Abstract
High grade glioma is a highly invasive brain tumor and recurrence is almost inevitable, even after radical resection of the tumor mass. Cytotoxic immune responses and immunological memory induced by immunotherapy might prevent tumor recurrence. Dendritic cells (DCs) are professional antigen-presenting cells of the innate immune system with the potential to generate robust antigen-specific T cell immune responses. DC-based immunotherapeutic strategies have been intensively studied in both preclinical and clinical settings. Although advances have been made in the experimental use of DCs, there are still considerable challenges that need to be addressed for clinical translation. In this review, we describe the variability of regimens currently available for DC-based immunotherapy and then review strategies to optimize DC therapeutic efficacy against glioma.
Keywords: glioma, malignant, immunotherapy, dendritic cell, cancer
Introduction
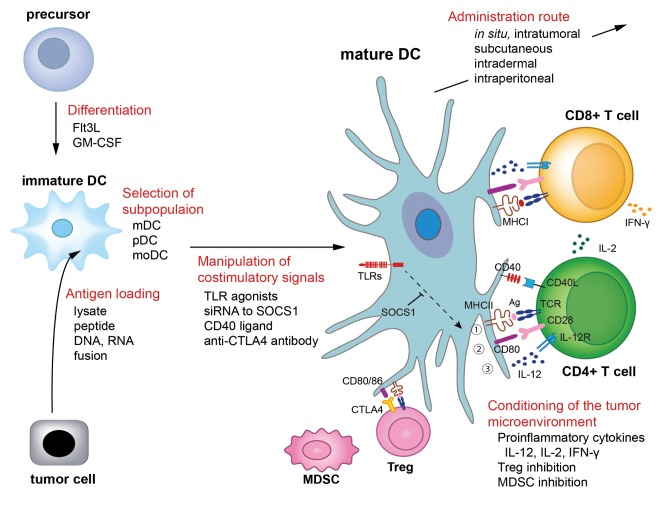
High grade glioma (HGG) is one of the most lethal malignant tumors in humans.115) Despite aggressive treatment by radical surgical resection combined with temozolomide and bevacizumab chemotherapy and radiotherapy, the prognosis for patients with HGG remains unsatisfactory with a median survival of less than 2 years.65,108) The infiltrative nature of the tumor into the brain parenchyma hampers its complete surgical resection and relapse of the tumor is almost inevitable. Tumor antigen-specific immune cells can identify and attack infiltrating tumor cells to control tumor regrowth through immunological memory and immune surveillance.41,60) Dendritic cells (DCs), the most potent antigen-presenting cell (APC), and T cells are the dominant effector cells that inhibit tumor progression. In this context, the development of clinically effective DC-based immunotherapy is a major focus for specific immunotherapy in HGG.112) While there are a wide variety of regimens that generate tumor-specific effector immune responses in the context of DC-based immunotherapy, only a limited number have been tested in clinical trials to date.111) In this review, we summarize the regimens used for DC-based immunotherapy including (i) DC differentiation, (ii) selection of DC subpopulations, (iii) antigen loading of DCs, (iv) manipulation of costimulatory and coinhibitory signals via DCs, (v) conditioning of the tumor microenvironment, (vi) administration route of DCs as shown in Fig. 1. We also review the strategies for optimizing the therapeutic efficacy of DC-based immunotherapy.
Fig. 1.
Dendritic cell (DC)-based immunotherapeutic strategies for glioma. DCs are the professional antigen-presenting cells that generate robust antigen-specific T cell immune responses. There are a wide variety of regimens that generate anti-glioma immune responses in the context of DC-based immunotherapy. Bone-marrow derived precursors are differentiated into DCs by Flt3L or GM-CSF. DCs are heterogenous cell populations that include mDC, pDC, and moDC. These subpopulations act differently and have synergistic effects in anti-tumor immunity. DCs can also be subdivided according to CD8α or NK1.1 expression. DCs are to be loaded with tumor antigens derived from eitherwhole tumor cell lysate, peptide, DNA, RNA, or tumor-DC fusion. MHC-antigen complex are recognized by TCR on T cells (signal 1). Tumor-loaded DCs are then pulsed with maturation stimuli to increase the expression of costimulatory molecules such as CD80 (signal 2) and to increase the secretion of proinflammatory cytokines such as IL-12 (signal 3). These three signals are essential to generate robust anti-tumor T cell responses. IL-2 derived from CD4+ helper T cells stimulates CD8+ cytotoxic T cells, which then secrete IFN-γ and exhibit potent cytolytic activity against glioma cells. Inhibition of immune regulatory components such as Treg or MDSC enhances anti-tumor immunity. Administration route of DCs influences the therapeutic efficacy of DC-based immunotherapies. Optimization of a DC-based immunotherapeutic regimen is critical for the development of clinically relevant immunotherapy for glioma. Flt3L represents fms-like tyrosine kinase 3 ligand. Ag: antigen, CTLA-4: cytotoxic T-lymphocyte antigen 4, DC: dendtiric cell, GM-CSF: glanulocyte monocyte-colony stimulating factor, IFN: interferon, IL: interleukin, mDC: myeloid DC, MDSC: myeloid-derived suppressor cell, MHC: major histocompatibility class, moDC: monocyte-derived DC, pDC: plasmacytoid DC, siRNA: small interfering RNA, SOCS1: suppressor of cytokine signaling 1, TCR: T cell receptor, TLR: toll-like receptor, Treg: regulatory T cell.
Dendritic Cell Differentiation
DCs can present and cross-present antigenic peptides in the context of major histocompatibility class (MHC) II and MHC I molecules, respectively, and can prime both CD4+ T helper cells and CD8+ cytotoxic T cells.90,91) Cross-presentation of antigens to CD8+ T cells is primarily performed by DCs. Furthermore, DCs are not only sentinels in T cell immune responses, but can also function as strong activators of natural killer (NK) cells and NK T cells,44,100) thus linking innate and adaptive immunity. The type 1 polarizing DC (DC1) subset plays an important role in tumor immunity by directing effector T cell responses to a T helper type 1 (Th1) phenotype and the DC2 subset is associated with immunity against extracellular antigens and wound healing. DC1 polarization induces the abundant production of interleukin (IL)-12p70 heterodimer and IL-23, secretion of the chemokine MIP-1, and preferential expression of Delta-4 Notch ligand.77) Such DC1 products are highly associated with chemo-attraction and the activation of Th1-type CD4+ and CD8+ T cells. Furthermore, IL-12p70 production is critical for the sensitization of high-avidity T cells that directly recognize and kill tumor targets.38,71,77)
DC differentiation from bone marrow precursors can be induced by granulocyte macrophage colony-stimulating factor (GM-CSF) or fms-like tyrosine kinase-3 ligand (Flt3L). Flt3L expands both DC1 and DC2 subsets with a significantly higher percentage and number of DC1 than DC2 cells, while GM-CSF preferentially expands the DC2 subset.83,114) Isolated DC1 from Flt3L-injected mice had significantly higher levels of IL-12p40 than IL-10, while the converse occurred with DC2. Both Flt3L and GM-CSF increased the number of naïve and memory T cells in mice, but the number of memory CD4+ and CD8+ T cells was significantly increased by Flt3L compared to GM-CSF. While GM-CSF increased the frequency of both Th1 and Th2 cytokine-producing cells, Flt3L significantly augmented the frequency of Th1 cells.83,114)
