Abstract
Dual pH-multichannel intraluminal impedance (pH-MII) is a sensitive tool for evaluating overall gastroesophageal reflux disease, and particularly for permitting detection of nonacid reflux events. pH-MII technology is especially useful in the postprandial period or at other times when gastric contents are nonacidic. pH-MII was recently recognized by the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition as being superior to pH monitoring alone for evaluation of the temporal relation between symptoms and gastroesophageal reflux. In children, pHMII is useful to correlate symptoms with reflux (particularly nonacid reflux), to quantify reflux during tube feedings and the postprandial period, and to assess efficacy of antireflux therapy. This clinical review is simply an evidence-based overview addressing the indications, limitations, and recommended protocol for the clinical use of pH-MII in children.
Keywords: acid gastroesophageal reflux, bolus contact time, gastroesophageal reflux, gastroesophageal reflux disease, multichannel intraluminal impedance, nonacid gastroesophageal reflux
Gastroesophageal reflux (GER), defined as the retrograde passage of gastric contents into the esophagus, is a normal physiological phenomenon that occurs in healthy children several times per day after meals and lasts <3 minutes (1). Simple physiological GER becomes a disease, gastroesophageal reflux disease (GERD), when it causes troublesome symptoms and/or complications (2).
In otherwise healthy infants, the prevalence of regurgitation has been reported to be 50% at age 0 to 3 months, peaking to 67% at 4 months, and then declining to <5% by age 10 to 12 months (3,4). Van Howe and Storms (5) reported that symptoms of GER, as measured by the Infant Gastroesophageal Reflux Questionnaire Revised, decrease with age in the first 6 months of life in otherwise healthy infants.
Although most infants outgrow symptoms of GER, for some older children typical GER symptoms such as heartburn, epigastric pain, and regurgitation persist at 1.8% to 7.2% and 2.3% at ages 3 to 9 years and with a prevalence of 5.2%, 5.0%, and 8.2 at ages 10 to 17 years, respectively (6). Parents’ perception of their older children's GER symptoms can have the prevalence as high as 28% when less-specific symptoms such as nonfocal abdominal pain are taken into account (6). This wider range in older children is likely attributed to the changing repertoire of GER symptoms as compared with infants (7). Recent studies clearly showed that between 2000 and 2005, the annual incidence of GERD diagnosis among infants (age 1 year or younger) more than tripled (from 3.4% to 12.3%) and increased by 30% to 50% in other age groups (8).
Children with GERD may present with symptoms that can be minor, such as heartburn or regurgitation, to more complicated diseases, such as erosive esophagitis, esophageal stricture, or Barrett esophagus (9,10). In a single-center retrospective cross-sectional study, the frequency of erosive esophagitis was found to be slightly higher in male children and increase with age with no significant variations according to race or ethnicity. Hiatal hernia is the only endoscopic observation that predicts erosive esophagitis (11).
Severe GERD during childhood has a few well-known risk factors that include neurological disorders such as spastic quad-riplegia and cerebral palsy (12,13), congenital malformation such as esophageal atresia and tracheoesophageal fistula (14,15), chronic lung disease (16,17), and extraesophageal disease (18).
GERD has been implicated in several “extraesophageal” complications such as feeding disorders and respiratory disorders that include asthma, chronic cough, chronic hoarseness, other laryngeal disorders, and recurrent pneumonia (19–22).
Most GER episodes occur during transient relaxations of the lower esophageal sphincter (LES) with no associated swallowing (23–26). Those reflux episodes become pathological when protective mechanisms are altered: insufficient clearance and buffering of refluxate, delayed gastric emptying, abnormalities in epithelial restitution and repair, and decreased neural protective reflexes of the aerodigestive tract (27).
PH MONITORING
Intraluminal esophageal pH monitoring measures the frequency and duration of acid esophageal reflux episodes. By convention, a drop in intraesophageal pH <4.0 is considered an acid reflux episode. This cutoff was initially chosen because heartburn induced by acid perfusion of the esophagus in adults generally occurs at pH <4.0 (28). pH monitoring provides a quantitative measure of esophageal acid exposure with established normal ranges: a reflux index (RI) >7% is considered abnormal, an RI <3% is considered normal, and an RI between 3% and 7% is indeterminate (29). Because the severity of pathological acid reflux does not correlate consistently with symptom severity, it is likely that a continuum exists such that normal ranges should be regarded as guidelines for interpretation rather than absolutes.
The sensitivity and specificity of pH monitoring are not well established. In fact, pH monitoring has significant limitations because of its inability to detect nonacidic retrograde bolus movement in the esophagus, and in particular in infants who are frequently fed milk and/or milk-based formulas. Mitchell et al (30) found that the majority of reflux episodes went undetected by standard pH probe monitoring even after adjustments in the criteria for defining acid reflux episodes were made.
Gastric pH
Grant and Cochran (31) assessed only preterm milk-fed infants and found a median gastric pH of <4 for 8.2% of the time (range 2.0%–41.2%). Even when standard-fed infants were examined, Washington and colleagues found that a gastric pH was <4 for approximately 40% of the time (32). On the basis of these studies, gastric pH is >4 for as long as 92% of the time; if reflux occurred during this time, the pH probe would fail to detect it.
Proportion of Acid and Nonacid Reflux
Other studies showed that the occurrence of nonacid (pH ≥ 7) and/or weakly acidic reflux (4 ≤ pH <7) varies between 45% and 90% in children and infants (33–36). In a study of 28 children (mean age 6.5±5.6 years) with respiratory symptoms, Rosen and Nurko (34) found that 45% of GER events were nonacid. Mousa and colleagues reported that in infants with apparent life-threatening events or apnea 48% of total GER events were nonacid (35). In an evaluation of preterm infants with apnea, Magista et al (36) found that 76% of reflux events were weakly acidic (4≤ pH <7). In infants with respiratory symptoms or recurrent regurgitation, Wenzl et al (33) reported that of GER episodes were nonacid (pH ≥4).
Significance of Nonacid Reflux
In light of recent data suggesting an association between nonacidic reflux and respiratory symptoms (33–35), the inability of pH monitoring to detect nonacidic reflux could prove detrimental in clinical diagnosis. For example, in a study of GER and respiratory disorders in infants, Wenzl et al (33) found that 78% of the GER episodes that were temporally associated with breathing irregularities were nonacid (pH ≥4). Moreover, in a study of children (mean age 6.5 years) with respiratory symptoms such as cough, tachypnea, and wheezing, Rosen and Nurko (34) found that respiratory symptoms occurred more frequently when GER was nonacidic (pH ≥4). Therefore, the capacity to test for a potential correlation between GER and extraesophageal symptoms using esophageal pH monitoring remains questionable.
Impedance Technique
Multichannel intraluminal impedance (MII) detects GER episodes based on changes in electrical resistance to the flow of an electrical current between 2 electrodes placed on the MII probe, when a liquid, semisolid, or gas bolus moves between them. Combined esophageal pH monitoring and impedance (pH-MII) offer several advantages over a standard pH probe. First, it is able to detect reflux regardless of its pH value. Second, it is able to distinguish swallows (antegrade flow) from authentic GER (retrograde flow). Third, it can detect accurately the height of the refluxate. Fourth, it is able to determine whether the refluxate is liquid, gas, or mixed (both liquid and gas). Fifth, it can still measure symptom association with GER even while the patient is taking acid-suppression medications. This nonacidic reflux following acid-suppression therapy had previously gone undetected by standard pH probe analysis (37).
Protocols for Performing pH-MII in Children
MII-pH can be performed via an ambulatory or a stationary method. The ambulatory system provides a more physiological environment because it allows the patient to move about normally and to perform routine activities, unimpeded by bulky instrumentation; this permits replication of symptoms in patients. For example, “exercise-induced” symptoms can be detected while the patient jogs or works out in the gymnasium. Patients usually fast for a minimum of 3 hours before testing to avoid vomiting and aspiration during catheter placement
Catheters
In general, various “age-appropriate” catheters are used for impedance studies. The 3 that are most commonly used include the infant (0–2 years), the pediatric (age 2–10 years), and the adult (>10 years old) catheters. Each catheter has a diameter of 2.13 mm (6.4 French). There are 7 impedance sensors positioned along the length of each catheter. Each sensor is in the form of a 4-mm cylindrical ring. The segment between each pair of sensors, known as the impedance sensor spacing, corresponds to 1 recording impedance channel. The presence of 7 impedance sensors results in 6 corresponding impedance channels. The pH electrode is positioned in the center of either the most distal impedance sensor spacing (infant and pediatric catheters) or the impedance sensor spacing that is immediately proximal to it (adult catheters). There are also catheters with 2 pH-measuring points.
