The reddened appearance of lesional psoriasis skin reflects increases in angiogenesis and although this increase is unlikely to be the principle cause of psoriasis it may provide new therapeutic targets. VEGF is a potent mediator of angiogenesis [1], elevated VEGF is found in lesional skin and sera of psoriasis patients and correlates with disease severity and several VEGF genetic polymorphisms have been identified associated with early onset psoriasis.
VEGF contributions to psoriasis pathogenesis are multiple and include increasing cutaneous vascular permeability and inflammation, promotion of cutaneous leukocyte infiltration, eliciting pro-inflammatory T cell differentiation, and activating and inducing keratinocyte (KC) proliferation. Thus, VEGF serves autocrine and paracrine functions in psoriasis, promoting pro-inflammatory feedback loops between KCs, endothelial cells, infiltrating T cells and monocytes which together exacerbate cell-mediated immune inflammatory reactions.
VEGF importance in psoriasis is further evidenced by the development of a psoriasiform phenotype in KC-VEGF overexpressing mice, where skin phenotypes similar to human psoriasis develop following oxazalone [2] or TPA [3] treatment or tape stripping in young and spontaneously in older mice [4]. In each model, KC-derived VEGF and angiogenesis increased prior to lesion development, and in lesional skin, immunocyte infiltrate included T cells and macrophages [2, 4]. Targeted VEGF-inhibition improved the skin disease, however dermal T cells remained present in one study [4], and VEGFR1 or VEGFR2-specific inhibition individually failed to modify the inflammatory response, but together resolved the psoriasiform phenotype [2].
Despite recent reports of patients experiencing psoriasis remission during bevacizumab treatment for colon cancer [5] and following sorafenib treatment for renal cell carcinoma [6], targeting VEGF in psoriasis has received minimal attention. Using the KC-Tie2 psoriasiform mouse model, we investigated the efficacy of anti-VEGF treatment in skin inflammation. KC-Tie2 mice spontaneously develop skin disease that phenocopies human psoriasis, including increases in angiogenesis, VEGF (~3-fold; P<0.02), psoriasis-related pro-inflammatory leukocytes and cytokines, and disease resolution following CsA treatment [7].
Adult KC-Tie2 mice and littermate controls were treated with subcutaneous VEGF-Trap or control FC protein (25mg/kg; n=8/group; Regeneron Pharmaceuticals, Tarrytown, NY) 2x/week for 4 weeks similar to what has previously been done [4]. VEGF-Trap is a soluble fusion protein that binds VEGF with high affinity, has an extended in vivo half-life in mice and consists of portions of human VEGFR1/R2 and the Fc domain of human IgG1. All animal protocols were approved by the Case Western Reserve University IACUC.
Skin was examined histologically using H&E, immunohistochemistry and at the RNA and protein levels as previously described [7, 8].
All data are presented as mean ± SEM. Data were tested for normality and statistical significance calculated using either a Student t test or a Mann– Whitney U test, as appropriate. Significance was defined as P≤0.05.