To increase the proportion and function of the DC1 subset in GM-CSF treated progenitor cells, Mailliard et al. developed a novel protocol, in which bone marrow cells were cultured with GM-CSF followed by interferon (IFN)-γ, IFN-α, IL-4, and polyinosinic-polycytidylic acid (polyI:C) stabilized by lysine and carboxymethylcellulose (polyICLC).71) Such α-type-1 polarized DCs produced abundant IL-12 compared to the normal DC1 subset and were resistant to immunosuppressive environments created by regulatory T cells (Tregs). Okada et al. reported an α-type-1 polarized DC vaccine loaded with tumor antigens was useful for controlling relapse in a mouse model of HGG in a CXCL-10 dependent manner.38) Based on these results, Akiyama et al. performed a phase I clinical trial of DC vaccination using α-type 1 DCs, which showed some promise in HGG patients.2) Cohen et al. utilized IL-6 to improve the expansion and anti-tumor immune stimulation of Flt3L-generated DCs.28) Exposure of fresh mouse bone marrow to Flt3L + IL-6 triggered the massive expansion of CD34+ progenitor cells, and committed nearly all cells to subsequent DC differentiation. Such programming included trafficking into the tumor, the subsequent spontaneous up-regulation of MHC/costimulatory molecules, as well as the secretion of Th1 polarizing cytokines such as IL-12. Moreover, proliferative conditioning with Flt3L + IL-6 conferred progressive resistance to many tumor-associated immunosuppressive factors such as IL-10, vascular endothelial growth factor, and prostaglandin E2. Furthermore, Flt3L + IL-6 induced signal transducer and activator of transcription (STAT)3-dependent global DC differentiation, whereas GM-CSF + IL-4 induced STAT5-dependent monocytic differentiation, which subsequently differentiated into granulocytes, macrophages or DCs.28) Despite the superiority of Flt3L to GM-CSF in terms of DC1 polarization, the clinical efficacy of Flt3L-conditioned DCs was not necessarily superior to GM-CSF conditioned DCs. Weigel et al. compared the therapeutic efficacy of DCs generated using GM-CSF + IL-4 supplemented with lipopolysaccharide (LPS) and DCs generated using Flt3L supplemented with LPS as a vaccination against acute myeloid leukemia (AML). The anti-AML effect was superior in GM-CSF + IL4 + LPS-treated groups compared with Flt3L + LPS groups.114) In contrast, Mineharu et al. compared the therapeutic efficacy of DC vaccination regimens and showed that Flt3L + IL-6 + CpG generated DCs were comparable or more effective compared to GM-CSF + IL-4 + CpG generated DCs.74) The combination of Flt3L and GM-CSF to mobilize DC precursors was also analyzed,9,11) but the potential of such precursors to achieve DC1 polarization is presently unclear. Flt3L + GM-CSF mobilization was recently reported to inhibit the infiltration of DCs into mouse tumors, and such DCs also activated Tregs and promoted tumor tolerance.28)
In most clinical trials for HGG, DCs were generated by GM-CSF + IL-4 and the efficacy of Flt3L-generated DC subsets has not been tested thus far.112) A phase I clinical trial of adenoviral mediated immunogene therapy using Flt3L in combination with cytotoxic gene therapy using thymidine kinase (TK) followed by gancyclovir administration is now underway.8,32,88,117) The clinical efficacy of DC vaccination using Flt3L-generated DCs should also be investigated in future trials.
Selection of DC Subpopulations
DCs are a heterogeneous population classified into distinct subsets according to patterns of cell surface antigen expression. The most commonly used classification of DC subsets in blood and the lymphatic system include myeloid DCs (mDCs), plasmacytoid DCs (pDCs), and monocyte-derived DCs (moDCs).39) The E-twenty six (ETS) transcription factor, PU.1, was reported to be a key regulator of DC development in both Flt3L and GM-CSF conditioning,122) but these DC differentiation factors act differently. Mouse hematopoietic progenitor cells cultured with Flt3L generate mDC and pDC subsets,17,76) whereas GM-CSF-cultured bone marrow cells differentiate into moDCs.28,51) mDCs are key regulators in the induction of anti-tumor immune responses, whereas pDCs are recognized as major producers of type I IFN. Lou et al. reported that CpG-activated pDCs prime antigen-specific CD8+ T cell responses in vivo, and generate memory T cells that combat tumor rechallenge. They also demonstrated that pDCs and mDCs synergistically enhanced antigen-specific anti-tumor immune responses. Synergy between pDCs and mDCs to activate T cells requires direct cell-to-cell contact between the two subsets and is dependent on MHC I expression by mDCs, but not pDCs, suggesting pDCs enhance the ability of mDCs to present antigens to T cells.69) However, interactions between moDCs and other DC subsets are not well studied and the optimal proportion of DC subsets for the induction of robust anti-tumor immunity remains to be investigated.
DCs are also classified based on their CD8α expression.103) The lymph node resident CD8α+ DC subset is specialized for cross-presentation of exogenous antigens to naïve CD8α+ T cells,33,53,86) whereas migratory CD8α− DCs are required for presentation by MHC class II to CD4+ T cells. 86) Recently, both CD8α+ and CD8α− DC subsets, but not pDCs, were shown to be effective at cross-presenting tumor antigens.73) It was speculated that migratory tumor DC subsets (CD8α−) with altered costimulatory receptor expression might contribute to the induction and regulation of tumor-specific responses. Moreover, Segura et al. reported that human BDCA1+ DCs, BDCA3+ DCs, and pDCs, which are homologous to mouse CD8α− DCs, CD8α+ DCs, and pDCs, respectively, all cross-presented soluble antigen efficiently, compared to macrophages.97) When bone marrow cells were cultured with both Flt3L and GM-CSF, few CD8α+ DCs or pDCs developed compared with cultures supplemented with Flt3L alone,121) again demonstrating the different actions of Flt3L and GM-CSF on DC progenitors.
Natural killer DCs (NKDCs) are a unique class of rodent and human immune cells that possess the characteristics of both NK cells and DCs.13,24–27) Flt3L is a potent inducer of functionally mature NKDCs in lymphoid and non-lymphoid organs. Flt3L-expanded NKDCs retain the unique ability to lyse tumor cells by IFN-γ release and induce more potent anti-tumor immune responses by CD4+ and CD8+ T cells when compared with spleen resident NKDCs.24) Thus, DCs can be subdivided into several subtypes according to their surface markers and characteristics, but the functions and interactions of each subtype remains to be clarified. Therefore, the individual properties and combinations of subsets that may best promote successful immunotherapy are poorly understood. As Flt3L-conditioned DCs and GM-CSF-conditioned DCs have distinct properties, the admixture of these cell populations might be a candidate for preparation of optimal DC preparations.