Several types of pH electrodes are available: antimony, ion-sensitive field effect transistor (ISFET), and glass electrodes. In vitro, antimony and glass pH electrodes are affected by different buffer components and temperature, respectively. In vivo, significant higher acid exposure times are obtained with glass electrodes compared with antimony and ISFET pH electrodes. ISFET electrodes produce stable in vitro measurements and result in the most accurate in vivo measurements of acid exposure time (38).
Diet
During testing, patients should be fed their regular diet every 3 to 4 hours, although acidic foods and drinks need to be avoided. Because of its ability to detect nonacid reflux, pH-MII testing can be performed in infants and children who require more frequent feedings or in patients who require continuous tube feedings. Patients should avoid very hot or very cold beverages or foods, acidic juices, and carbonated beverages. Extreme temperatures may interfere with the sensitivity of the pH probe (eg, hot increases and cold decreases sensitivity) (29). Even though MII can differentiate swallows from GER, mealtimes are typically excluded from the analysis except when patients are being evaluated for feeding-related symptoms. Unlike with esophageal pH monitoring, patients can be fed either continuously (to stomach or jejunum) or with boluses during pH-MII testing. Because these feeds do not generate swallows and because nothing is passing antegrade over the catheter, interpretation of impedance waves during feed periods is feasible and is often helpful to assess a possible relation between symptoms and feeds (39).
Position of the Probe
The pH-MII catheter is typically passed transnasally into the esophagus following a 3-hour fast. For proper/correct positioning, different methods can be used. Esophageal manometry, for example, has been used to locate the LES and determine its distance from the nares (40). There are other methods for proper positioning of the probe such as the Strobel formula (41) for infants younger than 1 year (length from nares to LES in centimeters = 5 + 0.252 [height]). In adults, the position of the probe is most accurately determined using manometry (42) or endoscopy (43).
The accuracy of results obtained depends on proper positioning of the pH electrode. We recommend a method that is consistent with the experience and expertise of the members of each center and the availability of special equipment (eg, fluoroscopy, manometry). Proper positioning of the catheter must be checked by radioscopy during a full respiratory cycle (because the tip of the electrode moves during inspiration and expiration).
Use of Acid-suppression Medications
The question of whether the patient should be studied on or off antireflux medications depends on the indications for the study itself. The study may be performed on acid-blocking medications, particularly in intractable patients, and when symptom association with nonacid reflux is being tested. When pharmacological intervention is being considered, the patient must be tested while off antireflux medication. Typically, the wash out period for antireflux medications is 7 days for proton pump inhibitors, 3 days for histamine-receptor antagonists, and 2 days for prokinetics (44,45).
Recent studies in adults are conflicting regarding the clinical value of performing pH-MII studies on or off acid suppression therapy. Zerbib et al (46) found that pH-MII detected symptom-GER association by 10% over a standard pH probe in untreated patients and 33% over a standard pH probe during acid-suppression therapy. Hemmink et al (47) used pH-MII to study 30 adult patients (both on and off proton pump inhibitor therapy) to determine whether there was concordance between the studies with respect to symptoms-association probability (SAP) significance. The authors found concordance in 14 patients; 7 patients had negative SAPs both on and off therapy and 7 patients had positive SAPs both on and off therapy. Eight patients had discordance between the 2 studies; 5 patients had a positive SAP off therapy (but not on therapy) and 3 patients had a positive SAP on therapy (but not off therapy). Because there was a higher rate of SAP positivity off therapy, the authors argue that studies should be performed off therapy. There are no pediatric studies that address this issue and the practice varies based on the institution.
Use of Nasogastric Tubes During the Procedure
If the study needs to be done with a nasogastric (NG) tube in place, then one should be mindful of the fact that the number of reflux episodes may be artificially inflated because of stenting of the LES. When an NG tube passes through the LES, postprandial reflux increases by nearly 70% compared with studies wherein the tube is in a nasoesophageal position (48,49). These results were obtained in children with neurological impairments; those who were fed with NG tubes experienced more postprandial reflux than patients who were fed orally. Moreover, more than half of the reflux events in NG-fed patients were nonacidic and would therefore have gone undetected had a pH probe alone been used (50).
Pacifiers
Pacifiers stimulate the flow of saliva and the downward contractions of the esophagus, thus reducing the time it takes to move gastric fluid back to where it belongs. There is, however, no statistically significant evidence that supports the use of pacifiers for reducing reflux (51). One of the complications of sucking a pacifier during pH-MII testing is that the sucking results in a rapid succession of swallows that are visible in all of the impedance channels. This makes it extremely difficult to detect reflux episodes and can result in an autoscan that falsely detects reflux. As a result, pacifier use should be limited or, if that is not possible, the tracing needs to be manually reviewed to ensure that false-positive reflux events are deleted.
Gum Chewing
There is new research that shows that postmeal gum chewing reduces acid in the esophagus and quells heartburn symptoms in people with chronic reflux problems. Gum stimulates saliva production, which theoretically works to neutralize acid remaining in the larynx and esophagus (52). As with pacifier sucking, the use of gum should be limited because of the increased number of swallows that it produces.
CPAP
It has been shown that nasal continuous positive airway pressure (nasal CPAP) significantly reduces reflux. Studies in adults with obstructive sleep apnea syndrome and reflux have demonstrated a significant decrease in reflux episodes during treatment with nasal CPAP (53,54). Therefore, children and infants who depend on nasal CPAP are not candidates for assessment by pHMII monitoring.
DEFINITIONS USED IN IMPEDANCE TESTING
Reflux Episodes
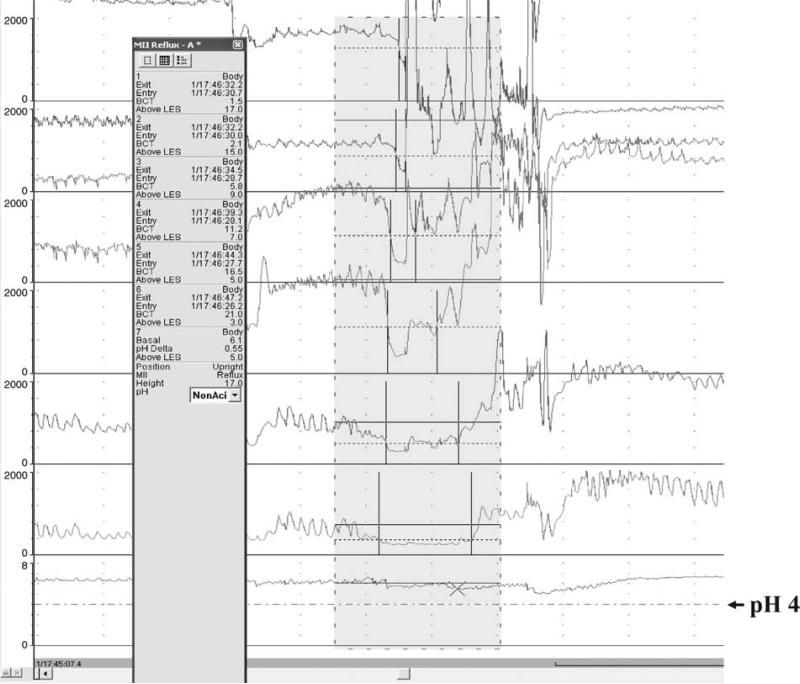
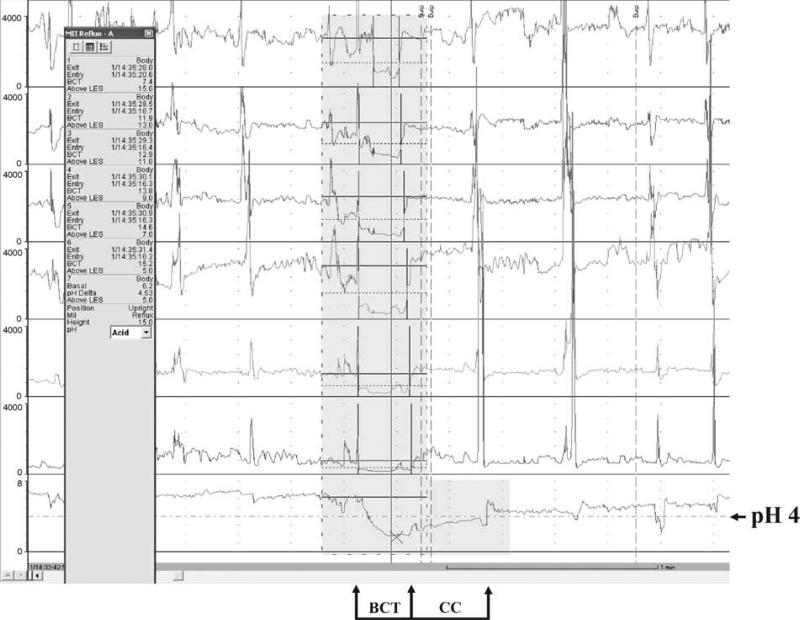
Impedance is measured in ohms. By expert opinion, a reflux episode by impedance is defined as fall in intraluminal impedance of ≤50% of baseline that progresses retrograde across 2 or more of the distal-most channels. An episode is considered acid (AGER) when the esophageal pH decreases and remains ≥4 for at least 5 seconds. An episode is considered nonacid (NAGER) when the pH increases, remains unchanged, or decreases by <1 pH unit while remaining ≥4 (Figs. 1 and 2) (55).
