Abstract
The expression of connexins in the ciliary epithelium is consistent with gap junctions between the pigmented (PE) and nonpigmented ciliary epithelium (NPE) that form when connexon hemichannels from adjacent cells pair to form a channel. Here we present evidence that suggests undocked connexons may form functional hemichannels that permit exchange of substances between NPE and the aqueous humor. Intact porcine eyes were perfused via the ciliary artery and propidium iodide (PI) (MW 668) was added to the aqueous humor compartment as a tracer. After calcium-free solution containing PI was introduced into the aqueous humor compartment for 30 min, fluorescence microscopy revealed PI in the NPE cell layer. PI entry into the NPE was inhibited by calcium and by the connexin antagonist 18α-glycyrrhetinic acid (18-AGA). Studies also were carried out with cultured porcine NPE. Under normal conditions, little PI entered the cultured cells but calcium-free medium stimulated PI accumulation and the entry was inhibited by 18-AGA. In cells loaded with calcein (MW 622), calcium-free solution stimulated calcein exit. 18-AGA partially suppressed calcein exit in calcium-free medium. Connexin 43 and connexin 50 proteins were detected by western blot analysis in both native and cultured NPE. In the intact eye, immunolocalization studies revealed connexin 50 at the basolateral, aqueous humor-facing, margin of the NPE. In contrast, connexin 43 was observed at the junction of the PE and NPE layer and on the basolateral membrane of PE. The results point to functional hemichannels at the NPE basolateral surface. It is feasible that hemichannels might contribute to the transfer of substances between the ciliary epithelium cytoplasm and aqueous humor.
Keywords: Connexin hemichannels, nonpigmented ciliary epithelium, porcine
1. Introduction
The ciliary epithelium, located at the surface of the ciliary processes, is responsible for the secretion of the aqueous humor (AH) and is an essential barrier to diffusion of high molecular weight solutes from the blood to the interior of the eye. There are two cell layers, the pigmented epithelium (PE) on the stromal (blood) side and the nonpigmented epithelium (NPE) on the aqueous humor side. In common with most epithelial tissues, gap junctions permit communication between adjacent cells and thus coordination of cell function. Gap junctions form when connexin proteins on the plasma membrane of neighboring cells connect to make channels that bridge between neighboring ciliary epithelial cells, allowing the cell-cell transfer of cytoplasmic molecules. In the ciliary processes it is important to recognize that gap junctions also connect NPE and PE cells. Thus while the two cell types are developmentally quite distinct and remain different throughout life, gap junctions enable cells in the two layers to function in some respects as a syncytium (Coca-Prados et al., 1992; Coffey et al., 2002; Edelman et al., 1994; Raviola and Raviola, 1978; Wolosin et al., 1997). In a coordinated manner, ionic solutes cross the bilayer by entering the PE, passing through gap junctions to the NPE, then moving from the NPE to the posterior chamber. The currently accepted model is that transcellular flow of ions establishes a voltage that drives paracellular ion flow and causes an obligatory osmotic flow of water from blood to aqueous (Civan and Macknight, 2004). Various ion transport mechanisms at the PE basolateral surface are responsible for uptake while efflux occurs by means of different transporters at the NPE basolateral surface. The mechanism of aqueous humor formation relies on gap junctions at the interface between the apical surfaces of the PE and NPE.
The structural organization of gap junctions requires connexin proteins to assemble in a highly specific way (Evans and Martin, 2002; Kumar and Gilula, 1996; White et al., 1994). Six-membered connexin structures, called connexons, form hemichannels in the plasma membrane and a gap junction forms when hemichannels on the surface of one cell connect to a hemichannels on the surface of an adjacent cell. However, some cells display connexin proteins on surfaces that do not face a neighboring cell (Saez et al., 2010; Scemes, 2012). If hemichannels were to form at these regions they would connect, if they opened, to the extracellular fluid. Depending on their selectivity and gating, such undocked or unapposed hemichannels could be a conduit for dissolved substances to pass out of the cell. Hemichannels are reported in a range of tissues and serve diverse functions. For example, connexin 43 hemichannels contribute to the release of molecules that signal between glial cells (Giaume et al., 2013). Connexin 37 forms hemichannels in platelets that release substances which play a role in hemostasis (Vaiyapuri et al., 2012). In the lens, connexin 46 and 50 hemichannels in fiber cells are believed to be important for tissue transparency (Beahm and Hall, 2002; Ebihara et al., 2011; Zampighi, 2003) while hemichannels in the epithelium release ATP in response to hyposmotic shock (Shahidullah et al., 2012a). Here we present evidence pointing to the existence of functional connexin hemichannels in porcine NPE. The putative hemichannels occur at the basolateral surface, where the NPE faces the aqueous humor. It is feasible that hemichannels might contribute to the release of substances from the ciliary epithelium into the aqueous humor.
2. Materials and methods
2.1. Animal tissue procurement and use
Fresh porcine eyes, purchased from the University of Arizona Meat Science Laboratory were transported to the laboratory on ice. Post mortem time ranged from 30 – 60 min. The use of porcine tissue was approved by the University of Arizona Institutional Animal Care and Use Committee and conformed to the ARVO Resolution for the Use of Animals in Ophthalmic and Vision Research.
2.2. Solutions and reagents
HEPES-buffered DMEM, fetal bovine serum and newborn calf serum and TO-PRO®-3 Iodide, were purchased from Invitrogen (Carlsbad, CA, USA). 18-α-Glycyrrhetinic acid, Fluorescein isothiocyanate–dextran (average mol wt 4,000), propidium iodide, choline chloride, calcein-AM and other salts and chemicals used to prepare Krebs solution were purchased from Sigma-Aldrich (St. Louis, MO).