Antigen Loading on DCs: Autophagosomes and a Cocktail of Tumor-Associated Antigens
Effective uptake and loading of tumor-associated antigens (TAAs) onto MHC complexes of DCs and expansion of DC subgroups that can efficiently prime naïve T cells play a critical role in the therapeutic efficacy of DC vaccination. Therefore, it is of critical importance to optimize the preparation of tumor cell antigens. Tumor antigens can be loaded on DCs as different forms including DNA, RNA, peptides, proteins and lysates, or DCs fused with tumor cells. Owing to the heterogeneous properties of HGG cells and poor identification of HGG-specific tumor antigens, most clinical trials of DC vaccination in HGG utilized whole tumor lysates instead of artificially-synthesized peptides as a source of TAA.56,111,119)
Whole tumor lysates have been mostly generated by irradiation (apoptosis) or freeze-thawing (necrosis) of tumors. Apoptotic bodies can enhance antigen cross-presentation more effectively than necrotic tumor lysates.19,96) However, loading of DCs with apoptotic bodies of HGGs can also increase the risk of inducing tolerogenic DCs via the cyclooxygenase-2 (COX2) pathway.1) Recently, autophagic tumor lysates and autophagosomes were introduced as a source of TAAs and DCs loaded with purified autophagosomes from autophagic tumor cells induced tumor-specific immune responses.67) Autophagy not only provides variable tumor antigens but regulates the selective release of high-mobility group B1 (HMGB1), an endogenous pattern recognition receptor (PRR) that induces DC maturation.109) A comparative analysis of autophagic, apoptotic, and necrotic tumor lysates in terms of anti-tumor immunity showed that triggering autophagy and/or apoptosis to generate tumor cell lysates increased the immunogenicity of tumor cells and enhanced the delivery of TAAs to DCs when compared to necrotic tumor cell lysates.74) The therapeutic efficacy of vaccination with DCs loaded with autophagic tumor cell lysates or DCs with apoptotic tumor cell lysates were superior to DCs loaded with necrotic tumor cell lysates.74) Protective immunity against intracranial glioma growth was also observed following immunization with RNA-loaded DCs or DCs fused with tumor cells.52,111) Ashley et al. reported the induction of antitumor immunity by DCs pulsed with tumor extracts or tumor RNA, with no significant difference between the two strategies in eliciting antitumor T cell responses and prolonging the survival of glioma-bearing mice.6) Finally, Parajuli et al. systemically compared the efficacy of vaccination with DCs loaded with tumor antigens from different sources. They showed that DCs fused with tumor cells, DCs pulsed with apoptotic tumor cells, and DCs pulsed with tumor RNA induced superior tumor cytolytic activities in peripheral blood mononuclear cells compared to DCs pulsed with necrotic lysates. In addition, DCs pulsed with apoptotic lysates induced the greatest expansion of tumor-specific lymphocytes.82)
The identification of TAAs and a better understanding of human leukocyte antigen (HLA) restriction in HGG have enhanced the production of DCs loaded with multiple glioma-related antigens, selected according to the HLA genotype of individual patients. Glioma-associated antigens identified to date include epidermal growth factor receptor isoform III (EGFRvIII), tenascin, survivin, the alpha-2 chain of IL-13 receptor (IL-13Ra2 chain), gp100, melanoma antigen (MAGE)-1 and MAGE-3, WT-1, HER2, EphA2 and YKL-40. The characteristics of these antigens have been previously reviewed.36,45,119) In a clinical setting, Okada et al. treated HLA-A2 positive patients with DCs pulsed with HLA-A2 peptides including EphA2, IL-13R-a2, YKL-40, and gp100.78) Akiyama et al. developed a DC vaccination regime using a cocktail of five synthetic peptides (WT-1, HER2, MAGE-A3, and MAGE-A1 or gp100) restricted to HLA-A2 or A24 for recurrent HGG patients with HLA-A2 or A24 genotype.2) Another option for antigen loading is the genetic modification of DCs, which allows multi-epitope presentation of full-length TAAs without requiring knowledge of the patient’s HLA genotype.16) Dendreon’s Provenge (sipuleucel-T), the first US Food and Drug Administration (FDA)-approved immunotherapy for hormone-refractory prostate cancer, consists of a DC-enriched product (B cells, monocytes, and NK cells are also included) cultured ex vivo with a recombinant fusion protein containing prostatic acid phosphatase (PSA) and GM-CSF,104) suggesting that antigen loading with TAAs is clinically relevant. Taken together, immunization with DCs pulsed with a cocktail of glioma-associated antigens or autophagosomes extracted from glioma cells might be a promising approach to treat HGG patients.
Manipulation of Costimulatory and Coinhibitory Signals via DCs
In addition to the first signal provided through T cell receptors that recognize antigenic peptide-MHC molecules on DCs, a second signal induced by interactions between costimulatory ligands on T cells and their receptors on DCs are required for robust T cell responses22,64). In the absence of costimulatory molecule interactions, antigen-specific T-cells become hyporesponsive, a state characterized as anergy.54) The upregulation of costimulatory molecules is, therefore, an attractive approach for generating therapeutic immunity to combat malignancies.35) Costimulatory molecules belong to two major families: B7/CD28 family and tumor necrosis factor (TNF)/TNF receptor family. B7/CD28 family molecules are involved in the initiation of cell-mediated immune responses, and TNF/TNF receptor family members are involved in the later phases of T-cell activation. B7 molecules expressed on DCs include CD80 (B7-1), CD86 (B7-2), inducible costimulator (ICOS) ligand (B7h), programmed death 1 ligand (PD-L1 or B7-H1), PD-L2 (B7-DC), B7-H3, and B7-H4. TNF/TNF receptor family includes 4-1BB ligand, OX-40, glucocorticoid-induced tumor necrosis factor receptor (GITR), LIGHT, and CD27. These molecules have been well characterized in previous review articles.22,35,43,120)
It is well known that a variety of single agents, including Toll-like receptor (TLR) agonists, CD40 ligand, CD70, GITR ligand, OX-40 ligandDi, and calcium ionophores, can increase the expression of costimulatory molecules on DCs.12,77,118) There are a wide variety of TLR agonists including Pam3Cys (TLR1/2 agonist), FSL-1 and MALP2 (TLR2/6 agonist), polyI:C (TLR3 agonist), LPS and monophosphoryl lipid A (TLR4 agonists), imiquimod and R848 (TLR7 agonists), and class B CpG oligodeoxynucleotide (CpG; TLR9 agonist). It should be noted that these TLR agonists do not uniformly stimulate anti-tumor immune responses. For example, cDCs respond to most TLR agonists, whereas pDCs are more sensitive to agonist CpG stimulation compared with all other agonists used. Simultaneous ligation of TLR1/2 and TLR3 was the most optimal at inducing a DC1 phenotypic maturation for Flt3L-generated DCs, whereas TLR3/4 + TLR7/9 ligations were optimal for GM-CSF + IL-4-generated DCs.68) The therapeutic effect of TLR agonists depends partly on the expression of TLRs on tumor cells or immune cells in the tumor microenvironment. Intratumoral injection of CpG showed the best therapeutic effect for GL261 glioma cells and Pam3Cys or R848 also produced a significant survival benefit, whereas polyI:C or purified LPS stimulation alone was not effective.42) Recent evidence suggests that combination of different classes of immunostimulants act synergistically to increase anti-tumor immune responses. For example, CD40 and TLR ligands are synergistic and this combination of immunostimulants can significantly suppress tumor growth in mice.107) Optimal regimens for the upregulation of costimulatory molecules require further investigation.
CD80 and CD86 are among the most well characterized costimulatory molecules. They bind two surface molecules expressed on T cells, CD28, and CTLA-4. Notably, in contrast to the costimulatory signal derived from CD28, the engagement of CTLA-4 by CD80 or CD86 induces a negative regulation of the immune response, leading to immune tolerance.43) Thus, the balance between activating and inhibitory signals derived from the engagement of CD28 and CTLA-4, respectively, is crucial to assure protective immunity against cancer. Therefore, in addition to the upregulation of CD80 and CD86 by immunostimulants, blockade of signaling transduced through CTLA-4 is necessary to maximize anti-tumor immune reactions. Indeed, a phase III study showed that the CTLA-4 antibody, ipilimumab, either alone or in combination with gp100 vaccine, improved overall survival compared with gp100 alone in patients with metastatic melanoma who had undergone previous treatment 47) and the drug was subsequently approved by the FDA. PD-L1, PD-L2, and B7-H4 also function as negative regulators of T cell immune responses. Aberrant expression of PD-L1 has been reported in many human cancers including glioblastoma and melanoma,22,120) and the expression of PD-L1 correlates with a poor prognosis for patients.22) Antibody blockade of PD-L1 and PD-L2 on DCs improved the proliferation and cytokine production of CD4+ T cells18) and a phase II clinical trial using anti-PD-L1 antibodies for non-small cell lung carcinoma is currently underway.110) Thus, suppression of negative costimulatory molecules (also called coinhibitory molecules) seems to be a promising approach to increase the therapeutic efficacy of DC-based immunotherapy. However, as indicated by the low response rate and the risks of severe side-effects of ipilimumab, further research is necessary to determine the optimal regimen to manipulate costimulatory/coinhibitory molecules.