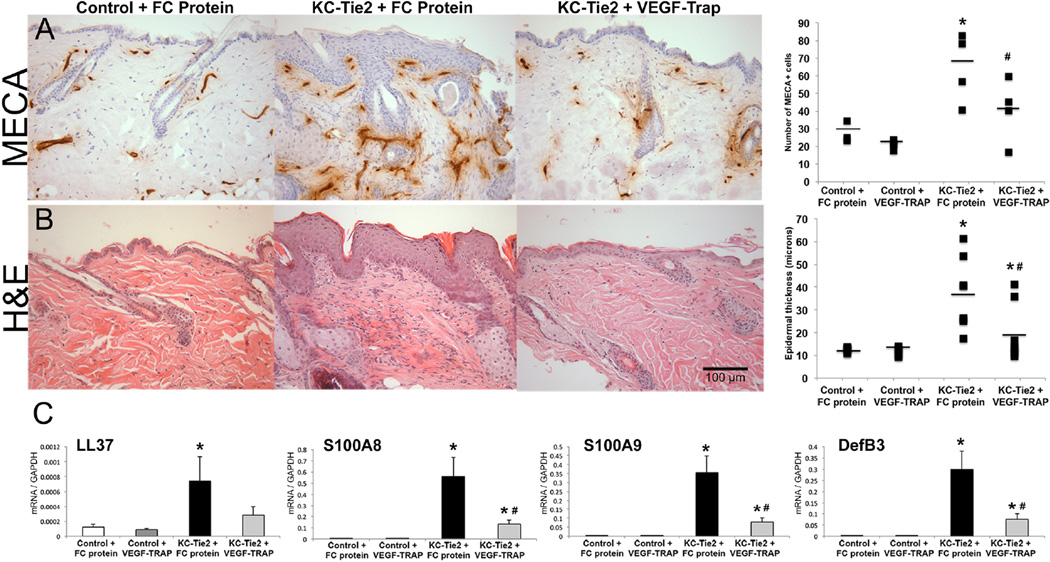
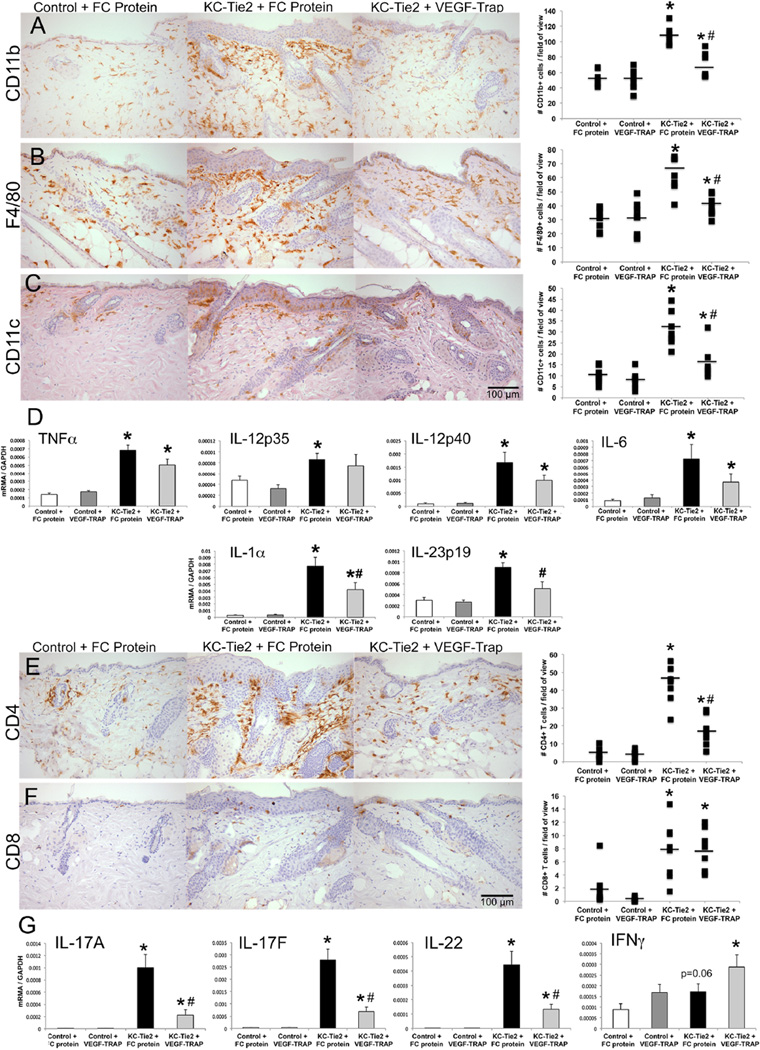
VEGF-Trap significantly reduced dermal angiogenesis to control levels in KC-Tie2 mice (P=0.04 vs KC-Tie2+FC protein) with concomitant reductions in acanthosis (47%; P=0.03 vs KC-Tie2+FC protein) and decreases in innate defense genes S100A8/A9 and DefB3 (all P<0.03; Fig. 1). Despite significant decreases in cutaneous CD11b+, CD11c+ and F4/80+ cells in VEGF-Trap treated KC-Tie2 mice (P<0.001 vs KC-Tie2+FC protein), levels remained significantly increased compared to control littermates (P<0.04). Myeloid cell-derived cytokines, TNFα, IL-1α, IL-6 and IL-12 remained elevated following VEGF-Trap treatment whereas IL-23 expression returned to control mouse levels (P=0.02 vs KC-Tie2+FC protein; Fig. 2). Interestingly, CD4+ T cells decreased (63%; P=0.001) in VEGF-Trap treated KC-Tie2 mouse skin, accompanied by significant reductions in IL-17A, IL-17F and IL-22 (all P<0.007; Fig. 2), whereas CD8+ T cells remained elevated along with IFNγ levels.
