. These results suggest that therapeutic targeting of miR-30d down-regulation may be explored as a novel approach to preventing drug resistance or reinforcing the efficacy of chemotherapeutic drugs such as cisplatin in ATC.
Footnotes
Supported by grants from National Natural Sciences Foundation of China (81072146; 81101913), Natural Science Foundation of Jiangsu provincial Colleges and Universities (12KJD310005), Science and Technology Foundation of Suzhou City (SYS201319), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), US Public Health Service (R01CA135038), and by Elsa Pardee Foundation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaha AR. Implications of prognostic factors and risk groups in the management of differentiated thyroid cancer. Laryngoscope. 2004;114:393–402. doi: 10.1097/00005537-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 [see commetns] Cancer. 1998;83:2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quintavalle C, Donnarumma E, Iaboni M, Roscigno G, Garofalo M, Romano G, Fiore D, De Marinis P, Croce CM, Condorelli G. Effect of miR-21 and miR-30b/c on TRAIL-induced apoptosis in glioma cells. Oncogene. 2012 doi: 10.1038/onc.2012.410. [DOI] [PubMed] [Google Scholar]
- 5.Baraniskin A, Birkenkamp-Demtroder K, Maghnouj A, Zollner H, Munding J, Klein-Scory S, Reinacher-Schick A, Schwarte-Waldhoff I, Schmiegel W, Hahn SA. MiR-30a-5p suppresses tumor growth in colon carcinoma by targeting DTL. Carcinogenesis. 2012;33:732–739. doi: 10.1093/carcin/bgs020. [DOI] [PubMed] [Google Scholar]
- 6.Gaziel-Sovran A, Segura MF, Di Micco R, Collins MK, Hanniford D, Vega-Saenz de Miera E, Rakus JF, Dankert JF, Shang S, Kerbel RS, Bhardwaj N, Shao Y, Darvishian F, Zavadil J, Erlebacher A, Mahal LK, Osman I, Hernando E. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell. 2011;20:104–118. doi: 10.1016/j.ccr.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu D, Takeshita F, Hino Y, Fukunaga S, Kudo Y, Tamaki A, Matsunaga J, Takahashi RU, Takata T, Shimamoto A, Ochiya T, Tahara H. miR-22 represses cancer progression by inducing cellular senescence. J Cell Biol. 2011;193:409–424. doi: 10.1083/jcb.201010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao J, Liang L, Huang S, Ding J, Tan N, Zhao Y, Yan M, Ge C, Zhang Z, Chen T, Wan D, Yao M, Li J, Gu J, He X. MicroRNA-30d promotes tumor invasion and metastasis by targeting Galphai2 in hepatocellular carcinoma. Hepatology. 2010;51:846–856. doi: 10.1002/hep.23443. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi N, Uemura H, Nagahama K, Okudela K, Furuya M, Ino Y, Ito Y, Hirano H, Inayama Y, Aoki I, Nagashima Y, Kubota Y, Ishiguro H. Identification of miR-30d as a novel prognostic maker of prostate cancer. Oncotarget. 2012;3:1455–1471. doi: 10.18632/oncotarget.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwertheim S, Sheu SY, Worm K, Grabellus F, Schmid KW. Analysis of deregulated miRNAs is helpful to distinguish poorly differentiated thyroid carcinoma from papillary thyroid carcinoma. Horm Metab Res. 2009;41:475–481. doi: 10.1055/s-0029-1215593. [DOI] [PubMed] [Google Scholar]
- 11.Visone R, Pallante P, Vecchione A, Cirombella R, Ferracin M, Ferraro A, Volinia S, Coluzzi S, Leone V, Borbone E, Liu CG, Petrocca F, Troncone G, Calin GA, Scarpa A, Colato C, Tallini G, Santoro M, Croce CM, Fusco A. Specific microRNAs are downregulated in human thyroid anaplastic carcinomas. Oncogene. 2007;26:7590–7595. doi: 10.1038/sj.onc.1210564. [DOI] [PubMed] [Google Scholar]
- 12.Marton S, Garcia MR, Robello C, Persson H, Trajtenberg F, Pritsch O, Rovira C, Naya H, Dighiero G, Cayota A. Small RNAs analysis in CLL reveals a deregulation of miRNA expression and novel miRNA candidates of putative relevance in CLL pathogenesis. Leukemia. 2008;22:330–338. doi: 10.1038/sj.leu.2405022. [DOI] [PubMed] [Google Scholar]
- 13.Esposito F, Tornincasa M, Pallante P, Federico A, Borbone E, Pierantoni GM, Fusco A. Down-regulation of the miR-25 and miR-30d contributes to the development of anaplastic thyroid carcinoma targeting the polycomb protein EZH2. J Clin Endocrinol Metab. 2012;97:E710–718. doi: 10.1210/jc.2011-3068. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Klionsky DJ. Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells. Annu Rev Biochem. 2000;69:303–342. doi: 10.1146/annurev.biochem.69.1.303. [DOI] [PubMed] [Google Scholar]