In addition to negative costimulatory molecules (or coinhibitory molecules) such as PD-L1, DCs express a variety of molecules that may suppress antigen presentation or T cell activation and functions. Silencing of these molecules by siRNA is a powerful strategy to augment DC-mediated anti-tumor immunity. A20, a negative regulator of TLR and TNF receptor signaling pathway involved in the stimulation of T cell-mediated responses15,66) and suppressor of cytokine signaling 1 (SOCS1), a negative regulator of signaling through IFN-γ, IL-2, IL-6, or IL-12, stimulators of T cell expansion,98) have been studied for this purpose.16,72) Antigen-loaded DCs, silenced for either A20 or SOCS1 by siRNA, activated large numbers of effector T cells, which correlated with the inhibition of tumor growth in mice.48,98,105) Surface molecules with direct suppressive effects on T cells are also attractive targets.16) Notch ligands and DC-derived immunoglobulin receptor 2 (DIgR2) are two main targets. Silencing of Delta1, a Notch ligand, by siRNA enhanced cytokine production by CD4+ T-cells in response to polyclonal T cell receptor activation.106) Immunization of mice with antigen-pulsed, DIgR2-silenced DCs elicited more potent antigen-specific CD4+ and CD8+ T cell responses, thus showing an improved therapeutic efficacy.99)
Conditioning of the Tumor Microenvironment
In addition to cognate antigen recognition (signal 1) and costimulation (signal 2), DC-derived soluble factors create a third signal (signal 3) to condition the immune microenvironment. Cytokines and chemokines secreted from DCs are critical for immune polarization and recruitment of accessory leukocyte populations. Priming and activity of anti-tumor T-cell responses ideally occur in Th1-polarized microenvironments, achieved by the presence of cytokines such as type I IFN (IFN-α and IFN-β), IFN-γ, and IL-12p70 as well as the presence of leukocytes such as CD8+ T cells, Th1-polarized CD4+ helper T cells, and NK cells.57) DC-derived IL-12p70 stimulates IFN-γ production in naïve T cells, thereby promoting Th1 responses that overcome immune tolerance against tumor cells. Insug et al. reported protective immunity against intracranial glioma model induced by lysate- or RNA- loaded DCs was strengthened by adding recombinant IL-12,52) although IL-12 boosting may not have an additive effect when using Th1 polarized DCs that secrete abundant IL-12.75) A survival benefit of combining lysate-pulsed DC vaccination and IFN-β gene therapy was demonstrated by Saito et al.93) Similarly, Okada et al. revealed that the sequential intratumoral delivery of an IFN-α encoding adenoviral vector and bone-marrow derived ex vivo cultured syngeneic DCs induced long-term survival and specific cytotoxic T lymphocyte activity in a mouse glioma model.78) Supplementation of Th1 cytokines such as IL-2 and IFN-γ also has an impact on cytotoxic T lymphocyte responses and CD8+ T cell-mediated immunological memory, as these cytokines augmented the therapeutic efficacy of Flt3L-mediated gene therapy in a refractory rat glioma model.75) Thus, cytokine treatment is a powerful tool to induce robust anti-tumor cytotoxic and memory T cell responses.
For the robust expansion of immunogenic DCs, signals 1, 2, and 3 are all necessary. Insufficiency of any of these three signals and/or the presence of immunosuppressive conditioning such as IL-10 or IL-27 leads to the induction of tolerogenic DCs, which express coinhibitory molecules and secrete immunosuppressive cytokines, thus inducing tolerance conditions.55) Care should also be taken for the sequential order of signal input in DCs. Cytokine-mediated maturation of DCs before pulsation by PRR such as TLR could lead to the expansion of undifferentiated T-cell populations and the induction of antigen-specific tolerance.10,55) Matured DCs have a decreased capacity for antigen uptake. Soluble factors secreted by tolerogenic DCs attract Tregs to the tumor microenvironment. These factors include the chemokines CCL17 and CCL22, which bind to CCR4 and CCR8 receptors on Treg cells, respectively.50) Therefore, blockade of CCL17 and CCL22 could be an option to reduce Treg cell migration to the tumor microenvironment, thus sustaining sufficient anti-tumor immunity.
Accordingly, one active mechanism whereby DCs induce tolerance is through the induction of Tregs. Therefore, inhibition of Treg induction and function is a fascinating strategy to boost anti-tumor immunity and consequently Treg biology has been extensively studied. Maes et al. reported that depletion of CD25+ Tregs by anti-CD25 treatment strongly enhanced the efficacy of DC vaccination.70) In contrast, depletion of Tregs using a CD25-targetting strategy interfered with the clonal expansion of tumor antigen specific T lymphocytes and decreased the efficacy of DC-based in situ immunogene therapy in a large glioma model.30) Because CD25 is not a specific marker for Tregs, Foxp3, a more specific marker exclusively expressed by Tregs, could be a target for Treg depletion. However, Foxp3 is intranuclear and therefore cannot be easily depleted using immunoglobulins. Anti-CTLA4 antibody is another option to eliminate Treg cells, as discussed in the previous section. Recently, the local delivery of inhibitors to NF-κB combined with immunogene therapy using Flt3L and TK was reported to induce Foxp3+ Treg suppression and Th1 cytokine production in the tumor microenvironment, resulting in potent anti-tumor T cell responses and prolonged survival.75) Importantly, although Foxp3+ T cells are the most understood subtype of Treg, other subtypes have been identified including Foxp3- IL-10+ T cells (named Tr1 or T regulatory type-1), IL-35+ T, and transforming growth factor-β-producing Th3 cells. The role of DCs in the induction of these regulatory cells has been reviewed elsewhere.63,85)
Myeloid-derived suppressor cells (MDSCs) are another negative regulator of anti-tumor immunity.79) Raychaudhuri et al. reported in 2011 that glioblastoma patients have increased MDSC counts (CD33+ HLA-DR–) in the peripheral blood when compared to normal donors.89) Normal human monocytes acquire MDSC-like properties when cocultured with glioma cells in vitro.92) MDSCs from PBMCs isolated with anti-CD33/CD15-coated beads significantly restored T-cell function.89) These findings indicate a significant role for MDSCs in immune tolerance in patients with glioblastoma. Fujita et al. reported that COX-2 inhibition or anti-Gr1 antibody blocked the development of MDSCs (CD11b+ Gr1+) and the CCL2-mediated accumulation in the tumor microenvironment, which delayed tumor development in a mouse glioma model.37) Of note, accumulating evidence suggest that several chemotherapeutic agents such as 5-fluorouracil,113) docetaxel,61) gemcitabine,40) and sunitinib malate, a receptor tyrosine kinase inhibitor,81) could reverse MDSC-mediated immune suppression in murine tumor models. However, caution is necessary since some chemotherapeutic agents increase the number of MDSCs; i.e., standard doxorubicin and cyclophosphamide chemotherapy was associated with increased numbers of MDSCs in breast cancer patients.34) Other compounds such as polyphenol E95) or all-trans-retinoic acid49) decrease the number of MDSCs in mice and humans, respectively.
Prevention or reversal of immune tolerance in the tumor microenvironment is one of the most powerful approaches to combat HGG. However, the molecular mechanisms that balance immunogenicity/immunosuppression are more complex than expected and remain to be fully elucidated. A further understanding of the mechanisms and development of novel modalities to control immune tolerance is mandatory to improve the therapeutic efficacy of DC-based immunotherapies.
Administration Route
Tumor cell immunogenicity depends upon the microenvironment in which the cells grow. Therefore, the vaccine administration route is of critical importance. In conventional vaccination paradigms, tumor cells are manipulated ex vivo before being reintroduced to the patient. Vaccination is given to patients by intradermal (i.d.), subcutaneous (s.c.), intramuscular (i.m.), or intratumoral (i.t.) injections. An alternative approach is the direct introduction of immunostimulatory molecules such as Flt3L into the tumor microenvironment (in situ), with or without combination with cytotoxic treatments to expose variable tumor antigens, known and unknown, to induce potent tumor-specific immune responses.3,4,7,21,23,29,31,41,58–60,117) Bonnotte et al. compared s.c and i.d. injection in the flank in a colon carcinoma model and showed that most i.d. injections prevented tumor growth against primary and secondary tumor challenges, whereas s.c. injection was associated with progressive tumor growth.14) Kudo-Saito et al. compared s.c and i.t injection in a mouse colon adenocarcinoma model implanted with MC38 cells expressing human carcinoembryonic antigen (CEA). Mice were treated by s.c. priming with replication-competent recombinant vaccinia virus that contained the CEA transgene and transgenes for a triad of T-cell costimulatory molecules (B7–1, ICAM-1, and LFA-3; designated TRICOM) followed by i.t. boosting with replication-defective recombinant fowlpox that contained CEA and TRICOM or each treatment alone. The anti-tumor activity induced by i.t. vaccination was superior to that induced by s.c. vaccination. In addition they demonstrated that an s.c. priming vaccination, followed by i.t. boosting vaccinations was superior to either s.c. or i.t. vaccination alone.62) Mineharu et al. also showed a synergistic effect between s.c. and i.t. injection in rat glioma models. They administered subcutaneous vaccination with Flt3L + IL-6 generated DCs pulsed with tumor cells killed by adenovirus-expressing thymidine kinase (Ad-TK) and gave an intratumoral injection of Ad-TK/Flt3L as in situ immunogene therapy. Combination of in situ immunogene therapy and subcutaneously injected vaccination induced more potent anti-tumor immune responses and better therapeutic efficacy than either treatment alone.74) They speculated that intratumoral injection of immunostimulatory cytokines such as Flt3L prolonged the survival of subcutaneously administered DCs.