FIGURE 1.
Nonacid gastroesophageal reflux event.
FIGURE 2.
Acid gastroesophageal reflux event.
Adult data have suggested that the category of nonacid reflux be divided into weakly acidic reflux (4≤ pH <7) and nonacid reflux (pH ≥7). Currently, there are no outcome studies to determine whether there is any clinical significance in subdividing reflux into 3 categories. Therefore, pH-MII allows the detection of 3 main reflux events: acid reflux detected by both pH electrode and impedance sensors, nonacid reflux detected only by the impedance sensors, and pH-only episodes that are detected only by the pH electrode and not by the impedance sensors.
Characteristics of Nonacid Reflux
The characteristics of nonacidic reflux can differ considerably throughout a 24-hour period. This variation is largely influenced by feeding and the age of the subject. Sifrim et al (56) evaluated postprandial reflux in 30 healthy adult controls and 28 patients with GERD. The investigators reported that the rate of nonacid reflux was similar in patients with GERD (median of 11 [range 8–16]/24 hours) and controls (median of 13 [range 8.5–19]/24 hours) (56). In addition, nonacid reflux comprised one third of all reflux events in both groups and occurred frequently early after a meal (within approximately 30 minutes) (56).
Nonacid reflux is more likely to occur during feeding and during the first postprandial hour. Condino et al (57) found that in infants (mean age 7 months, range 2–11 months) with GER symptoms, the proportion of nonacid reflux decreased from 61% during the first postprandial hour to 39% during the second post-prandial hour and finally to 29% after 2 hours postprandial. Moreover, nonacid reflux events occurred with greater frequency in younger infants. Fifty-four percent of GER episodes were nonacid in infants ages 2 to 3 months, and 45% were nonacid in infants ages 8 to 11 months.
Regarding the proximal migration of nonacid reflux, available data show that the height of refluxate varies considerably with the age of the patient. In adult patients, Shay et al (58) reported that among 60 healthy adults, 24% of nonacid GER reached the proximal esophagus compared with 34% of acid GER. Conversely, Rosen and Nurko (34) found that in children (mean age at time of study 6.5 years) with persistent respiratory symptoms, 75% of nonacidic GER reach the proximal esophagus compared with only 8.8% of acid GER. In a study of 16 neurologically impaired children (median age 23 months) (9 NG-fed, 7 fed orally) using 12-hour pHMII recordings, Del Buono et al (50) found that 52.4% of those GER episodes that reached the proximal esophagus were nonacid reflux episodes. In a study of infants (median age 4 months, range 1–19 months), Mousa et al (35) found that acid reflux reached the hypopharynx significantly more often (P = 0.018) than nonacid reflux.
With increasing numbers of studies demonstrating a possible association between nonacid GER and respiratory disorders (33–35,59), the need to develop and implement a treatment strategy is critical.
pH-only Acid Gastroesophageal Reflux Events
Use of pH-MII reveals a unique class of acid reflux event wherein drops in intraluminal pH in the distal esophagus do not correspond to coordinate drops in impedance. These “pH-only” events (POEs) occur regularly in infants and have been found to contribute significantly to total esophageal acid exposure due to reflux (60–62).
Several mechanisms for POEs have been suggested. In the first, it has been suggested that some POEs may be the result of short-column acid reflux episodes that ascend only as far as the distal-most impedance channel (channel 6—the location of the pH electrode in the infant and pediatric catheter) or perhaps even midway into the next channel (channel 5—the location of the pH electrode for the adult catheters) (62). In either case, the extent of the proximal ascension of these short-column acid events would not be sufficient to be detectable by pH-MII. In the second possible mechanism, it has been suggested that some POEs may be the result of low-volume acid reflux episodes; such episodes would be sufficient to register a drop in pH to <pH 4 but would fail to reach a threshold volume for detection by impedance (63,64). It has been suggested in the third possible mechanism that some POEs may be the residuals of previous impedance-detectable acid reflux episodes that were not completely cleared (61). In the fourth, it has been suggested that some POEs may be the result of esophageal shortening during swallowing or esophageal spasms (64); esophageal shortening may occasionally result in descending movement of the catheter through the LES into the acid pool of the proximal stomach (65–69). In the fifth, the adult literature has suggested that POEs may be artifacts from swallowing acidic contents or relaxations of the LES during swallowing that allow small amounts of acid into the distal esophagus. Rosen et al (62) examined 700 POEs of which 45% were not associated with swallows, whereas 55% were associated with swallows.
The duration of POEs is the period during which intraluminal pH in the distal esophagus remains <4. Minimum duration is 5 seconds. Strings of POEs separated by latency periods of <5 seconds are considered to be a single continuous event.
Composition of Refluxate
The composition of the refluxate may be important clinically because some data suggest that certain types of reflux may predis-pose patients to have symptomatic GER episodes. For example, gas reflux events with weak acidity appear to be more common among patients with reflux-attributed laryngeal lesions as compared with patients with GERD and controls (70). In evaluating for GER-symptom associations, Loots et al (71) found that when gas bolus GER was included in the analysis, the number of patients with positive symptom findings increased. This positive finding based on the method of GER detection was consistent for both infants and children; infants were more frequently symptom positive than were children. The presence of gas, however, may provide important clinical insight such as the presence of aerophagia, which may be masquerading as GERD.
Proximal Extent of Reflux Migration
Impedance monitoring permits the measurement of the proximal height reached by the refluxate. In general, the height reached by the refluxate is considered to be localized to the distal esophagus if it is confined to the 2 most distal impedance channels (impedance channels 5 and 6). The refluxate is considered to be proximal if it reaches either or both of the most proximal channels (channels 1 and/or 2).
Clearance of Gastroesophageal Reflux
MII-pH permits measurement of the time interval required for the reflux episode to be cleared from the esophagus (clearance time). Both the clearance of the reflux detected by impedance and the clearance of the reflux detected by the pH electrode can be determined. Clearance of many acid GER events occurs in 2 phases: the first phase is referred to as volume clearance (also referred to as bolus clearance time or bolus contact time) and the second phase is chemical clearance (CC) (72). During volume clearance, the bulk of the refluxed bolus is extruded from the esophagus by swallowing and peristalsis (primary and secondary). During chemical clearance, the acidified esophageal mucosa is neutralized by swallowed bicarbonate-rich saliva and possibly esophageal secretions that may include bicarbonate and protein (69,73,74). The duration of volume clearance is the period during which intraluminal impedance in the distal esophagus is <50% of baseline impedance. The duration of CC is the period beginning at the point at which volume clearance is completed and ends at the point at which intraluminal pH in the distal esophagus returns to pH 4. Total duration of these “2-phase” acid reflux events is the sum of volume clearance and CC components (61,72). Occasionally, CC occurs concurrently with bolus contact; duration of these “single-phase” acid reflux events is considered to be the duration of volume clearance (61,72).