Figure 1. VEGF-Trap treated KC-Tie2 mice have decreased angiogenesis, acanthosis and innate defense gene expression.
Representative back skin sections from control and KC-Tie2 mice treated with control FC protein (25mg/kg) or VEGF-Trap (25mg/kg) immunostained using the endothelial cell-specific antibody (MECA) (A) or stained with H&E (B). Quantification of angiogenesis (MECA+ blood vessel number) (A), epidermal thickness (B) and qRT-PCR gene expression analyses of psoriasis-related innate defense molecules (C) are presented (n=8/group; mean ± SEM). * P< 0.05 vs Control + FC protein; # P<0.05 vs KC-Tie2 + FC protein. Scale bar = 100µm.
Figure 2. VEGF-Trap treated KC-Tie2 mice have fewer cutaneous infiltrating myeloid cells, CD4+ T cells and reduced IL-23 and Th17 cytokines.
Representative back skin sections from control and KC-Tie2 mice treated with control FC protein (25mg/kg) or VEGF-Trap (25mg/kg) immunostained using antibodies targeting CD11b (A), F4/80 (B), CD11c (C), CD4 (E) and CD8 (F) antigen. Quantification of skin infiltrating myeloid cells (A-C), T cells (E, F) and qRT-PCR gene expression analyses of myeloid-derived cytokines (D) and T cell derived cytokines (G) are presented (n=8/group; mean ± SEM). * P< 0.05 vs Control + FC protein; # P<0.05 vs KC-Tie2 + FC protein. Scale bar = 100µm.
Together, these data provide in vivo evidence for efficacy of VEGF-Trap treatment for psoriasis and suggest disease improvement may occur via VEGFmediated effects on the IL-23/Th17 axis of inflammation. These findings are consistent with VEGF inhibition in two prior genetic VEGF-overexpressing models of skin inflammation [2, 4], as well as recent evidence showing VEGF-inhibition (using G6-31) efficacy in the KC-specific JunB:c-Jun knockout mouse [9]. However, we provide mechanistic insight into how VEGF-mediated inhibition occurs, identifying reductions in Th17 cell presence and activation (via decreased CD4+ T cells and IL-17A/F and IL-22) in VEGF-Trap treated mice; possibly as a result of decreased levels of IL-23 but not IL-12. This observation validates and expands upon a prior report demonstrating decreased infiltrating CD3/4+ T cells and reductions in IL-22 [9]. Although significant decreases in myeloid cell infiltrates were observed in VEGF-Trap treated KC-Tie2 mouse skin, and decreased IL-23, other myeloid-cell derived cytokines, including TNFα, IL-12, IL-1α and IL-6 remained elevated, potentially explaining the significant improvement, but not complete reversal of acanthosis, Th1 presence (and increased IFNγ) and skin disease severity. This is reminiscent of prior work where significant improvement in skin disease severity, but not complete reversal, was found in KC-Tie2 mice treated with antibodies targeting TNFα [8]. Interestingly, recent attempts blocking both VEGF and TNFα, using a novel fusion protein targeting both molecules, called Valpha [3] has proven highly effective at reversing/preventing TPA-induced acanthosis and dermal lymph/angiogenesis in KC-VEGF animals at levels greater than either TNFα (Enbrel) or VEGF (VEGF-Trap) inhibition alone. VEGF-Trap also inhibits PlGF-signaling events [4], and PlGF expression levels were also elevated in KC-Tie2 mouse skin (21.66 ± 6.6pg/ml KC-Tie2 vs 8.4 ± 2.7pg/ml controls; P=0.002), thus the VEGF-Trap effects reported here likely reflect VEGF- and PlGF-inhibition. Interestingly, our observation that CD8+ T cells remain elevated along with IFNγ levels in KC-Tie2 mice treated with VEGF-Trap suggests disease severity can improve despite sustained Th1 activity if Th17 cell depletion is sufficient, and is consistent with recent clinical trials showing excellent efficacy of IL-17 inhibition in psoriasis patients.