The brain is an immune privileged site that lacks resident DCs. Therefore, it is important to determine which lymph nodes is the optimal destination of injected-DCs. Evidence suggested that priming of T cells by DCs within the cervical lymph nodes induced an integrin homing pattern towards intracerebral locations.20)
The advantage of ex vivo cultures of DCs is that a specific subtype of DC can be amplified and tolerogenic DCs can possibly be excluded. Genetic engineering of DCs is also a fascinating option to improve the quality of DC vaccination. Alternatively, the advantage of in situ immunotherapy is that a cocktail of cytokines secreted from DCs in the tumor microenvironment can be fully utilized. Although no clinical trial testing the combination treatment of intratumoral and extratumoral administration of DC-based immunotherapy has been reported, such an approach may have beneficial effects.
Clinical Translation
Clinical trials of DC-based immunotherapy for HGG have been extensively reviewed previously46,111,112,119) and some trials are currently underway.5,8) Although only phase I/II trial results have been reported, substantial progress has been made. Wheeler et al. reported that responders to autologous DC vaccination, who exhibited at least two standard deviations above mean prevaccine IFN-γ production after the third vaccination, had longer survival compared to non-responders. This indicates the importance of genetic or other biomarkers to identify responders prior to the initiation of treatment.116) In this context, Prins et al. stratified the study population in their clinical trial according to the genetic expression signature of tumor cells (mesenchymal, proneural, and proliferative signatures as proposed by Heidi et al.84)). Results from their phase I clinical trial suggested that glioblastoma cells with a mesenchymal gene expression signature, the worst prognostic phenotype,101,102) might be a good candidate for autologous DC vaccination, as these cells induced higher numbers of CD3+ and CD8+ tumor-infiltrating lymphocytes compared with tumor cells with other gene expression signatures. Furthermore, patients with mesenchymal signatures had significantly extended survival compared to a historical control cohort with the same signature, whereas no survival difference was observed in those with a proneural gene expression signature.87) Thus, identification of proper prognostic and predictive biomarkers will help determine which patients are the best candidates for DC-based immunotherapies.
Although still in phase I trial, autologous DC vacci-nation combined with s.c. injection of imiquimod or polyICLC, respective agonists of TLR7 and TLR3, showed a median overall survival of 35.9 months with three long time survivors > 6 years among 15 newly diagnosed glioblastoma patients.87) This result gives us hope that the optimization of a DC-based immunotherapeutic regimen could improve the prognosis of patients with HGG. Additionally, there are several emerging therapeutic strategies against glioma including virotherapy, gene therapy, stem cell-based therapies, and nanotechnology94) as reviewed by Auffinger et al.8) Integration of these multiple therapeutic modalities including conventional chemotherapy80) into DC-based therapy along with the stratification of patients according to molecular and genetic diagnosis will be necessary to gain a significant clinical benefit in new HGG treatments.
In summary, DCs are a heterogeneous cell population that displays a wide range of characteristics and immune regulatory systems that balance a highly complex system of inflammatory and inhibitory immune reactions in the tumor microenvironment. There are a wide variety of regimens to be tested to optimize DC preparations, adjuvant immune stimulation, and immune tolerance inhibition. Preclinical and clinical studies to identify optimal combinations of DC-based immunotherapies with various therapeutic strategies including chemotherapy, gene therapy, and other types of cellular therapies are also warranted. Continual upgrading of treatment regimens as well as increased understanding of the molecular and genetic characteristics of gliomas will help boost the development of clinically relevant DC-based immunotherapies.
Acknowledgments
The work related to the review in MGCs and PRLs laboratories is funded by grants from the NINDS/NIH and the Phase One Foundation.
References
- 1). Akasaki Y, Liu G, Chung NH, Ehtesham M, Black KL, Yu JS: Induction of a CD4+ T regulatory type 1 response by cyclooxygenase-2-overexpressing glioma. J Immunol 173: 4352– 4359, 2004. [DOI] [PubMed] [Google Scholar]
- 2). Akiyama Y, Oshita C, Kume A, Iizuka A, Miyata H, Komiyama M, Ashizawa T, Yagoto M, Abe Y, Mitsuya K, Watanabe R, Sugino T, Yamaguchi K, Nakasu Y: α-type-1 polarized dendritic cell-based vaccination in recurrent high-grade glioma: a phase I clinical trial. BMC Cancer 12: 623, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Ali S, Curtin JF, Zirger JM, Xiong W, King GD, Barcia C, Liu C, Puntel M, Goverdhana S, Lowenstein PR, Castro MG: Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther 10: 1071– 1084, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Ali S, King GD, Curtin JF, Candolfi M, Xiong W, Liu C, Puntel M, Cheng Q, Prieto J, Ribas A, Kupiec-Weglinski J, van Rooijen N, Lassmann H, Lowenstein PR, Castro MG: Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res 65: 7194– 7204, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Ardon H, Van Gool SW, Verschuere T, Maes W, Fieuws S, Sciot R, Wilms G, Demaerel P, Goffin J, Van Calenbergh F, Menten J, Clement P, Debiec-Rychter M, De Vleeschouwer S: Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunol Immunother 61: 2033– 2044, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E: Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med 186: 1177– 1182, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Assi H, Candolfi M, Baker G, Mineharu Y, Lowenstein PR, Castro MG: Gene therapy for brain tumors: basic developments and clinical implementation. Neurosci Lett 527: 71– 77, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Auffinger B, Thaci B, Nigam P, Rincon E, Cheng Y, Lesniak MS: New therapeutic approaches for malignant glioma: in search of the Rosetta stone. F1000 Med Rep 4: 18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Avigan D, Wu Z, Gong J, Joyce R, Levine J, Elias A, Richardson P, Milano J, Kennedy L, Anderson K, Kufe D: Selective in vivo mobilization with granulocyte macrophage colony-stimulating factor (GM-CSF)/granulocyte-CSF as compared to G-CSF alone of dendritic cell progenitors from peripheral blood progenitor cells in patients with advanced breast cancer undergoing autologous transplantation. Clin Cancer Res 5: 2735– 2741, 1999. [PubMed] [Google Scholar]
- 10). Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM: Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood 108: 2655– 2661, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Berhanu A, Huang J, Alber SM, Watkins SC, Storkus WJ: Combinational FLt3 ligand and granulocyte macrophage colony-stimulating factor treatment promotes enhanced tumor infiltration by dendritic cells and antitumor CD8(+) T-cell cross-priming but is ineffective as a therapy. Cancer Res 66: 4895– 4903, 2006. [DOI] [PubMed] [Google Scholar]
- 12). Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM: Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med 196: 1627– 1638, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Bonmort M, Dalod M, Mignot G, Ullrich E, Chaput N, Zitvogel L: Killer dendritic cells: IKDC and the others. Curr Opin Immunol 20: 558– 565, 2008. [DOI] [PubMed] [Google Scholar]
- 14). Bonnotte B, Gough M, Phan V, Ahmed A, Chong H, Martin F, Vile RG: Intradermal injection, as opposed to subcutaneous injection, enhances immunogenicity and suppresses tumorigenicity of tumor cells. Cancer Res 63: 2145– 2149, 2003. [PubMed] [Google Scholar]
- 15). Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A: The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol 5: 1052– 1060, 2004. [DOI] [PubMed] [Google Scholar]