2.3. Antibodies
Rabbit polyclonal anti-connexin 43 antibody was purchased from Sigma-Aldrich (1:10,000 for WB and 1:400 for immunofluorescence). Mouse monoclonal anti-connexin 50 antibody (2.0 μg/ml for WB and 20 μg/ml for immunofluorescence) and mouse monoclonal anti-connexin 26 (1:1000 WB; 1:200 IHC) was purchased from Invitrogen Immunodetection (Carlsbad CA). Rabbit polyclonal anti-connexin 37 antibody (1:2500 for WB and 10 μg/ml for IHC) was purchased from Alphadiagnostic (Owings Mills, MD), rabbit polyclonal anti-connexin 40 antibody (WB 1:1000) was purchased from Millipore (Billerica, MA) and from Abcam (Cambridge, MA) (WB 1:500). Rabbit polyclonal anti-connexin2 6 (WB1:1000; IHC 1:100); mouse monoclonal anti0connexin 31 antibody (WB1:1000; IHC 1:100); rabbit polyclonal anti-connexin 26/30/32 (WB 1:1000; IHC 1:100), rabbit polyclonal anti-connexin 40 antibody (WB1:1000; IHC 1:100) were purchased from Santa Cruz Biotechnologies Inc. (Santa Cruz, CA). Rabbit polyclonal anti-β-actin antibody was obtained from Cell Signaling Technology (Danvers, MA). Mouse monoclonal anti-β-actin antibody (1:3000) was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz. CA). Goat anti-rabbit IRDye 680 (1:20,000) or goat anti-mouse IRDye 800 (1:20,000) conjugated secondary antibodies were purchased from LI-COR Biosciences (Lincoln, Nebraska USA). Alexa Fluor 546 goat anti-mouse IgG; Alexa Fluor 488 goat anti-rabbit IgG was from molecular probes (Eugene, OR, USA) (1:200).
2.4. Intact eye preparation
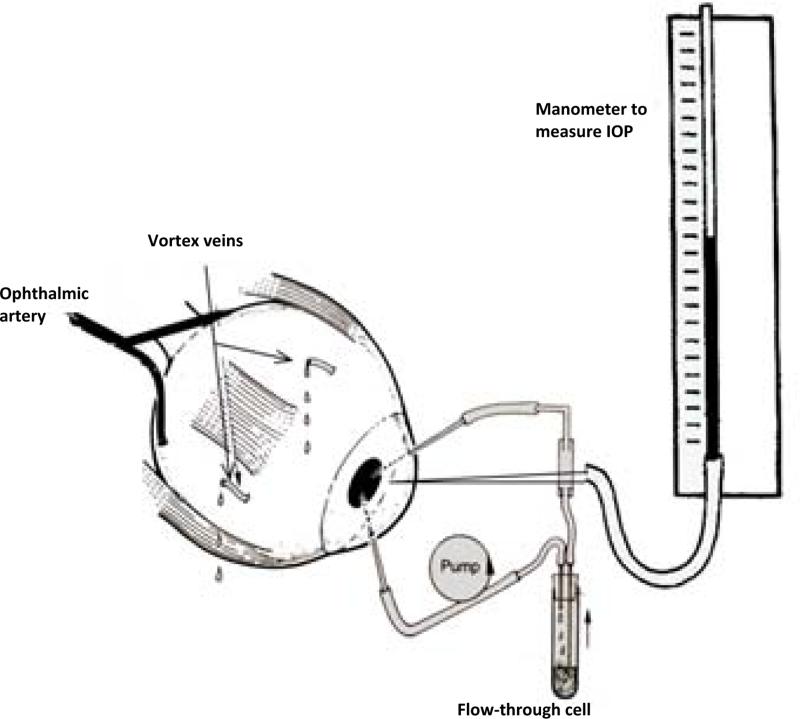
Fresh pig eyes were obtained from a local abattoir and perfused using method described in earlier studies on aqueous humor formation and multifocal electroretinogram (Shahidullah et al., 2003; Shahidullah et al., 2005). The eye was placed on a water-filled circulating warming jacket maintained at 37°C and covered with an insulated plastic cup. The ophthalmic artery was cannulated and the eye perfused with solution at 37°C, that contained (in mM) NaCl, 118; KCl, 4.0; MgSO4, 1.2; CaCl2, 2.0; NaHCO3, 25; KH2PO4, 1.2; glucose, 10; reduced glutathione, 1.0; ascorbate 0.05 and allopurinol, 1.8, at pH 7.4. The solution was continuously bubbled with O2 containing 5% CO2. Allopurinol, a xanthine oxidase inhibitor, was included to suppress oxidative damage and reperfusion injury. Using a peristaltic pump (Watson Marlow, 505S) perfusion flow was increased stepwise to1.5 ml.min−1 over a period of 40 – 60 min. The arterial perfusion pressure was continuously monitored using a digital pressure transducer and never exceeded 140 mmHg. On reaching the optimum arterial flow rate, perfusion was continued for a further 40 min which we know from previous studies is sufficient to establish a steady arterial pressure and AH secretion rate (Wilson et al., 1993). The anterior chamber was then perfused with propidium iodide solution to study uptake by the ciliary epithelium (see below). A schematic diagram of the perfused eye preparation is shown (Fig. 1).
Figure 1.
Diagram of the intact eye preparation that shows the position of the needles used to pump aqueous humor substitute through the aqueous humor compartment and to measure intraocular pressure (IOP).