- 15.Zhu H, Wu H, Liu X, Li B, Chen Y, Ren X, Liu CG, Yang JM. Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells. Autophagy. 2009;5:816–823. doi: 10.4161/auto.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng Y, Ren X, Zhang Y, Patel R, Sharma A, Wu H, Robertson GP, Yan L, Rubin E, Yang JM. eEF-2 kinase dictates cross-talk between autophagy and apoptosis induced by Akt Inhibition, thereby modulating cytotoxicity of novel Akt inhibitor MK-2206. Cancer Res. 2011;71:2654–2663. doi: 10.1158/0008-5472.CAN-10-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Cheng Y, Ren X, Zhang L, Yap KL, Wu H, Patel R, Liu D, Qin ZH, Shih IM, Yang JM. NAC1 modulates sensitivity of ovarian cancer cells to cisplatin by altering the HMGB1-mediated autophagic response. Oncogene. 2012;31:1055–1064. doi: 10.1038/onc.2011.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, Zhong X, Tanyi JL, Shen J, Xu C, Gao P, Zheng TM, DeMichele A, Zhang L. mir-30d Regulates multiple genes in the autophagy pathway and impairs autophagy process in human cancer cells. Biochem Biophys Res Commun. 2013;431:617–622. doi: 10.1016/j.bbrc.2012.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu D, Yang Y, Liu Q, Wang J. Inhibition of autophagy by 3-MA potentiates cisplatin-induced apoptosis in esophageal squamous cell carcinoma cells. Med Oncol. 2011;28:105–111. doi: 10.1007/s12032-009-9397-3. [DOI] [PubMed] [Google Scholar]
- 20.Ren JH, He WS, Nong L, Zhu QY, Hu K, Zhang RG, Huang LL, Zhu F, Wu G. Acquired cisplatin resistance in human lung adenocarcinoma cells is associated with enhanced autophagy. Cancer Biother Radiopharm. 2010;25:75–80. doi: 10.1089/cbr.2009.0701. [DOI] [PubMed] [Google Scholar]
- 21.Chang Y, Yan W, He X, Zhang L, Li C, Huang H, Nace G, Geller DA, Lin J, Tsung A. miR-375 inhibits autophagy and reduces viability of hepatocellular carcinoma cells under hypoxic conditions. Gastroenterology. 2012;143:177–187. e178. doi: 10.1053/j.gastro.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Frankel LB, Wen J, Lees M, Hoyer-Hansen M, Farkas T, Krogh A, Jaattela M, Lund AH. microRNA-101 is a potent inhibitor of autophagy. EMBO J. 2011;30:4628–4641. doi: 10.1038/emboj.2011.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borst P, Rottenberg S, Jonkers J. How do real tumors become resistant to cisplatin? Cell Cycle. 2008;7:1353–1359. doi: 10.4161/cc.7.10.5930. [DOI] [PubMed] [Google Scholar]
- 24.Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, Yin XM, Dong Z. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int. 2008;74:631–640. doi: 10.1038/ki.2008.214. [DOI] [PubMed] [Google Scholar]
- 25.Harhaji-Trajkovic L, Vilimanovich U, Kravic-Stevovic T, Bumbasirevic V, Trajkovic V. AMPK-mediated autophagy inhibits apoptosis in cisplatin-treated tumour cells. J Cell Mol Med. 2009;13:3644–3654. doi: 10.1111/j.1582-4934.2009.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su SF, Chang YW, Andreu-Vieyra C, Fang JY, Yang Z, Han B, Lee AS, Liang G. miR-30d, miR-181a and miR-199a-5p cooperatively suppress the endoplasmic reticulum chaperone and signaling regulator GRP78 in cancer. Oncogene. 2012 doi: 10.1038/onc.2012.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husted S, Sokilde R, Rask L, Cirera S, Busk PK, Eriksen J, Litman T. MicroRNA expression profiles associated with development of drug resistance in Ehrlich ascites tumor cells. Mol Pharm. 2011;8:2055–2062. doi: 10.1021/mp200255d. [DOI] [PubMed] [Google Scholar]
- 28.Braun J, Hoang-Vu C, Dralle H, Huttelmaier S. Downregulation of microRNAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene. 2010;29:4237–4244. doi: 10.1038/onc.2010.169. [DOI] [PubMed] [Google Scholar]
- 29.Ketley A, Warren A, Holmes E, Gering M, Aboobaker AA, Brook JD. The miR-30 microRNA family targets smoothened to regulate hedgehog signalling in zebrafish early muscle development. PLoS One. 2013;8:e65170. doi: 10.1371/journal.pone.0065170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaragosi LE, Wdziekonski B, Brigand KL, Villageois P, Mari B, Waldmann R, Dani C, Barbry P. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol. 2011;12:R64. doi: 10.1186/gb-2011-12-7-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]