- 16). Boudreau JE, Bonehill A, Thielemans K, Wan Y: Engineering dendritic cells to enhance cancer immunotherapy. Mol Ther 19: 841– 853, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Brasel K, De Smedt T, Smith JL, Maliszewski CR: Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood 96: 3029– 3039, 2000. [PubMed] [Google Scholar]
- 18). Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ: Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 170: 1257– 1266, 2003. [DOI] [PubMed] [Google Scholar]
- 19). Brusa D, Garetto S, Chiorino G, Scatolini M, Migliore E, Camussi G, Matera L: Post-apoptotic tumors are more palatable to dendritic cells and enhance their antigen cross-presentation activity. Vaccine 26: 6422– 6432, 2008. [DOI] [PubMed] [Google Scholar]
- 20). Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, Aurrand-Lions M, Rüegg C, Dietrich PY, Walker PR: Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity 22: 175– 184, 2005. [DOI] [PubMed] [Google Scholar]
- 21). Candolfi M, King GD, Yagiz K, Curtin JF, Mineharu Y, Muhammad AK, Foulad D, Kroeger KM, Barnett N, Josien R, Lowenstein PR, Castro MG: Plasmacytoid dendritic cells in the tumor microenvironment: immune targets for glioma therapeutics. Neoplasia 14: 757– 770, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Capece D, Verzella D, Fischietti M, Zazzeroni F, Alesse E: Targeting costimulatory molecules to improve antitumor immunity. J Biomed Biotech 2012: 926321, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Castro MG, Candolfi M, Kroeger K, King GD, Curtin JF, Yagiz K, Mineharu Y, Assi H, Wibowo M, Ghulam Muhammad AK, Foulad D, Puntel M, Lowenstein PR: Gene therapy and targeted toxins for glioma. Curr Gene Ther 11: 155– 180, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Chaudhry UI, Katz SC, Kingham TP, Pillarisetty VG, Raab JR, Shah AB, DeMatteo RP: In vivo overexpression of Flt3 ligand expands and activates murine spleen natural killer dendritic cells. FASEB J 20: 982– 984, 2006. [DOI] [PubMed] [Google Scholar]
- 25). Chauvin C, Josien R: Dendritic cells as killers: mechanistic aspects and potential roles. J Immunol 181: 11– 16, 2008. [DOI] [PubMed] [Google Scholar]
- 26). Chauvin C, Philippeau JM, Hémont C, Hubert FX, Wittrant Y, Lamoureux F, Trinité B, Heymann D, Rédini F, Josien R: Killer dendritic cells link innate and adaptive immunity against established osteosarcoma in rats. Cancer Res 68: 9433– 9440, 2008. [DOI] [PubMed] [Google Scholar]
- 27). Chen L, Calomeni E, Wen J, Ozato K, Shen R, Gao JX: Natural killer dendritic cells are an intermediate of developing dendritic cells. J Leukoc Biol 81: 1422– 1433, 2007. [DOI] [PubMed] [Google Scholar]
- 28). Cohen PA, Koski GK, Czerniecki BJ, Bunting KD, Fu XY, Wang Z, Zhang WJ, Carter CS, Awad M, Distel CA, Nagem H, Paustian CC, Johnson TD, Tisdale JF, Shu S: STAT3- and STAT5-dependent pathways competitively regulate the pan-differentiation of CD34pos cells into tumor-competent dendritic cells. Blood 112: 1832– 1843, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Curtin JF, King GD, Candolfi M, Greeno RB, Kroeger KM, Lowenstein PR, Castro MG: Combining cytotoxic and immune-mediated gene therapy to treat brain tumors. Curr Top Med Chem 5: 1151– 1170, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Curtin JF, Candolfi M, Fakhouri TM, Liu C, Alden A, Edwards M, Lowenstein PR, Castro MG: Treg depletion inhibits efficacy of cancer immunotherapy: implications for clinical trials. PLoS ONE 3: e1983, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Curtin JF, King GD, Barcia C, Liu C, Hubert FX, Guillonneau C, Josien R, Anegon I, Lowenstein PR, Castro MG: Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol 176: 3566– 3577, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, Edwards MR, Michelsen KS, Kroeger KM, Liu C, Muhammad AK, Clark MC, Arditi M, Comin-Anduix B, Ribas A, Lowenstein PR, Castro MG: HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med 6: e10, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). de Brito C, Tomkowiak M, Ghittoni R, Caux C, Leverrier Y, Marvel J: CpG promotes cross-presentation of dead cell-associated antigens by pre-CD8α+ dendritic cells [corrected]. J Immunol 186: 1503– 1511, 2011. [DOI] [PubMed] [Google Scholar]
- 34). Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ: Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 58: 49– 59, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Driessens G, Kline J, Gajewski TF: Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev 229: 126– 144, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Dunn GP, Dunn IF, Curry WT: Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun 7: 12, 2007. [PMC free article] [PubMed] [Google Scholar]
- 37). Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, Ohlfest JR, Okada H: COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res 71: 2664– 2674, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Fujita M, Zhu X, Ueda R, Sasaki K, Kohanbash G, Kastenhuber ER, McDonald HA, Gibson GA, Watkins SC, Muthuswamy R, Kalinski P, Okada H: Effective immunotherapy against murine gliomas using type 1 polarizing dendritic cells—significant roles of CXCL10. Cancer Res 69: 1587– 1595, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K: Development of monocytes, macrophages, and dendritic cells. Science 327: 656– 661, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Ghansah T, Vohra N, Kinney K, Weber A, Kodumudi K, Springett G, Sarnaik AA, Pilon-Thomas S: Dendritic cell immunotherapy combined with gemcitabine chemotherapy enhances survival in a murine model of pancreatic carcinoma. Cancer Immunol Immunother 62: 1083– 1091, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Ghulam Muhammad AK, Candolfi M, King GD, Yagiz K, Foulad D, Mineharu Y, Kroeger KM, Treuer KA, Nichols WS, Sanderson NS, Yang J, Khayznikov M, Van Rooijen N, Lowenstein PR, Castro MG: Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin Cancer Res 15: 6113– 6127, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Grauer OM, Molling JW, Bennink E, Toonen LW, Sutmuller RP, Nierkens S, Adema GJ: TLR ligands in the local treatment of established intracerebral murine gliomas. J Immunol 181: 6720– 6729, 2008. [DOI] [PubMed] [Google Scholar]
- 43). Greenwald RJ, Freeman GJ, Sharpe AH: The B7 family revisited. Annu Rev Immunol 23: 515– 548, 2005. [DOI] [PubMed] [Google Scholar]
- 44). Gustafsson K, Junevik K, Werlenius O, Holmgren S, Karlsson-Parra A, Andersson PO: Tumour-loaded α-type 1-polarized dendritic cells from patients with chronic lymphocytic leukaemia produce a superior NK-, NKT- and CD8+ T cell-attracting chemokine profile. Scand J Immunol 74: 318– 326, 2011. [DOI] [PubMed] [Google Scholar]
- 45). Hashiba T, Izumoto S, Kagawa N, Suzuki T, Hashimoto N, Maruno M, Yoshimine T: Expression of WT1 protein and correlation with cellular proliferation in glial tumors. Neurol Med Chir (Tokyo) 47: 165– 170; discussion 170, 2007. [DOI] [PubMed] [Google Scholar]
- 46). Heimberger AB, Sampson JH: Immunotherapy coming of age: what will it take to make it standard of care for glioblastoma? Neuro-oncology 13: 3– 13, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ: Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711– 723, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Hong B, Ren W, Song XT, Evel-Kabler K, Chen SY, Huang XF: Human suppressor of cytokine signaling 1 controls immunostimulatory activity of monocyte-derived dendritic cells. Cancer Res 69: 8076– 8084, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D: Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother 62: 909– 918, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D'Ambrosio D: Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med 194: 847– 853, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM: Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 176: 1693– 1702, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52). Insug O, Ku G, Ertl HC, Blaszczyk-Thurin M: A dendritic cell vaccine induces protective immunity to intracranial growth of glioma. Anticancer Res 22: 613– 621, 2002. [PubMed] [Google Scholar]