2.5. Propidium iodide uptake studies in the intact eye
After establishing steady state perfusion (see above), the anterior chamber was cannulated with three 23G needles. The first needle was connected via fine silicon tubing (0.5 mm internal diameter) passing through a Watson-Marlow peristaltic pump to the inlet of a 95 μl quartz flow-through cell. The outlet of the flow-through cell was then connected to the anterior chamber via silicon tubing and a second 23G needle to constitute the anterior chamber circulating system. Before cannulating the anterior chamber with the needles, the circulating system was filled, with 1.04 ml of aqueous humor substitute (AHS) containing (in mM): NaCl, 110; KCl, 3; CaCl2, 1.4; MgCl2, 0.5; KH2PO4, 0.9; NaHCO3, 30; glucose, 6; ascorbic acid 3, equilibrated with 95% O2 - 5% CO2 and the pH adjusted to 7.56 plus 30.7 μM propidium iodide. On the basis of our previous studies that showed the average anterior chamber volume in pig was 237 μl (Shahidullah et al., 2005), the final concentration of propidium iodide in the anterior chamber was ~25 μM. Care was taken so that no bubble remains in the circulating system. In calcium-free AHS, CaCl2 was replaced with an equivalent molar concentration of choline chloride and 1 mM EGTA was added. The third needle connected the anterior chamber, again via silicon tubing, to a water manometer to continuously measure intraocular pressure (IOP) which ranged between 9 – 15 mm Hg. Eyes with IOP that exceeded 15 mmHg were excluded from the study. By means of the peristaltic pump, the AHS containing propidium iodide was circulated through the anterior chamber of the eye preparation. The two needles in the anterior chamber (input and output) were kept wide apart to optimize fluid mixing. Using this approach, the aqueous humor compartment was perfused for 40 min with control or calcium-free AHS containing propidium iodide.
The eyes were removed at the end of the perfusion period and then ciliary body was isolated and dissected following a procedure described below. Cut sections of ciliary body (~5 mm) were prepared in frozen blocks in OCT medium by cooling in a slurry prepared from crushed dry ice and 2-methyl butane. This slurry, when at equilibrium (no bubbling), gives a freezing temperature of ~90°C. The ciliary body blocks thus prepared were either stored at −85°C for future use or immediately cut into 5-7 μm sections and fixed on slides. The specimens were then examined and photographed using a Zeiss Axiovert 200M epifluorescence microscope fitted with a Carl Zeiss Axiocam digital camera.
2.6. Isolation and culture of porcine NPE
Porcine non-pigmented ciliary epithelium (NPE) was established in primary culture using a modification of our technique described previously (Shahidullah et al., 2007). Fresh porcine eyes were dissected to obtain the entire ring of NPE that remained attached to the vitreous, leaving the pigmented cell (PE) layer attached to the ciliary body. The NPE was separated from the vitreous using fine scissors then cut into 1-2 mm pieces. NPE from 5-7 eyes was pooled and transferred to a 90 mm petri dish containing 15 ml of 0.015% collagenase A and 500 U/ml of hyaluronidase (Sigma St. Louis, MO) in a collagenase buffer containing (in mM): NaCl 66.7, KCl 13.4, HEPES 3.8, CaCl2 4.8, pH 7.4. The petri dish was placed on a rotary shaker in a 37°C incubator for 5-7 min then removed from the shaker and the collagenase and hyaluronidase neutralized by adding excess (7 ml) of a 1:1 mixture of newborn calf serum (NCS) and fetal bovine serum (FBS). NPE cells were dispersed by gentle trituration using a round-tipped Pasteur pipette and pelleted by centrifugation at 2,000 rpm (670 × g) for 10 min at 4°C. The pellet was dispersed and the cells incubated without changing the medium for 3-4 days in a small volume (3 ml) of Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich, St Louis, MO) containing 10% FBS and 100 IU/ml gentamycin at 37°C in 5% CO2/95% air. Thereafter the medium was changed every alternate day. The cells grew to confluence in 7-10 days. At confluence the cells were trypsinized and seeded at a density of 2.5×104 cells.cm2 for subsequent passage. Passage 2 – 4 cells were used in the studies reported here. Cell viability was assessed by a Trypan blue exclusion test.
2.7. Propidium iodide uptake studies in cultured NPE
Cells were plated at a density of 2.5 ×104/sq.cm2 and confluent monolayers were obtained either on 35 mm dish for qualitative epifluorescence microphotography or on 24 well plates for quantitative measurement of fluorescence. Prior to use, the cell monolayers were serum-starved for 2 hours in serum-free DMEM and then the medium was replaced with Krebs solution that contained (in mM) 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 25 NaHCO3, 2.0 CaCl2, 1 MgCl2, and 5.5 glucose, equilibrated with 5% CO2 and adjusted to pH 7.4 (normal Krebs solution). Cells were allowed to equilibrate in Krebs solution for 1 h. Experiments were carried out in the same Krebs solution at 37°C in a humidified incubator with 95% air and 5% CO2. Cells were incubated for 30 min with 25 μM of propidium iodide or fluorescein-dextran (MW, 4000) in normal Krebs solution or nominally calcium-free Krebs solution. In calcium-free solution CaCl2 was omitted and replaced with an equivalent amount of choline chloride. Some cells also were exposed to 18-AGA (100 μM) added 20 min beforehand. At the end of the incubation period, the cells were washed 3 times with normal Krebs solution to remove external propidium iodide then examined and photographed using a Zeiss Axiovert 200M epifluorescence microscope fitted with a Carl zeiss Axiocam digital camera. Microscope and camera settings were kept unchanged in photographing control and treated samples. Alternately, the cells (those grown on 24-well plates) were washed two times with Krebs solution and then homogenized by sonication in 300 μl of distilled water using a Misonix S3000 sonicator (6W power setting, 4 strokes of 15 sec each with a 5 sec interval). Using a Varioscan Flash® plate reader, fluorescence intensity was measured in 150 μl aliquots of the homogenate transferred to a flat-bottom 96-well black plate at excitation/emission wavelengths of 535nm/617nm. Protein concentration in the sample was measured using a BCA protein assay kit (Pierce, Rockford, Illinois) based on a published method (Smith et al., 1985) using bovine serum albumin (BSA) as the standard. The results were expressed as fluorescence intensity/mg of protein.