ANALYSIS
Software Analysis
The use of the pH-MII technology in clinical practice is time-consuming because of the manual evaluation of the impedance tracing that requires expertise. For a visual analysis, consecutive frames of 2 to 4 minutes are analyzed, representing 360 to 720 frames for a 24-hour recording. The duration of analysis ranges from 1 to 3 hours depending on the experience of the investigator and the number of reflux episodes to be analyzed.
Software (AutoScan [version 5.0.9]/BioView Analysis, Sandhill Scientific, Highlands Ranch, CO) has been validated versus visual analysis and is the most commonly used: overall, it showed a good agreement, however, an inaccurate evaluation of association between nonacid reflux episodes and symptoms occurred in 20% of cases due to detection of more NAGER episodes than visual analysis (7). There are no reports to determine how accurately the software performs with pediatric populations.
More recently, Medical Measurement Systems has developed alternative commercial software (Ohmega; Dover, NH), but there are no validation studies yet to show how the software performs compared to manual analysis.
Indications and Limitations of MII-pH Testing
Arguments in the literature suggest that pH-MII detects more reflux events than pH monitoring alone. However, there are no prospective studies that have determined whether the additional detection, particularly of nonacid reflux, changes the outcome or influences the type of therapy used to treat patients.
Currently, pH-MII testing is clinically indicated in intractable patients who have discrete symptoms such as chest or abdominal pain, cough, apparent life-threatening events, apnea, choking, and intermittent stridor. There is also an important application for impedance in determining the correlation between symptoms and reflux in patients who have not responded to therapy, or in patients in whom there may be an association between symptoms and nonacid reflux events. Moreover, there is a clear indication in patients that are tube fed because the majority of reflux during tube feeding is nonacidic (75). Another use is to measure the efficacy of acid-suppression medications; because of its ability to record air boluses transiting throughout the esophagus, MII is a valuable tool to differentiate patients with aerophagia from patients with GER.
Validation and Reproducibility
Studies combining impedance monitoring with videofluoroscopy, bolus transit, and pressure measurements have validated the accuracy of impedance to measure and evaluate esophageal bolus transit (76). Impedance was found to be sensitive for detecting intraluminal flow. Small amounts (0.1 mL) of swallowed liquid provoke variations of intraluminal impedance in neonates (77); however, the evaluation of esophageal motor activity in children experiencing esophageal symptoms (eg, dysphagia, swallowing disorders, chest pain) by MII in interventional outcome studies has not been reported.
The day-to-day reproducibility of 24-hour pH-MII recordings is relatively poor in children (78). Conversely, reproducibility has been reported as fairly good in adults (number of events, overall acidity, and gas-liquid composition of reflux) during 24-hour pHMII studies (46) as well as for 90-minute postprandial recordings (total number of reflux) (79). During 3- to 6-hour studies in prematures, pH-MII has been reported to have a high level of intraand interobserver agreement (80). Studies of observer agreement during 24-hour pH/MII recordings have not been reported.
Normal Values
Normal values of pH-MII have been reported in premature infants (81) and adults (46,58,82) (Table 1). In 2004, Shay et al (58) conducted a multicenter study of 60 healthy volunteers and determined “normal” values to reference in the assessment of pH-MII monitoring results. The authors used the 95th percentile findings as the upper limit of normal for 24-hour MII/pH parameters at 5 and 15 cm above the LES; the upper limit of normal for total, acidic, weakly acidic and nonacid reflux were 73, 55, 26, and 1, respectively (58). Zerbib et al (46) found similar numbers in normal adults with the upper limit of normal for healthy adults for total, acidic, weakly acidic and nonacid reflux being 75, 50, 33, and 15, respectively.
TABLE 1.
Normal parameters of esophageal impedance studies
| References | Age median (range) | AGER index, % | Median no. total GER (n) | Median % of total that were AGER | pH probe distance from LES, cm | % Reaching proximal esophagus |
|---|---|---|---|---|---|---|
| Lopez-Alonso et al (81)* | 32 wk median gestation (12 days ATOS) | 5.59 | 71 | 25.4 | 2 | 90 |
| Shay et al (58) | 39 y (22-62 y) | 1.20 | 30 | 18 | 5 | 58 |
| Zerbib et al (46) | 35 y (18-72 y) | 1.60 | 44 | 22 | 5 | 22 |
AGER = acid gastroesophageal reflux; GER = gastroesophageal reflux; LES = lower esophageal sphincter.
Study was done with a nasogastric tube in place.
In contrast, Zentilin et al (82) posited that there is need for a better definition of normal values relative to the meal composition, its energy content, and timing; their study of 25 healthy Italian adults (median age 29 years, range 22–67 years) on a Mediterranean diet and without reflux symptoms produced different results. The researchers found that half of all reflux events were weakly acidic episodes. This is in contrast to results obtained by Shay et al (58) and Zerbib et al (46), who found that only one third of total reflux events were weakly acidic. The authors concluded that their study provided normal values of pH-MII monitoring that are suitable for countries in which people have dietary habits similar to those of Italians.
Normal values that were reported for preterm infants vary substantially from the values noted for the adult population. In a study of healthy preterm infants, Lopez-Alonso et al (81) reported a median of 71 reflux events; 73% of which were considered weakly acidic and 25% were acidic. In this study, the 95th percentile for total events was 100 with an upper limit of normal for the percentage of acid reflux of 52% and the percentage of weakly acidic reflux of 98%. Rosen et al (83) compared patients with eosinophilic esophagitis (n = 10) and control patients (patients with normal pH recordings, normal esophageal biopsies, and no gastrointestinal symptoms; n = 10) and found that the 95th percentile for total events in patients with eosinophilic esophagitis and control patients was 80 and 69, respectively, which is similar to adult data (83). Large studies are needed to confirm the range of normal values in children.
To accurately assess GER using pH-MII, a standard definition of normal values for pH-MII monitoring should be identified. The lack of normal values and the high day-to-day variability of pHMII in children currently limit the usefulness of the number of reflux episodes. To date, symptom-reflux association analysis is the only method that can identify possible association between GER and a short-lived symptom with sudden onset, such as cough, regurgitation, chest pain, and apnea.
Impedance to Evaluate Medication or Treatment Efficacy
pH-MII may be a useful tool to evaluate the effect of reflux therapies. Using pH-MII, Orr et al (84) found that the use of esomeprazole reduced overall reflux events, but nonacidic reflux events were more likely to occur during the treatment. Similarly, Mainie et al (85) reported on 12 adults with frequent heartburn who were studied during the postprandial period. Treatment with the acid-suppression drug omeprazole resulted in a significant reduction in the number of acid reflux events but there was a shift from acid to nonacid reflux such that the total number of reflux events was unchanged (85). In an effort to find a therapy that effectively treats nonacid reflux, Vela et al (86) assessed the effect of baclofen, a γ-aminobutyric acid agonist that reduces transient LES relaxations, on both acid and nonacid reflux and their associated symptoms. The authors found that the use of baclofen reduced both postprandial acid and nonacid GER and their associated symptoms.
In pediatrics, there are limited therapeutic data. A recent trial of famotidine therapy in infants (median age 5.3 months, range 1.3–10.5 months) who had GER showed a reduction in the number of regurgitation episodes and decreased crying time (87). However, many infants experienced neurological adverse effects including increased irritability, anorexia, and somnolence.
Jejunal feeds have been used in patients with GERD; however, a recent study reported that jejunal feeds may not reduce the frequency of GER episodes. Rosen et al (75) reported that in a group of children (mean age 75 months ± 62), significantly more reflux events occurred during jejunal feeds than during theintervals between feeds. However, there were fewer GERD-related hospitalizations after initiation of jejunal feeds and there were no significant differences between height and number of reflux events between jejunumfed patients and oral-feeding patients with GERD (75). Rosen et al (75) concluded that GERD complications may be associated with GERD characteristics other than the height of reflux and number of reflux events.