In conclusion, we demonstrate VEGF-Trap treatment improves chronic skin inflammation in KC-Tie2 mice and that disease resolution occurs in an IL-23/Th17 mediated manner. Although systemic VEGF inhibition is unlikely to become a frontline target for psoriasis drug development due in part to its potential side effects; our data provide proof of concept support for targeting this molecule and its multi-cellular functions in psoriatic skin. Targeting VEGF topically may provide an effective new approach for treating mild-to-moderate psoriasis skin lesions by inhibiting angiogenesis and reducing leukocyte infiltration and KC proliferation.
Acknowledgments
The authors apologize to colleagues whose work could not be cited because of the space constraints. We thank Dr. John Rudge (Regeneron Pharmaceuticals, Tarrytown, NY) for providing VEGF-Trap and Dr. Thomas McCormick for feedback on the manuscript.
Funding support: NLW is supported in part by grants from the National Institutes of Health (P30AR39750, P50AR05508, RO1AR063437; RO1AR062546) and the National Psoriasis Foundation.
Footnotes
Conflicts of Interest: None.
References Cited
- 1.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 2.Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, et al. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–1057. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- 3.Jung K, Lee D, Lim HS, Lee S-I, Kim YJ, Lee GM, et al. Double Antiangiogenic and Anti-inflammatory Protein Valpha Targeting VEGF-A and TNF-α in Retinopathy and Psoriasis. Journal of Biological Chemistry. 2011;286:14410–14418. doi: 10.1074/jbc.M111.228130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- 5.Akman A, Yilmaz E, Mutlu H, Ozdogan M. Complete remission of psoriasis following bevacizumab therapy for colon cancer. Clinical and experimental dermatology. 2009;34:e202–e204. doi: 10.1111/j.1365-2230.2008.02991.x. [DOI] [PubMed] [Google Scholar]
- 6.Fournier C, Tisman G. Sorafenib-associated remission of psoriasis in hypernephroma: case report. Dermatology online journal. 2010;16:17. [PubMed] [Google Scholar]
- 7.Wolfram JA, Diaconu D, Hatala DA, Rastegar J, Knutsen DA, Lowther A, et al. Keratinocyte but not endothelial cell specific overexpression of Tie2 leads to the development of psoriasis. Am J Pathol. 2009;174:1443–1458. doi: 10.2353/ajpath.2009.080858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward NL, Loyd CM, Wolfram JA, Diaconu D, Michaels CM, McCormick TS. Depletion of antigen-presenting cells by clodronate liposomes reverses the psoriatic skin phenotype in KC-Tie2 mice. The British journal of dermatology. 2011;164:750–758. [Google Scholar]
- 9.Schonthaler HB, Huggenberger R, Wculek SK, Detmar M, Wagner EF. Systemic anti-VEGF treatment strongly reduces skin inflammation in a mouse model of psoriasis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21264–21269. doi: 10.1073/pnas.0907550106. [DOI] [PMC free article] [PubMed] [Google Scholar]