2.8. Calcein efflux studies
Confluent monolayer was established on 96-well plates. Culture medium from each well was replaced with 100 μl of serum-free DMEM containing 5 μM calcein-AM (a cell permeable ester form of calcein) and the cells were placed in an incubator at 37° C (5% CO2) for 30 min to allow calcein accumulation within the cells. Calcein-AM is non-fluorescent and membrane permeant. Esterase-mediated cleavage of the acetoxymethyl ester group occurs inside the cell, trapping calcein which is fluorescent and relatively membrane impermeant. Preliminary experiments indicated that the 30 min loading period resulted in a near steady-state intracellular concentration of calcein (data not shown). After the loading period, the loading buffer was aspirated and the cells were rinsed 3× (200 μl each) at room temperature with normal Krebs solution. The calcein-loaded cells were then incubated at 37° C (5% CO2) for another 30 minutes to allow calcein exit in control or calcium-free Krebs solution or calcium-free solution containing 100 μM 18 α-glycyrrhetinic acid. The cells were then washed 3× (200 μl each) with ice-cold Krebs solution and lysed in 50 μl of a lysis buffer that contained (mM) 50 mannitol, 1 tris-base, pH 7.4 with HEPES, 1% Triton X-100 and 0.5% sodium dodecyl sulphate. Calcein that remained in the cells was measured by fluorescence determined at excitation/emission wavelengths of 495nm/525nm using a Varioskan Flash ® fluorescence plate reader. Protein content in each of the wells was determined with the BCA assay. Intracellular calcein was expressed as relative fluorescence/mg protein.
2.9. Western blot
Cells were lysed in RIPA buffer containing 50 mM HEPES, 150 mM NaCl, 1.0 mM EDTA, 10 mM sodium pyrophosphate, 2.0 mM sodium orthovanadate, 10 mM sodium fluoride, 10% glycerol, 1.0% Triton X-100, 1.0% sodium deoxycholate and 1 tablet/7 ml of Protease Inhibitor Cocktail (Roche Diagnostics, Indianapolis, IN, USA). The cell lysate was centrifuged at 13,000g for 30 min and the supernatant was added to Laemmli buffer. Then the mixture was placed on a 7.5% SDS-polyacrylamide mini-gel. After separation by electrophoresis, the proteins were transferred by electrophoresis to nitrocellulose membrane and the membrane was blocked for 1h with Odyssey blocking buffer (LI-COR, Lincoln, NE). The membrane was then incubated overnight at 4 °C with one of the anti-connexin antibodies and at dilution specified above. All antibodies were diluted in the blocking buffer (LI-COR Odyssey, Lincoln, NE). After three washes in TTBS (30 mM Tris, 150 mM NaCl, 0.5% (v/v) Tween-20 at pH 7.4), each membrane was incubated for 1 h with an appropriate secondary antibody conjugated with either IR Dye 680 goat anti mouse secondary antibody (1:20,000) (Alexa-Fluor, Invitrogen, Carlsbad, CA) or IR Dye 800 goat anti rabbit secondary antibody (Rockland, Gilbertsville, PA). Protein bands were visualized by infra-red laser scan detection (LI-COR Odyssey, Lincoln, NE).
2.10. Immunolocalization
Immunolocalization studies were carried out using cultured NPE or fresh pig eyes using an approach described earlier (Shahidullah et al., 2009). For eye studies, the cornea was removed then 4-6 mm of the anterior sclera was carefully peeled from the choroid all around the globe using a pair of curved scissors. The corneal remnant at the limbus was used as the handle to facilitate this dissection. The whole iris-ciliary body along with the lens and anterior vitreous was then removed. The tissue was placed in a petri dish (corneal face down) containing ice-cold solution that contained (in mM) NaCl 113, KCl 4.6, NaHCO3 21.0, MgSO4 0.6, D-glucose 7.5, glutathione (reduced form) 1.0, Na2HPO4 1.0, HEPES 10.0, and CaCl2 1.4, pH adjusted to 7.4. The lens was removed by cutting the zonules and vitreous was carefully trimmed off, then the iris was dissected away to leave the ciliary body which was fixed in formalin and used to prepare 5-7μm paraffin sections. For studies on cultured NPE, cells grown on specially designed chamber slides (Nalgene Nunc, Lab-Tek II Chamber Slides), were washed with phosphate buffered saline (PBS) containing 1.0mM MgCl2 and 0.1mM CaCl2 then fixed in acetone for 2 min at room temperature.
The fixed tissue sections or NPE cells were incubated at room temperature for 90 min in 10% goat serum in PBS (blocking buffer). Primary antibodies directed against either connexin 43 or connexin 50 were added for 24h at 4°C. Control specimens received only the blocking buffer. The specimens were washed with PBS and incubated 24h at 4°C with fluorescent secondary antibody (Alexa Fluor 488 or 546 conjugated to either goat anti rabbit or anti mouse IgG, 1:200 dilution). Nuclear counterstaining was carried out using TO-PRO-3 iodide (Invitrogen, Carlsbad, CA) and images were captured using a Zeiss LSM 510 meta-NLO confocal microscope (Carl Zeiss Inc., Thornwood, NY). Fluorescence excitation was achieved using 488, 543 and 633nm laser excitation wavelengths for FITC, rhodamine and TO-PRO-3 iodide respectively.
2.11. Statistical analysis
A two-sample t test was used to analyze unpaired data and paired ‘t’ test was used to compare paired samples. One way analysis of variance (ANOVA) followed by Bonferroni's post hoc multiple comparison tests was used to compare differences for more than two groups of data. A probability (P) value of <0.05 was considered significant.