Similarly, pH-MII testing has been used to evaluate noninvasive therapies such as body positioning and thickening of feeds. Using pH-MII, Wenzl et al (39) studied 14 infants who received thickened and thin feeds in an alternating fashion. The authors found that the amount of feed that was regurgitated out of the mouth was reduced with the thickened feed, but the number of reflux events and the height of the reflux events were not statistically different between the 2 groups (45). Corvaglia et al (88) studied 5 preterm infants who received alternating thin MBM and MBM that was thickened with starch and found that thickened feeds did not reduce the number of total, acidic, or nonacid reflux.
Several studies in infants have been performed to determine the effect of positioning on reflux. Studies by Omari et al (89), Wijk et al (90), and Corvaglia et al (91) on preterm infants in the right and left lateral decubitus positions found that, during the postprandial period, there were significantly more reflux in the right lateral decubitus position.
Analysis of Symptoms Association
The importance of determining whether a patient's reflux is pathological or within physiological limits has often been overemphasized in the literature, but the presence of pathological reflux does not provide evidence regarding the cause of the symptoms. Furthermore, because there is no normative pediatric pH-MII data, one of the main uses for pH-MII testing in children is symptom correlation, which does not rely on absolute cutoff values for normal versus abnormal.
To date, symptom-reflux association analysis is the only method that can adequately identify the association between reflux and short-lived symptoms with sudden onset, such as heartburn, regurgitation, chest pain, and cough. For other symptoms that do not have clear start and end times, such as chronic laryngitis, otitis media, recurrent pneumonia, hoarseness, or globus sensation, the utility of pH-MII is as yet unclear.
Different methods have been used to quantify the index of association between GER and different symptoms:
Symptom index (SI) is defined as the percentage of symptom episodes that are related to reflux: ([number of reflux-related symptom episodes total number of symptom episodes] × 100%) (92). The optimal threshold for SI is currently 50%. The SI and esophageal acid exposure do not necessarily correlate. The higher the frequency of reflux during recording time, the greater the likelihood that a symptom will be associated with reflux by chance alone. For this reason, the symptom sensitivity index (SSI) was proposed as an additional parameter.
SSI is defined as the percentage of symptom-associated reflux episodes: ([reflux episodes associated with symptoms episodes ÷ total number of reflux episodes] × 100%) (93). SSI values of ≥10% are considered to be positive. Calculation of both SI and SSI may yield discordant results. Both the SI and SSI depend on a temporal association between reflux and symptoms, and this temporal association requires the clinician or researcher to somewhat arbitrarily pick a time window during which symptoms are considered to be “related” to reflux. Adult studies have suggested that the optimal time window is 2 minutes, but this definition was based on statistical rather than outcome studies. To determine the optimal window, one needs to identify the symptom window that predicts response to acid-suppression therapy or to fundoplication.
The SAP/regression method is used to address the limitations of the SI and SSI (eg, both are strongly influenced by the frequency of either symptoms or reflux). The SAP determines whether the reflux–symptom correlation is statistically significant (94). The SAP is calculated by dividing the 24-hour data into consecutive 2-minute or 5-minute intervals (35). Then, for each interval, it is determined whether reflux occurred (R–) providing the number of 2-minute segments with (total R+) and total number of segments with no GER (total R–) reflux. Presence and absence of symptoms (S) will also be verified: labeled (total S+), and totalled (S–). The 24-hour study is divided into 4 types of segments: R+S+, R+S–, R–S+, and R–S–. Then, the Fisher exact test is used to calculate the probability (P) for each symptom. By statistical convention, a SAP of >95% is positive. SAP is a statistical parameter that quantifies the probability that the observed distribution is not brought about by chance. As with all of the other statistical tests for association, however, a statistically significant relation between 2 parameters does not necessarily imply causality.
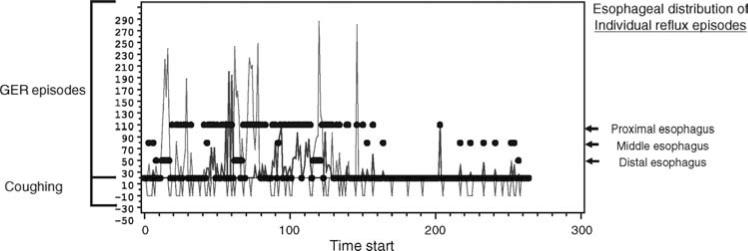
Regression analysis is also reported in the literature with results parallel to SAP (Fig. 3). Table 2 compares the advantages and disadvantages of each analysis method for the association between GER and other symptoms.
FIGURE 3.
Regression analysis plot. The temporal relation between the frequency and duration of gastroesophageal reflux (GER) and coughing episodes. Blue and red wave forms represent nonacid and acid gastroesophageal reflux episodes, respectively. Green wave forms represent coughing episodes.
TABLE 2.
Comparison between the methods of analyzing the association between gastroesophageal reflux and other symptoms
| SI | SSI | SAP | |
|---|---|---|---|
| Definition | Percent of reflux-associated symptom episodes | Percent of symptom-associated reflux episodes | Calculation of the statistical relation between symptoms and reflux episodes using Fisher exact test |
| Threshold (positive) | 50% | 10% | 95% |
| Advantage | Simple, understandable parameter; easy to calculate | Simple, understandable parameter; easy to calculate | Better relation between symptoms and reflux; uses all parameters |
| Disadvantage | Does not take the total number of reflux episodes into account | Does not take the total number of symptom episodes into account | Manual calculation is difficult |
SAP = symptom association probability; SI = symptom index; SSI = symptom sensitivity index.
CONCLUSIONS
pH-MII is a sensitive tool for evaluating overall reflux, and particularly for permitting detection of nonacid reflux events. pHMII was recently recognized by the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition as being superior to pH monitoring alone for evaluation of the temporal relations between symptoms and GER (2). pH-MII technology is especially useful in the postprandial period or at other times when gastric contents are nonacidic.
At the present time, in children, the primary use of pH-MII is to study intractable patients to establish whether nonacid reflux is contributing to the symptoms, to correlate symptoms with reflux (particularly nonacid reflux), to quantify reflux during tube feedings and the postprandial period, and to assess the efficacy of antireflux therapy. Its use is limited for determining whether a patient has pathological amounts of nonacid reflux because there are still limited normal values in pediatrics. It is also limited for determining the degree of reflux in patients with motility disorders or severe esophagitis because of limited data in such circumstances and for assessing the role of reflux in patients who have atypical symptoms with no distinct start time and stop time such as laryngitis.
Acknowledgments
Supported by NIH grants K24DK82792-1 (S.N.) and K23DK073713-01 (R.R.).