- 53). Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK: Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19: 47– 57, 2003. [DOI] [PubMed] [Google Scholar]
- 54). Jenkins MK, Schwartz RH: Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med 165: 302– 319, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55). Joffre O, Nolte MA, Spörri R, Reis e Sousa C: Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev 227: 234– 247, 2009. [DOI] [PubMed] [Google Scholar]
- 56). Johnson LA, Sampson JH: Immunotherapy approaches for malignant glioma from 2007 to 2009. Curr Neurol Neurosci Rep 10: 259– 266, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57). Kalinski P, Nakamura Y, Watchmaker P, Giermasz A, Muthuswamy R, Mailliard RB: Helper roles of NK and CD8+ T cells in the induction of tumor immunity. Polarized dendritic cells as cancer vaccines. Immunol Res 36: 137– 146, 2006. [DOI] [PubMed] [Google Scholar]
- 58). King GD, Kroeger KM, Bresee CJ, Candolfi M, Liu C, Manalo CM, Muhammad AK, Pechnick RN, Lowenstein PR, Castro MG: Flt3L in combination with HSV1-TK-mediated gene therapy reverses brain tumor-induced behavioral deficits. Mol Ther 16: 682– 690, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59). King GD, Muhammad AK, Curtin JF, Barcia C, Puntel M, Liu C, Honig SB, Candolfi M, Mondkar S, Lowenstein PR, Castro MG: Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro-oncology 10: 19– 31, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60). King GD, Muhammad AK, Larocque D, Kelson KR, Xiong W, Liu C, Sanderson NS, Kroeger KM, Castro MG, Lowenstein PR: Combined Flt3L/TK gene therapy induces immunological surveillance which mediates an immune response against a surrogate brain tumor neoantigen. Mol Ther 19: 1793– 1801, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61). Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY: A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res 16: 4583– 4594, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62). Kudo-Saito C, Schlom J, Hodge JW: Intratumoral vaccination and diversified subcutaneous/intratumoral vaccination with recombinant poxviruses encoding a tumor antigen and multiple costimulatory molecules. Clin Cancer Res 10: 1090– 1099, 2004. [DOI] [PubMed] [Google Scholar]
- 63). Kushwah R, Hu J: Role of dendritic cells in the induction of regulatory T cells. Cell Biosci 1: 20, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64). Lafferty KJ, Warren HS, Woolnough JA: A mediator acting as a costimulator for the development of cytotoxic responses in vitro. Adv Exp Med Biol 114: 497– 501, 1979. [DOI] [PubMed] [Google Scholar]
- 65). Lai A, Tran A, Nghiemphu PL, Pope WB, Solis OE, Selch M, Filka E, Yong WH, Mischel PS, Liau LM, Phuphanich S, Black K, Peak S, Green RM, Spier CE, Kolevska T, Polikoff J, Fehrenbacher L, Elashoff R, Cloughesy T: Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 29: 142– 148, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66). Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A: Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science 289: 2350– 2354, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67). Li Y, Wang LX, Yang G, Hao F, Urba WJ, Hu HM: Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res 68: 6889– 6895, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68). Lim SN, Kuhn S, Hyde E, Ronchese F: Combined TLR stimulation with Pam3Cys and Poly I: C enhances Flt3-ligand dendritic cell activation for tumor immunotherapy. J Immunother 35: 670– 679, 2012. [DOI] [PubMed] [Google Scholar]
- 69). Lou Y, Liu C, Kim GJ, Liu YJ, Hwu P, Wang G: Plasmacytoid dendritic cells synergize with myeloid dendritic cells in the induction of antigen-specific antitumor immune responses. J Immunol 178: 1534– 1541, 2007. [DOI] [PubMed] [Google Scholar]
- 70). Maes W, Rosas GG, Verbinnen B, Boon L, De Vleeschouwer S, Ceuppens JL, Van Gool SW: DC vaccination with anti-CD25 treatment leads to long-term immunity against experimental glioma. Neuro-oncology 11: 529– 542, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71). Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P: alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res 64: 5934– 5937, 2004. [DOI] [PubMed] [Google Scholar]
- 72). Mao CP, Wu TC: Inhibitory RNA molecules in immunotherapy for cancer. Methods Mol Biol 623: 325– 339, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73). McDonnell AM, Prosser AC, van Bruggen I, Robinson BW, Currie AJ: CD8alpha+ DC are not the sole subset cross-presenting cell-associated tumor antigens from a solid tumor. Eur J Immunol 40: 1617– 1627, 2010. [DOI] [PubMed] [Google Scholar]
- 74). Mineharu Y, King GD, Muhammad AK, Bannykh S, Kroeger KM, Liu C, Lowenstein PR, Castro MG: Engineering the brain tumor microenvironment enhances the efficacy of dendritic cell vaccination: implications for clinical trial design. Clin Cancer Res 17: 4705– 4718, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75). Mineharu Y, Muhammad AK, Yagiz K, Candolfi M, Kroeger KM, Xiong W, Puntel M, Liu C, Levy E, Lugo C, Kocharian A, Allison JP, Curran MA, Lowenstein PR, Castro MG: Gene therapy-mediated reprogramming tumor infiltrating T cells using IL-2 and inhibiting NF-κB signaling improves the efficacy of immunotherapy in a brain cancer model. Neurotherapeutics 9: 827– 843, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76). Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O'Keeffe M, Shao QX, Chen WF, Villadangos JA, Shortman K, Wu L: Cutting edge: generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol 174: 6592– 6597, 2005. [DOI] [PubMed] [Google Scholar]
- 77). Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A: Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol 6: 769– 776, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78). Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, Zeh H, Holtzman MP, Reinhart TA, Whiteside TL, Butterfield LH, Hamilton RL, Potter DM, Pollack IF, Salazar AM, Lieberman FS: Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol 29: 330– 336, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79). Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK: Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol 22: 275– 281, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80). Osuka S, Takano S, Watanabe S, Ishikawa E, Yamamoto T, Matsumura A: Valproic acid inhibits angiogenesis in vitro and glioma angiogenesis in vivo in the brain. Neurol Med Chir (Tokyo) 52: 186– 193, 2012. [DOI] [PubMed] [Google Scholar]
- 81). Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan PY, Chen SH: The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res 69: 2514– 2522, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82). Parajuli P, Mathupala S, Sloan AE: Systematic comparison of dendritic cell-based immunotherapeutic strategies for malignant gliomas: in vitro induction of cytolytic and natural killer-like T cells. Neurosurgery 55: 1194– 1204, 2004. [DOI] [PubMed] [Google Scholar]
- 83). Parajuli P, Mosley RL, Pisarev V, Chavez J, Ulrich A, Varney M, Singh RK, Talmadge JE: Flt3 ligand and granulocyte-macrophage colony-stimulating factor preferentially expand and stimulate different dendritic and T-cell subsets. Exp Hematol 29: 1185– 1193, 2001. [DOI] [PubMed] [Google Scholar]
- 84). Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K: Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9: 157– 173, 2006. [DOI] [PubMed] [Google Scholar]
- 85). Pletinckx K, Döhler A, Pavlovic V, Lutz MB: Role of dendritic cell maturity/costimulation for generation, homeostasis, and suppressive activity of regulatory T cells. Front Immunol 2: 39, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86). Pooley JL, Heath WR, Shortman K: Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8- dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J Immunol 166: 5327– 5330, 2001. [DOI] [PubMed] [Google Scholar]