3. Results
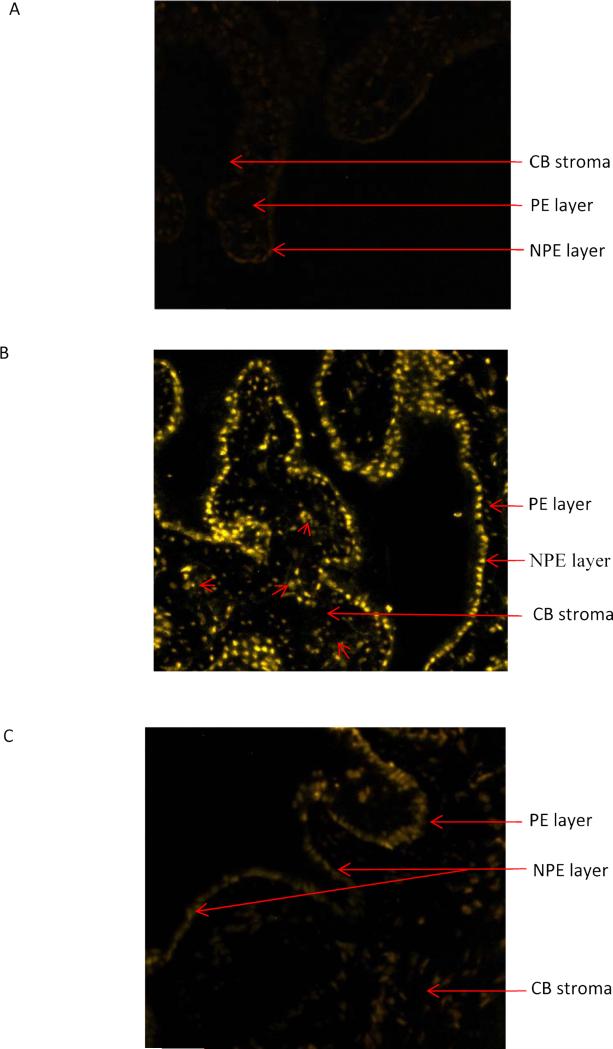
Propidium iodide (MW 668) was used as a tracer in uptake studies carried out to examine functional hemichannels in the ciliary processes. Intact porcine eyes were perfused via the ophthalmic artery in a preparation (Fig. 1) that sustains aqueous humor formation and a physiological IOP of 9-15 mmHg (Wilson et al., 1993). For 40 min the aqueous humor compartment was exchanged with normal, calcium-containing, medium (aqueous humor substitute) containing propidium iodide. Under these conditions only trace amounts of propidium iodide were detected by fluorescence in the ciliary processes (Fig 2A). In contrast, propidium iodide was readily detectable in the ciliary processes when calcium-free medium was introduced into the aqueous humor compartment (Fig. 2B). When propidium iodide was introduced into the aqueous humor compartment under calcium-free conditions, propidium iodide was readily detectable in nuclei of the NPE cell layer. Propidium iodide stained nuclei were hard to discern in the PE layer but were detectable in some areas within the stroma. To examine the contribution of connexin hemichannels to the entry of propidium iodide into the NPE, calcium-free medium containing 100 μM 18α-glycyrrhetinic acid (18-AGA) was introduced into the aqueous humor compartment. Under these conditions, propidium iodide was almost undetectable in both the NPE and PE layers (Fig. 2C).
Figure 2.
Propidium iodide (PI) uptake by ciliary processes in the intact porcine eye. The aqueous humor compartment was perfused with control medium for 90 min. After this equilibration period, the eyes were perfused for 40 min with control or calcium-free medium that contained propidium iodide (~25 μM). Panel A shows propidium iodide detected by confocal microscopy in 7 micron thick sections obtained from eyes perfused with control medium. Panel B shows propidium iodide detected in eyes perfused with calcium-free medium plus 1 mM EGTA. In eyes perfused with calcium-free medium propidium iodide is detectable mainly in the NPE cell layer. In some regions, propidium iodide is also detected in the PE (arrows) and ciliary process stroma (arrowheads). Panel C shows the results obtained from eyes perfused with calcium-free medium containing 18α-glycyrrhetinic acid (100 μM) (AGA).
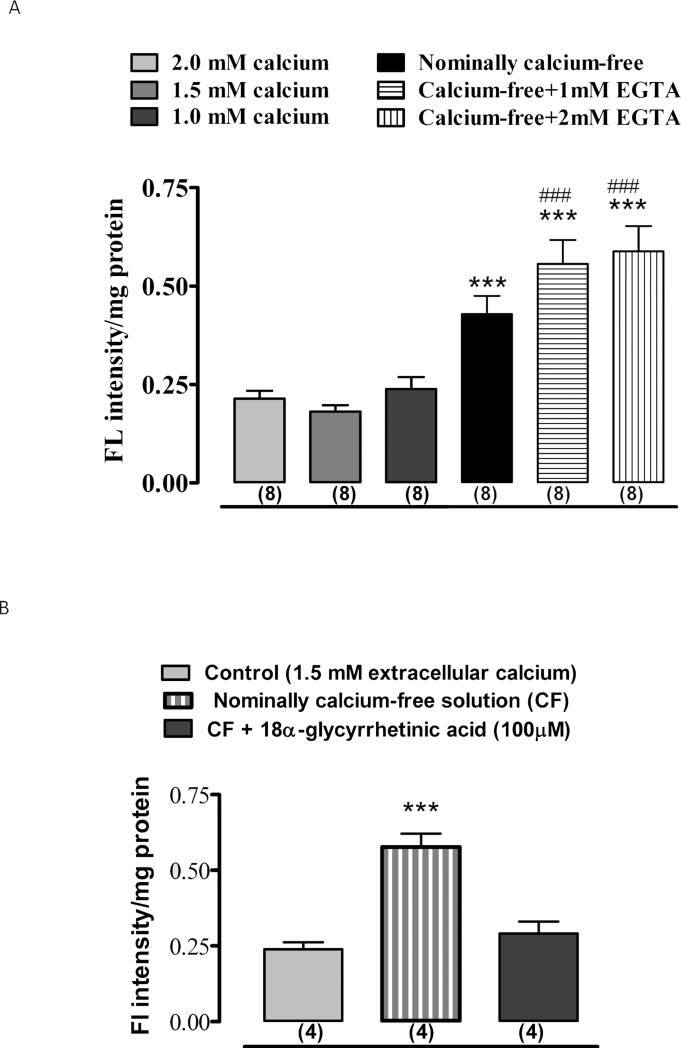
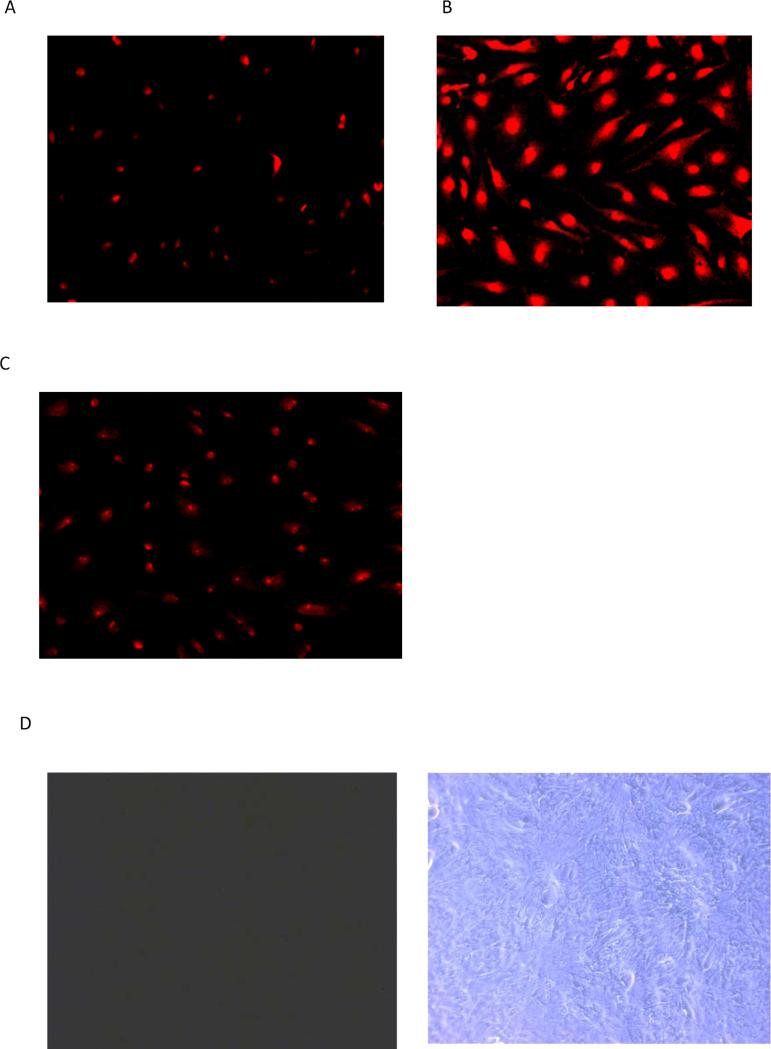