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Sherman P, Hassall E, Fagundes-Neto U, et al. A global evidence-based consensus on the definition of gastroesophageal reflux disease in children. Am J Gastroenterol. 2009;104:1278–95. doi: 10.1038/ajg.2009.129. [DOI] [PubMed] [Google Scholar]
- 2.Vandenplas Y, Rudolph C, Di Lorenzo C, et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49:498–547. doi: 10.1097/MPG.0b013e3181b7f563. [DOI] [PubMed] [Google Scholar]
- 3.Hassall E. Decisions in diagnosing and managing chronic gastroesophageal reflux disease in children. J Pediatr. 2005;146:S3–12. doi: 10.1016/j.jpeds.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Nelson SP, Chen EH, Syniar GM, et al. Prevalence of symptoms of gastroesophageal reflux during infancy. A pediatric practice-based study. Arch Pediatr Adolesc Med. 1997;151:569–72. doi: 10.1001/archpedi.1997.02170430035007. [DOI] [PubMed] [Google Scholar]
- 5.Van Howe RS, Storms MR. Gastroesophageal reflux symptoms in infants in a rural population: Longitudinal data over the first six months. BMC Pediatr. 2010;10:7. doi: 10.1186/1471-2431-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson SP, Chen EH, Syniar GM, et al. Prevalence of symptoms of gastroesophageal reflux during childhood. Arch Pediatr Adolesc Med. 2000;154:150–4. doi: 10.1001/archpedi.154.2.150. [DOI] [PubMed] [Google Scholar]
- 7.Gold BD. Gastroesophageal reflux disease: could intervention in childhood reduce the risk of later complications. Am J Med. 2004;117:23S–9S. doi: 10.1016/j.amjmed.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Nelson SP, Kothari S, Wu EQ, et al. Pediatric gastroesophageal reflux disease and acid-related conditions: trends in incidence of diagnosis and acid suppression therapy. J Med Econ. 2009;12:348–55. doi: 10.3111/13696990903378680. [DOI] [PubMed] [Google Scholar]
- 9.Hungin AP, Raghunath A, Wiklund I. Beyond heartburn: a review of the spectrum of reflux-induced disease. Fam Pract. 2005;22:591–603. doi: 10.1093/fampra/cmi061. [DOI] [PubMed] [Google Scholar]
- 10.El-Serag HB, Johanson JF. Risk factors for the severity of erosive esophagitis in Helicobacter pylori-negative patients with gastroesophageal reflux disease. Scand J Gastroenterol. 2002;37:899–904. doi: 10.1080/003655202760230847. [DOI] [PubMed] [Google Scholar]
- 11.El-Serag HB, Hill C, Jones R. Systematic review: the epidemiology of gastro-oesophageal reflux disease in primary care, using the UK General Practice Research Database. Aliment Pharmacol Ther. 2009;29:470–80. doi: 10.1111/j.1365-2036.2008.03901.x. [DOI] [PubMed] [Google Scholar]
- 12.Winters C, Jr, Spurling TJ, Chobanian SJ, et al. Barrett's esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology. 1987;92:118–24. [PubMed] [Google Scholar]
- 13.Sonnenberg A, El-Serag HB. Clinical epidemiology and natural history of gastroesophageal reflux disease. Yale J Biol Med. 1999;72:81–92. [PMC free article] [PubMed] [Google Scholar]
- 14.Jones R. Gastro-oesophageal reflux disease in general practice. Scand J Gastroenterol Suppl. 1995;211:35–8. doi: 10.3109/00365529509090292. [DOI] [PubMed] [Google Scholar]
- 15.Bretagne JF, Honnorat C, Richard-Molard B, et al. Comparative study of characteristics and disease management between subjects with frequent and occasional gastro-oesophageal reflux symptoms. Aliment Pharmacol Ther. 2006;23:607–16. doi: 10.1111/j.1365-2036.2006.02811.x. [DOI] [PubMed] [Google Scholar]
- 16.Locke GR, Talley NJ, Fett SL, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–56. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 17.Jones R, Ballard K. Healthcare seeking in gastro-oesophageal reflux disease: a qualitative study. Eur J Gastroenterol Hepatol. 2008;20:269–75. doi: 10.1097/MEG.0b013e3282f2a5bd. [DOI] [PubMed] [Google Scholar]
- 18.Garca Rodrguez LA, Perez Gutthann S. Use of the UK general practice research database for pharmacoepidemiology. Br J Clin Pharmacol. 1998;45:419–25. doi: 10.1046/j.1365-2125.1998.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poelmans J, Tack J. Extraoesophageal manifestations of gastroesophageal reflux. Gut. 2005;54:1492–9. doi: 10.1136/gut.2004.053025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tolia V, Wuerth A, Thomas R. Gastroesophageal reflux disease. Review of presenting symptoms, evaluation, management, and outcome in infants. Dig Dis Sci. 2003;48:1723–9. doi: 10.1023/a:1025486710231. [DOI] [PubMed] [Google Scholar]
- 21.Harding SM. Recent clinical investigations examining the association of asthma and gastroesophageal reflux. Am J Med. 2003;115:39S–44S. doi: 10.1016/s0002-9343(03)00191-8. [DOI] [PubMed] [Google Scholar]
- 22.Irwin RS, Madison JM. Anatomical diagnostic protocol in evaluating chronic cough with specific reference to gastroesophageal reflux disease. Am J Med. 2000;108:126S–30S. doi: 10.1016/s0002-9343(99)00351-4. [DOI] [PubMed] [Google Scholar]
- 23.Kawahara H, Dent J, Davidson G. Mechanisms responsible for gastroesophageal reflux in children. Gastroenterology. 1997;113:399–408. doi: 10.1053/gast.1997.v113.pm9247456. [DOI] [PubMed] [Google Scholar]
- 24.Dent J, Holloway RH, Toouli J, et al. Mechanisms of lower oesophageal sphincter incompetence in patients with symptomatic gastroesophageal reflux. Gut. 1998;29:1020–8. doi: 10.1136/gut.29.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Werlin SL, Dodds WJ, Hogan WJ, et al. Mechanisms of gastroesophageal reflux in children. J Pediatr. 1980;97:244–9. doi: 10.1016/s0022-3476(80)80482-3. [DOI] [PubMed] [Google Scholar]
- 26.Omari T. Gastro-oesophageal reflux disease in infants and children: new insights, developments and old chestnuts. J Pediatr Gastroenterol Nutr. 2005;41(Suppl 1):S21–3. doi: 10.1097/01.scs.0000180292.89483.cf. [DOI] [PubMed] [Google Scholar]
- 27.Vandenplas Y, Hassall E. Mechanisms of gastroesophageal reflux and gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2002;35:119–36. doi: 10.1097/00005176-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Tuttle SG, Grossman MI. Detection of gastro-esophageal reflux by simultaneous measurement of intraluminal pressure and pH. Proc Soc Exp Biol Med. 1958;98:225–7. doi: 10.3181/00379727-98-23998. [DOI] [PubMed] [Google Scholar]
- 29.Vandenplas Y. Oesophageal pH Monitoring for Gastroesophageal Reflux in Infants and Children. John Wiley & Sons; New York: 1992. pp. 235–44. [Google Scholar]
- 30.Mitchell DJ, McClure BG, Tubman TR, et al. Simultaneous monitoring of gastric and oesophageal pH reveals limitations of conventional oesophageal pH monitoring in milk fed infants. Arch Dis Child. 2001;84:273–6. doi: 10.1136/adc.84.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grant L, Cochran D. Buffering of gastric acid by milk feeds in preterm infants limits usefulness of oesophageal pH recordings.. Abstracts of the British Association of Perinatal Medicine 25th Scientific Meeting; September 2000. [Google Scholar]
- 32.Washington N, Spensley PJ, Smith CA, et al. Dual pH probe monitoring versus single pH probe monitoring in infants on milk feeds: the impact on diagnosis. Arch Dis Child. 1999;81:309–12. doi: 10.1136/adc.81.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wenzl TG, Schenke S, Peschgens T, et al. Association of apnea and nonacid gastroesophageal reflux in infants: investigations with the intraluminal impedance technique. Pediatr Pulmonol. 2001;31:144–9. doi: 10.1002/1099-0496(200102)31:2<144::aid-ppul1023>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 34.Rosen R, Nurko S. The importance of multichannel intraluminal impedance in the evaluation of children with persistent respiratory symptoms. Am J Gastroenterol. 2004;99:2452–8. doi: 10.1111/j.1572-0241.2004.40268.x. [DOI] [PubMed] [Google Scholar]