- 87). Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, Nelson SF, Liau LM: Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res 17: 1603– 1615, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88). Puntel M, Muhammad AK, Candolfi M, Salem A, Yagiz K, Farrokhi C, Kroeger KM, Xiong W, Curtin JF, Liu C, Bondale NS, Lerner J, Pechnick RN, Palmer D, Ng P, Lowenstein PR, Castro MG: A novel bicistronic high-capacity gutless adenovirus vector that drives constitutive expression of herpes simplex virus type 1 thymidine kinase and tet-inducible expression of Flt3L for glioma therapeutics. J Virol 84: 6007– 6017, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89). Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, Garcia J, Vogelbaum MA, Finke J: Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro-oncology 13: 591– 599, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90). Rock KL, Clark K: Analysis of the role of MHC class II presentation in the stimulation of cytotoxic T lymphocytes by antigens targeted into the exogenous antigen-MHC class I presentation pathway. J Immunol 156: 3721– 3726, 1996. [PubMed] [Google Scholar]
- 91). Rock KL, Gamble S, Rothstein L: Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science 249: 918– 921, 1990. [DOI] [PubMed] [Google Scholar]
- 92). Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, Lin Y, Dietz AB, Forsyth PA, Yong VW, Parney IF: Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro-oncology 12: 351– 365, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93). Saito R, Mizuno M, Nakahara N, Tsuno T, Kumabe T, Yoshimoto T, Yoshida J: Vaccination with tumor cell lysate-pulsed dendritic cells augments the effect of IFN-beta gene therapy for malignant glioma in an experimental mouse intracranial glioma. Int J Cancer 111: 777– 782, 2004. [DOI] [PubMed] [Google Scholar]
- 94). Saito R, Tominaga T: Convection-enhanced delivery: from mechanisms to clinical drug delivery for diseases of the central nervous system. Neurol Med Chir (Tokyo) 52: 531– 538, 2012. [DOI] [PubMed] [Google Scholar]
- 95). Santilli G, Piotrowska I, Cantilena S, Chayka O, D'Alicarnasso M, Morgenstern DA, Himoudi N, Pearson K, Anderson J, Thrasher AJ, Sala A: Polyphenol E enhances the antitumor immune response in neuroblastoma by inactivating myeloid suppressor cells. Clin Cancer Res 19: 1116– 1125, 2013. [DOI] [PubMed] [Google Scholar]
- 96). Scheffer SR, Nave H, Korangy F, Schlote K, Pabst R, Jaffee EM, Manns MP, Greten TF: Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int J Cancer 103: 205– 211, 2003. [DOI] [PubMed] [Google Scholar]
- 97). Segura E, Durand M, Amigorena S: Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J Exp Med 210: 1035– 1047, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98). Shen L, Evel-Kabler K, Strube R, Chen SY: Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol 22: 1546– 1553, 2004. [DOI] [PubMed] [Google Scholar]
- 99). Shi L, Luo K, Xia D, Chen T, Chen G, Jiang Y, Li N, Cao X: DIgR2, dendritic cell-derived immunoglobulin receptor 2, is one representative of a family of IgSF inhibitory receptors and mediates negative regulation of dendritic cell-initiated antigen-specific T-cell responses. Blood 108: 2678– 2686, 2006. [DOI] [PubMed] [Google Scholar]
- 100). Shimizu K, Fujii S: DC therapy induces long-term NK reactivity to tumors via host DC. Eur J Immunol 39: 457– 468, 2009. [DOI] [PubMed] [Google Scholar]
- 101). Shirahata M, Iwao-Koizumi K, Saito S, Ueno N, Oda M, Hashimoto N, Takahashi JA, Kato K: Gene expression-based molecular diagnostic system for malignant gliomas is superior to histological diagnosis. Clin Cancer Res 13: 7341– 7356, 2007. [DOI] [PubMed] [Google Scholar]
- 102). Shirahata M, Oba S, Iwao-Koizumi K, Saito S, Ueno N, Oda M, Hashimoto N, Ishii S, Takahashi JA, Kato K: Using gene expression profiling to identify a prognostic molecular spectrum in gliomas. Cancer Sci 100: 165– 172, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103). Shortman K, Heath WR: The CD8+ dendritic cell subset. Immunol Rev 234: 18– 31, 2010. [DOI] [PubMed] [Google Scholar]
- 104). Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM: Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 24: 3089– 3094, 2006. [DOI] [PubMed] [Google Scholar]
- 105). Song XT, Evel-Kabler K, Shen L, Rollins L, Huang XF, Chen SY: A20 is an antigen presentation attenuator, and its inhibition overcomes regulatory T cell-mediated suppression. Nat Med 14: 258– 265, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106). Stallwood Y, Briend E, Ray KM, Ward GA, Smith BJ, Nye E, Champion BR, McKenzie GJ: Small interfering RNA-mediated knockdown of notch ligands in primary CD4+ T cells and dendritic cells enhances cytokine production. J Immunol 177: 885– 895, 2006. [DOI] [PubMed] [Google Scholar]
- 107). Stone GW, Barzee S, Snarsky V, Santucci C, Tran B, Langer R, Zugates GT, Anderson DG, Kornbluth RS: Nanoparticle-delivered multimeric soluble CD40L DNA combined with Toll-Like Receptor agonists as a treatment for melanoma. PLoS ONE 4: e7334, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108). Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987– 996, 2005. [DOI] [PubMed] [Google Scholar]
- 109). Thorburn J, Horita H, Redzic J, Hansen K, Frankel AE, Thorburn A: Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ 16: 175– 183, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110). Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M: Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443– 2454, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111). Van Gool S, Maes W, Ardon H, Verschuere T, Van Cauter S, De Vleeschouwer S: Dendritic cell therapy of high-grade gliomas. Brain Pathol 19: 694– 712, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112). Vauleon E, Avril T, Collet B, Mosser J, Quillien V: Overview of cellular immunotherapy for patients with glioblastoma. Clin Dev Immunol 2010: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113). Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F: 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 70: 3052– 3061, 2010. [DOI] [PubMed] [Google Scholar]
- 114). Weigel BJ, Nath N, Taylor PA, Panoskaltsis-Mortari A, Chen W, Krieg AM, Brasel K, Blazar BR: Comparative analysis of murine marrow-derived dendritic cells generated by Flt3L or GM-CSF/IL-4 and matured with immune stimulatory agents on the in vivo induction of antileukemia responses. Blood 100: 4169– 4176, 2002. [DOI] [PubMed] [Google Scholar]
- 115). Wen PY, Kesari S: Malignant gliomas in adults. N Engl J Med 359: 492– 507, 2008. [DOI] [PubMed] [Google Scholar]
- 116). Wheeler CJ, Black KL, Liu G, Mazer M, Zhang XX, Pepkowitz S, Goldfinger D, Ng H, Irvin D, Yu JS: Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res 68: 5955– 5964, 2008. [DOI] [PubMed] [Google Scholar]
- 117). Xiong W, Candolfi M, Liu C, Muhammad AK, Yagiz K, Puntel M, Moore PF, Avalos J, Young JD, Khan D, Donelson R, Pluhar GE, Ohlfest JR, Wawrowsky K, Lowenstein PR, Castro MG: Human Flt3L generates dendritic cells from canine peripheral blood precursors: implications for a dog glioma clinical trial. PLoS ONE 5: e11074, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118). Xu S, Koski GK, Faries M, Bedrosian I, Mick R, Maeurer M, Cheever MA, Cohen PA, Czerniecki BJ: Rapid high efficiency sensitization of CD8+ T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. J Immunol 171: 2251– 2261, 2003. [DOI] [PubMed] [Google Scholar]
- 119). Xu X, Stockhammer F, Schmitt M: Cellular-based immunotherapies for patients with glioblastoma multiforme. Clin Dev Immunol 2012: 764213, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120). Zang X, Allison JP: The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res 13: 5271– 5279, 2007. [DOI] [PubMed] [Google Scholar]
- 121). Zhan Y, Vega-Ramos J, Carrington EM, Villadangos JA, Lew AM, Xu Y: The inflammatory cytokine, GM-CSF, alters the developmental outcome of murine dendritic cells. Eur J Immunol 42: 2889– 2900, 2012. [DOI] [PubMed] [Google Scholar]
- 122). Zhu XJ, Yang ZF, Chen Y, Wang J, Rosmarin AG: PU.1 is essential for CD11c expression in CD8(+)/CD8(−) lymphoid and monocyte-derived dendritic cells during GM-CSF or FLT3L-induced differentiation. PLoS ONE 7: e52141, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]