Because of the obstacles to quantifying propidium iodide uptake responses to calcium-free medium and 18-AGA in NPE of the intact eye, propidium iodide uptake studies were carried out using NPE cells in primary culture and quantified by measuring fluorescence intensity. Under normal conditions where the extracellular calcium concentration is 1.5 mM, cultured NPE cells largely excluded propidium iodide added to the culture medium (Fig. 3A). Propidium iodide accumulation was more than doubled in cells exposed to propidium iodide in nominally calcium-free medium and propidium iodide entry was greater still in calcium-free medium containing EGTA (Fig. 3A). Propidium iodide accumulation under calcium-free conditions was suppressed by presence of 18-AGA to the calcium-free medium (Fig. 3B). In additional experiments, microphotographs of non-confluent cultured NPE cells were taken after 30 min exposure to ~25 μM propidium iodide in normal calcium-containing Krebs solution, calcium-free Krebs solution or in calcium-free Krebs solution plus 18-AGA (100 μM). In normal calcium-containing medium only trace amount of propidium iodide was detectable (Fig. 4A). In contrast, propidium iodide was avidly taken up in calcium-free medium (Fig. 4B). When cells were pre-incubated with 18-AGA, propidium iodide uptake in calcium-free solution was markedly reduced (Fig. 4C). Consistent with the size limit of solute's passage through connexin hemichannels, when cells were exposed to fluorescein-dextran (MW ~4000), a larger solute than either propidium iodide, accumulation was not detectable under normal (Fig. 4D) or in calcium-free conditions (Fig. 4E).
Figure 3.
The sensitivity of propidium iodide uptake by cultured NPE to extracellular calcium and 18α-glycyrrhetinic acid. Cells were exposed to 25 μM propidium iodide for 30 min in control (calcium-containing) medium, nominally calcium-free medium or in calcium-free medium containing EGTA (1-2 mM) added to chelate trace amounts of calcium. Quantitative data on propidium iodide fluorescence under these conditions is shown in panel A. Panel B shows the results obtained from cells incubated in nominally calcium-free medium containing 18α-glycyrrhetinic acid (100 M).The values are the mean ± SE of results from 4-8 independent experiments. *** indicates a significant difference (p<0.001) from control and ### indicates a significant difference (p<0.001) from nominally calcium-free condition.
Figure 4.
Propidium iodide (PI) uptake by cultured NPE shown in low power micrographs. Panel A shows micrographs of cells exposed to propidium iodide (25 μM) for 30 min in control medium. Panel B shows micrographs of cells exposed to propidium iodide in nominally calcium-free medium. Panel C shows the results in calcium-free medium containing 18α-glycyrrhetinic acid (100 μM). Propidium iodide, which is associated with cell nuclei, was most abundant in the calcium-free group. In a separate experiment cells were exposed to fluorescein dextran (4000 μM) for 30 min in control or calcium-free medium then examined by fluorescence and phase contract microscopy. Cells from the control group and calcium-free group are shown in Panels D and E respectively with the phase contrast images on the right.