- 35.Mousa H, Woodley FW, Metheney M, et al. Testing the association between gastroesophageal reflux and apnea in infants. J Pediatr Gastroenterol Nutr. 2005;41:169–77. doi: 10.1097/01.mpg.0000173603.77388.84. [DOI] [PubMed] [Google Scholar]
- 36.Magista AM, Indrio F, Baldassarre M, et al. Multichannel intraluminal impedance to detect relationship between gastroesophageal reflux and apnoea of prematurity. Dig Liv Dis. 2007;39:216–21. doi: 10.1016/j.dld.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 37.Vela MF, Vela MF, Camacho-Lobato L, et al. Simultaneous intraesophageal impedance and pH measurement of acid and nonacid gastroesophageal reflux: effect of omeprazole. Gastroenterology. 2001;120:1599–606. doi: 10.1053/gast.2001.24840. [DOI] [PubMed] [Google Scholar]
- 38.Hemminka GJM, Weustena BLAM, Oorsb J, et al. Ambulatory oesophageal pH monitoring: a comparison between antimony, ISFET, and glass pH electrodes. Eur J Gastroenterol Hepatol. 2010;22:572–7. doi: 10.1097/MEG.0b013e328333139f. [DOI] [PubMed] [Google Scholar]
- 39.Wenzl TG, Schneider S, Scheele F, et al. Effects of thickening feeding on GER in infants: a placebo-control crossover study using intraluminal impedance. Pediatrics. 2003;114:e355–9. doi: 10.1542/peds.111.4.e355. [DOI] [PubMed] [Google Scholar]
- 40.Klingler PJ, Hinder RA, Wetscher GJ, et al. Accurate placement of the esophageal pH electrode for 24-hour pH monitoring using a combined pH/manometry probe. Am J Gastroenterol. 2000;95:906–9. doi: 10.1111/j.1572-0241.2000.01927.x. [DOI] [PubMed] [Google Scholar]
- 41.Strobel CT, Byrne WJ, Ament ME, et al. Correlation of esophageal lengths in children with height: application to the Tuttle test without prior esophageal manometry. J Pediatr. 1979;94:81–4. doi: 10.1016/s0022-3476(79)80361-3. [DOI] [PubMed] [Google Scholar]
- 42.Dobhan R, Castell DO. Normal and abnormal proximal esophageal acid exposure: results of ambulatory dual-probe pH monitoring. Am J Gastroenterol. 1993;88:25–9. [PubMed] [Google Scholar]
- 43.Gibbons TE, Corredor-Buchmann J, Smith CD. Placement of pH probes during upper endoscopy: direct visualization is accurate. J Ark Med Soc. 2009;105:183–4. 186. [PubMed] [Google Scholar]
- 44.Tutuian R, Maine I, Agrawal A, et al. Nonacid reflux in patients with chronic cough on acid: suppressive therapy. Chest. 2006;130:386–91. doi: 10.1378/chest.130.2.386. [DOI] [PubMed] [Google Scholar]
- 45.Maine I, Tutuian R, Shay S, et al. Acid and non-acid suppressive therapy in patients with persistent symptoms despite acid suppressive therapy: a multicenter study using combined ambulatory impedance-pH monitoring. Gut. 2006;55:1398–402. doi: 10.1136/gut.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zerbib F, des Varannes SB, Roman S, et al. Normal values and day-to-day variability of 24-h ambulatory oesophageal impedance-pH monitoring in a Belgian-French cohort of healthy subjects. Aliment Pharmacol Ther. 2005;22:1011–21. doi: 10.1111/j.1365-2036.2005.02677.x. [DOI] [PubMed] [Google Scholar]
- 47.Hemmink GJ, Bredenoord AJ, Weusten BL, et al. Esophageal pH-impedance monitoring in patients with therapy-resistant reflux symptoms: ‘on’ or ‘off’ proton pump inhibitor? Am J Gastroenterol. 2008;103:2446–53. doi: 10.1111/j.1572-0241.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 48.Peter CS, Weichers C, Bohnhorst B, et al. Influence of nasogastric tubes on GER in preterm infants: a multiple intraluminal impedance study. J Pediatr. 2002;141:277–9. doi: 10.1067/mpd.2002.126298. [DOI] [PubMed] [Google Scholar]
- 49.Lopez-Alonso M, Moya MJ, Cabo JA, et al. Acid and non-acid gastroesophageal reflux in newborns. Preliminary results using intraluminal impedance. Cir Pediatr. 2005;18:126–36. [PubMed] [Google Scholar]
- 50.Del Buono R, Wenzl TG, Rawat D, et al. Acid and non-acid gastroesophageal reflux in neurologically impaired children: investigation with the multiple intraluminal impedance procedure. J Pediatr Gastroenterol Nutr. 2006;43:331–5. doi: 10.1097/01.mpg.0000232333.77805.94. [DOI] [PubMed] [Google Scholar]
- 51.Orenstein SR. Effect of nonnutritive sucking on infant gastroesophageal reflux. Pediatr Res. 1988;24:38–40. doi: 10.1203/00006450-198807000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Smoak BR, Koufman JA. Effects of gum chewing on pharyngeal and esophageal pH. Ann Otol Rhinol Laryngol. 2001;110:1117–9. doi: 10.1177/000348940111001206. [DOI] [PubMed] [Google Scholar]
- 53.Kerr P, Shoenut JP, Millar T, et al. Nasal CPAP reduces gastroesophageal reflux in obstructive sleep apnea syndrome. Chest. 1992;102:1539–44. doi: 10.1378/chest.101.6.1539. [DOI] [PubMed] [Google Scholar]
- 54.Tawk M, Goodrich S, Kinasewitz G, et al. The effect of one week of continuous positive air pressure treatment in obstructive sleep apnea patient with concomitant gastroesophageal reflux. Chest. 2006;130:1003–8. doi: 10.1378/chest.130.4.1003. [DOI] [PubMed] [Google Scholar]
- 55.Skopnik H, Silny J, Heiber O, et al. Gastroesophageal reflux in infants: evaluation of a new intraluminal impedance technique. J Pediatr Gastroenterol Nutr. 1996;23:591–8. doi: 10.1097/00005176-199612000-00014. [DOI] [PubMed] [Google Scholar]
- 56.Sifrim D, Holloway R, Silny JH, et al. Composition of the post-prandial refluxate in patients with gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:647–55. doi: 10.1111/j.1572-0241.2001.03598.x. [DOI] [PubMed] [Google Scholar]
- 57.Condino AA, Sondheimer Pan Z, et al. Evaluation of infantile acid and nonacid gastroesophageal reflux using combined pH monitoring and impedance measurement. J Pediatr Gastroenterol Nutr. 2006;42:16–21. doi: 10.1097/01.mpg.0000188008.66752.72. [DOI] [PubMed] [Google Scholar]
- 58.Shay S, Tutuian R, Sifrim D, et al. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol. 2004;99:1037–43. doi: 10.1111/j.1572-0241.2004.04172.x. [DOI] [PubMed] [Google Scholar]
- 59.Sifrim D, Barnes N. GERD-related chronic cough: how to identify patients who will respond to antireflux therapy? J Clin Gastroenterol. 2010;44:234–6. doi: 10.1097/MCG.0b013e3181d06b2f. [DOI] [PubMed] [Google Scholar]
- 60.Woodley FW, Mousa H. Acid gastroesophageal reflux reports in infants: a comparison of esophageal pH monitoring and multichannel intraluminal impedance measurements. Dig Dis Sci. 2006;51:1910–6. doi: 10.1007/s10620-006-9179-0. [DOI] [PubMed] [Google Scholar]
- 61.Woodley FW, Mousa H. pH only acid reflux events in infants during later phases of the feeding cycle are less acidic and cleared more efficiently than classic two-phase acid reflux events. J Pediatr Gastroenterol Nutr. 2009;48:41–7. doi: 10.1097/MPG.0b013e31816f214a. [DOI] [PubMed] [Google Scholar]
- 62.Rosen R, Lord C, Nurko S. The sensitivity of multichannel intraluminal impedance and the pH probe in the evaluation of gastroesophageal reflux in children. Clin Gastroenterol Hepatol. 2006;4:167–72. doi: 10.1016/s1542-3565(05)00854-2. [DOI] [PubMed] [Google Scholar]
- 63.Peter CS, Wiechers C, Bohnhorst B, et al. Detection of small bolus volumes using multiple intraluminal impedance in preterm infants. J Pediatr Gastroenterol Nutr. 2003;36:381–4. doi: 10.1097/00005176-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 64.Fox M. Bravo wireless versus catheter pH monitoring systems. Gut. 2006;55:434–5. [PMC free article] [PubMed] [Google Scholar]