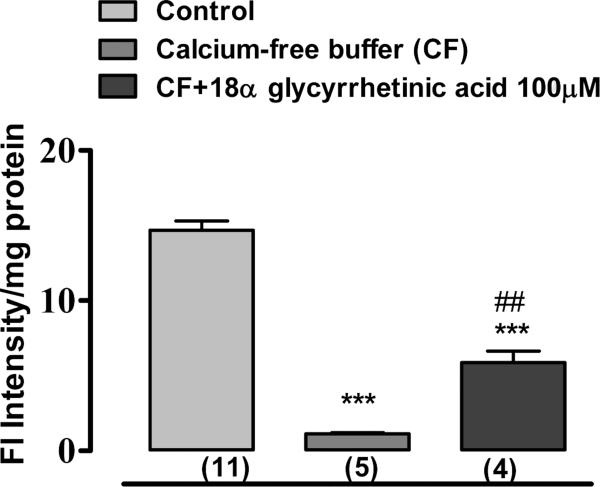
In separate experiments, hemichannel function was examined by monitoring loss of a tracer. Cultured NPE cells were loaded with calcein (MW 622) and the subsequent exit of the tracer was examined by measuring the amount of calcein remaining in the cells after 30 min in either normal or calcium-free conditions. The loss of calcein from cells exposed to calcium-free medium was markedly greater than the calcein loss seen in control cells (Fig. 5). 18-AGA caused significant partial inhibition of calcein exit in cells exposed to calcium-free medium.
Figure 5.
Loss of calcein from cultured porcine NPE. Cells loaded with calcein were incubated for 30 min in control medium, calcium-free medium or calcium-free medium containing 18α-glycyrrhetinic acid (100 M). Calcein that remained in the cells was quantified by measuring fluorescence. The values are the mean ± SE of results from 4-11 independent experiments. *** indicates a significant difference (p<0.001) from control, ## indicates a significant difference (p<0.01) from calcium-free condition.
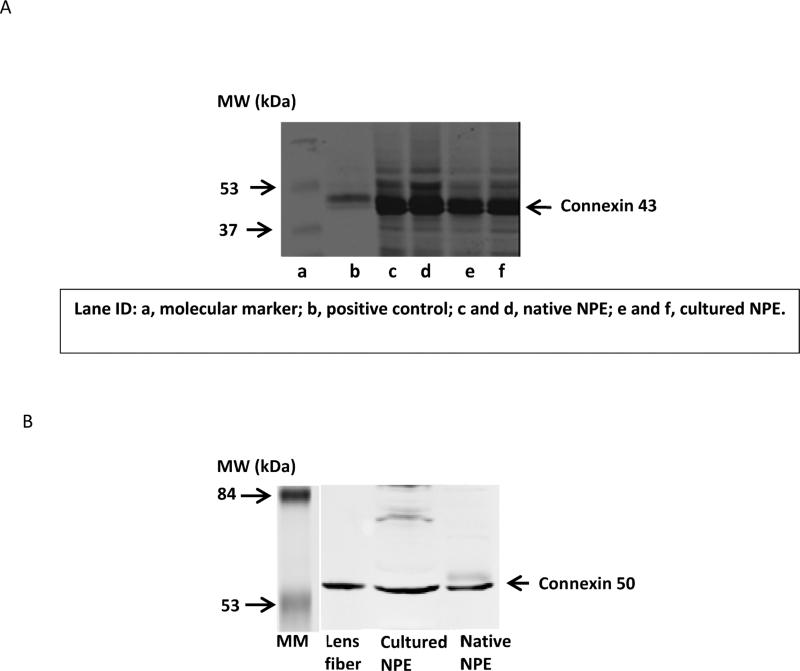
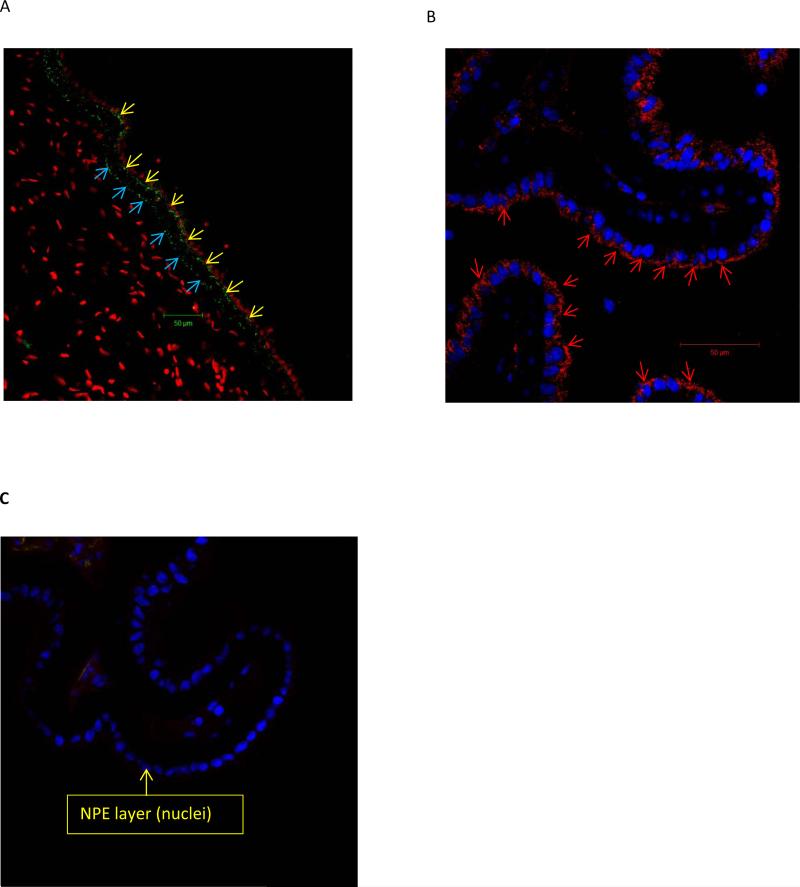
Western blot analysis was carried out to compare connexin protein expression in cultured NPE and native NPE isolated from fresh eyes (Fig. 6). Robust immunoreactive bands were observed for connexin 43 and connexin 50 but connexins 26, 32, 31, 37, and 40 were not detected. Immunolocalization studies were carried out to examine connexin 43 and connexin 50 in the ciliary processes. The distribution patterns of connexin 43 and connexin 50 were different. Connexin 43 was most abundant at the junction between the NPE and PE cell layers (Fig. 7A). Connexin 50 was detected mainly at the basolateral surface of the NPE including the region where the cells face the aqueous humor (Fig 7B).