- 65.Pouderoux P, Lin S, Kahrilas PJ, et al. Timing, propagation, coordination, and effect of esophageal shortening during peristalsis. Gastroenterology. 1997;112:1147–54. doi: 10.1016/s0016-5085(97)70125-2. [DOI] [PubMed] [Google Scholar]
- 66.Fletcher J, Wirz A, Henry E, et al. Unbuffered highly acidic gastric juice exists at the gastroesophageal junction after a meal. Gastroenterology. 2001;121:775–83. doi: 10.1053/gast.2001.27997. [DOI] [PubMed] [Google Scholar]
- 67.Pal A, Brasseur JG. The mechanical advantage of local longitudinal shortening on peristaltic transport. J Biomech Eng. 2002;124:94–100. doi: 10.1115/1.1427700. [DOI] [PubMed] [Google Scholar]
- 68.Fletcher J, Wirz A, Henry E, et al. Studies of acid exposure immediately above the gastro-oesophageal squamocolumnar junction: evidence of short segment reflux. Gut. 2004;53:168–73. doi: 10.1136/gut.2003.022160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Helm JF, Dodds WJ, Pelc LR, et al. Effect of esophageal emptying and saliva on clearance of acid from the esophagus. N Engl J Med. 1984;310:284–8. doi: 10.1056/NEJM198402023100503. [DOI] [PubMed] [Google Scholar]
- 70.Kawamura O, Aslam M, Rittmann T, et al. Physical and pH properties of gastroesophagopharyngeal refluxate: a 24-hour simultaneous ambulatory impedance and pH monitoring study. Am J Gastroenterol. 2004;99:1000–10. doi: 10.1111/j.1572-0241.2004.30349.x. [DOI] [PubMed] [Google Scholar]
- 71.Loots CM, Benninga MA, Davidson GP, et al. Addition of pH-Impedance monitoring to standard pH monitoring increases the yield of symptom association analysis in infants and children with gastroesophageal reflux. Pediatrics. 2009;154:248–52. doi: 10.1016/j.jpeds.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 72.Woodley FW, Fernandez S, Mousa H. Diurnal variation in the chemical clearance of acid gastroesophageal reflux in infants. Clin Gastroenterol Hep. 2007;5:37–43. doi: 10.1016/j.cgh.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Helm JF, Dodds WJ, Riedel DR, et al. Determinants of esophageal acid clearance in normal subjects. Gastroenterolgy. 1983;85:607–12. [PubMed] [Google Scholar]
- 74.Helm JF. Esophageal acid clearance. J Clin Gastroenterol. 1986;8(Suppl 1):5–11. doi: 10.1097/00004836-198606001-00003. [DOI] [PubMed] [Google Scholar]
- 75.Rosen R, Levine P, Nurko S. Do jejunal feeds decrease gastroesophageal reflux as detected by pH-MIII? J Pediatr Gastroenterol Nutr. 2007;45(suppl 7):E40. [Google Scholar]
- 76.Tutuian R, Castell DO. Combined multichannel intraluminal impedance and manometry clarifies esophageal function abnormalities: study in 350 patients. Am J Gastroenterol. 2004;99:1011–9. doi: 10.1111/j.1572-0241.2004.30035.x. [DOI] [PubMed] [Google Scholar]
- 77.Peter CS, Wiechers C, Bohrnorst B, et al. Detection of small bolus volumes using multichannel intraluminal impedance in preterm infants. J Pediatr Gastroenterol Nutr. 2003;36:381–4. doi: 10.1097/00005176-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 78.Dalby K, Nielsen RG, Markoew S, et al. Reproducibility of 24-hour combined multiple intraluminal impedance (MII) and pH measurements in infants and children. Evaluation of a diagnostic procedure for gastroesophageal reflux disease. Dig Dis Sci. 2007;52:2159–65. doi: 10.1007/s10620-006-9731-y. [DOI] [PubMed] [Google Scholar]
- 79.Bredenoord AJ, Weusten BL, Timmer R, et al. Reproducibility of multichannel intraluminal electrical impedance monitoring of gastroesophageal reflux. Am J Gastroenterol. 2005;100:265–9. doi: 10.1111/j.1572-0241.2005.41084.x. [DOI] [PubMed] [Google Scholar]
- 80.Peter CS, Sprodowski N, Ahlborn V, et al. Inter- and intra-observer agreement for gastroesophageal reflux detection in infants using multiple intraluminal impedance. Biol Neonate. 2004;85:11–4. doi: 10.1159/000074951. [DOI] [PubMed] [Google Scholar]
- 81.Lopez-Alonso M, Moya MJ, Cabo JA, et al. Twenty-four-hour esophageal impedance-pH monitoring in healthy preterm neonates: rate and characteristics of acid, weakly acidic, and weakly alkaline gastroesophageal reflux. Pediatrics. 2006;118:E299–308. doi: 10.1542/peds.2005-3140. [DOI] [PubMed] [Google Scholar]
- 82.Zentilin P, Iiritano E, Dulbecco P, et al. Normal values of 24-h ambulatory intraluminal impedance combined with pH-metry in subjects eating a Mediterranean diet. Dig Liver Dis. 2006;38:226–32. doi: 10.1016/j.dld.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 83.Rosen R, Furuta G, Fritz J, et al. Role of acid and nonacid reflux in children with eosinophilic esophagitis compared with patients with gastroesophageal reflux and control patients. J Pediatr Gastroenterol Nutr. 2008;46:520–3. doi: 10.1097/MPG.0b013e318158600c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Orr WC, Craddock A, Goodrich S. Acidic and non-acidic reflux during sleep under conditions of powerful acid suppression. Chest. 2007;131:460–5. doi: 10.1378/chest.06-1312. [DOI] [PubMed] [Google Scholar]
- 85.Mainie I, Tutian R, Shay S, et al. Acid and Non-acid gastroesophageal reflux in patients with persistent symptoms despite acid suppressive therapy: a multicenter study using combined ambulatory impedance-pH monitoring. Gut. 2006;55:1398–402. doi: 10.1136/gut.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vela M, Tutian R, Katz PO, et al. Baclofen decreases acid and non-acid post-prandial gastroesophageal reflux measured by combined multi-channel intraluminal impedance and pH. Aliment Pharmacol Ther. 2003;17:243–51. doi: 10.1046/j.1365-2036.2003.01394.x. [DOI] [PubMed] [Google Scholar]
- 87.Orenstein SR, Shalaby TM, Devandry SN, et al. Famotidine for infant gastro-oesophageal reflux: a multi-centre, randomized, placebo-controlled, withdrawal trial. Aliment Pharmacol Ther. 2003;17:1097–107. doi: 10.1046/j.1365-2036.2003.01559.x. [DOI] [PubMed] [Google Scholar]
- 88.Corvaglia L, Ferlini M, Rotatori R, et al. Starch thickening of human milk is ineffective in reducing the gastroesophageal reflux in preterm infants: a crossover study using intraluminal impedance. J Pediatr. 2006;148:265–8. doi: 10.1016/j.jpeds.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 89.Omari TI, Rommel N, Staunton E, et al. Paradoxical impact of body positioning on gastroesophageal reflux and gastric emptying in the premature neonate. J Pediatr. 2004;145:194–200. doi: 10.1016/j.jpeds.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 90.Wijk MP, Benninga MA, Dent J, et al. Effect of body position changes on post-prandial gastroesophageal reflux and gastric emptying in the healthy premature neonate. J Pediatr. 2007;151:585–90. doi: 10.1016/j.jpeds.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 91.Corvaglia L, Rotatori R, Ferlini M, et al. The effect of body positioning on gastroesophageal reflux in premature infants: evaluation by combined impedance and pH monitoring. J Pediatr. 2007;151:591–6. doi: 10.1016/j.jpeds.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 92.Wiener GJ, Richter JE, Copper JB, et al. The symptom index: a clinically important parameter of ambulatory 24-hr esophageal pH monitoring. Am J Gastroenterol. 1988;83:358–61. [PubMed] [Google Scholar]
- 93.Breumelhof R, Smout AJPM. The symptom sensitivity index: a valuable additional parameter in 24-hr esophageal pH recording. Am J Gastroenterol. 1991;86:160–4. [PubMed] [Google Scholar]
- 94.Weusten BL, Roelofs JM, Akkermans LM, et al. The symptom-association probability: an improved method for symptom analysis of 24-hour esophageal pH data. Gastroenterology. 1994;107:1741–5. doi: 10.1016/0016-5085(94)90815-x. [DOI] [PubMed] [Google Scholar]