Figure 6.
Western blot detection of connexin 43 (Panel A) and connexin 50 (Panel B) in freshly isolated porcine NPE and in cultured porcine NPE. Glutathione-S-transferase fusion protein containing the cytoplasmic carboxy-terminal tail of Connexin 43 (GST-Cx43) and porcine lens fiber were used as positive controls for connexin 43 and connexin 50, respectively. 60 μg of protein was loaded to each lane.
Figure 7.
Immunolocalization of connexin 43 and connexin 50 in porcine ciliary processes. The results show connexin 43 and connexin 50 detected by confocal microscopy in a control, untreated porcine eye. Connexin 43 (Panel A) was detectable at the junction between the NPE and PE layers (yellow arrows) and also at the basolateral (stroma-facing) margin of the PE (blue arrow). Connexin 50 (Panel B) was detectable at the basolateral (aqueous humor-facing) margin of the NPE (arrow heads). In contrast, the negative control (panel C), shows no staining.
4. Discussion
The two connexin proteins, connexin 43 and connexin 50, detected in porcine ciliary epithelium have been reported in the ciliary epithelium of humans and other mammals. For example, connexin 43 was reported in the human (Coca-Prados et al., 1992), bovine (Coca-Prados et al., 1992; Wang et al., 2010), mouse (Calera et al., 2009) and rat ciliary epithelium (Coffey et al., 2002) and connexin 50 was reported in the nonpigmented ciliary epithelium of rabbit (Wolosin et al., 1997). The localization of connexin 43 to the apical junctions between the PE and NPE is consistent with gap junctions that provide coupling between the two cell layers (Coffey et al., 2002). Connexin 50, on the other hand, was detected at the basolateral membrane of NPE and appeared in regions with no apparent cell-cell contact that face the aqueous humor. It is known from earlier studies that the NPE layer is rich in connexin 50 (Wolosin et al., 1997). It is possible that apparently unpaired basolateral connexins on the NPE have no functional significance unless they form connexons that happen to come into contact with connexons on an adjacent cell to form gap junctions that permit lateral solute movement in the NPE layer. However, findings from propidium iodide uptake studies in an intact eye preparation, as well as experiments in cultured NPE, suggest that NPE cells have functional connexin hemichannels that face the aqueous humor.
Studies on gating of various connexin hemichannels point to voltage-dependence and channel opening under low calcium conditions (Zhang et al., 2006). Here we showed nominally calcium-free conditions significantly increased the ability of propidium iodide to pass from the bathing solution into cultured NPE cells. Propidium iodide accumulation by cultured NPE was higher still in calcium-free solutions that contained EGTA to chelate trace amounts of calcium. Calcium concentration in nominally calcium-free solution has been estimated at ~ 5 μM (Lynch et al., 2008) and based on this value it can be calculated that 1 and 2 mM EGTA reduce the calcium concentration to < 1 nM (Schoenmakers et al., 1992). Propidium iodide was found to enter the NPE cell layer when calcium-free propidium iodide-containing solution was introduced into the aqueous humor compartment of intact eyes. Because of its size and structure, propidium iodide (MW 667) can pass through open hemichannels but otherwise is unable to cross the plasma membrane. On this basis, the findings are consistent with the opening of functional hemichannels in the NPE under calcium-free conditions. The connnexin inhibitor 18-AGA significantly reduced the magnitude of the increase of propidium uptake in calcium-free conditions but did not fully prevent the response. The findings point to the contribution of functional connexin hemichannels to propidium iodide uptake by the NPE while leaving open the possibility of other propidium iodide entry mechanisms. In other studies, a component of propidium uptake resistant to 18-AGA has been attributed to pannexin hemichannels (Shahidullah et al., 2012b).
While propidium iodide in the aqueous humor compartment was able to enter the NPE layer, at least under calcium-free conditions, it was far less detectable in the PE layer. However, we cannot rule out the possibility that its detection in PE cells was obscured by pigment. For this reason, lack of propidium iodide detection in the PE layer does not eliminate the possibility of cell-cell passage of propidium iodide through NPE-PE gap junctions, or rule out functional hemichannels in the PE formed by basolateral connexin 43. Indeed, in the present investigation, immunolocalization studies showed evidence of connexin 43 at the basolateral surface of the PE in regions that face the ciliary process stroma. The trace amount of PI that was observed in the ciliary process stroma might have entered by crossing through the ciliary epithelium bilayer. It is also possible that removal of extracellular calcium might alter the tight junctions between the NPE cells, leading to paracellular movement of propidium iodide (PI) from the aqueous humor compartment toward the ciliary process stroma. An additional possible pathway for propidium iodide movement into the ciliary process stroma is entry via the iris base where a route for solute movement has been proposed (Candia and Alvarez, 2008; Freddo, 2001).
The case for hemichannels was strengthened by studies on the exit of calcein (MW 622). When cultured NPE cells loaded with calcein were subjected to calcium-free solution, calcein exit increased significantly. Consistent with the presence of functional hemichannels that open in calcium-free solution, calcein exit was reduced by ~ 50% by the connexin inhibitor 18-AGA. Although 18-AGA is not particularly selective among connexin subtypes (Srinivas, 2009), it has been shown to be an effective inhibitor of both gap junctions (Davidson et al., 1986; Guan et al., 1996; Yamamoto et al., 1998) and connexin hemichannels (Faigle et al., 2008; Velasquez Almonacid et al., 2009) in a wide variety of cells and tissues. The fact that calcium-free stimulation of calcein exit was not entirely eliminated by 18-AGA suggests an alternate route. Pannexin hemichannels feasibly could contribute to calcein exit but their opening has been found to be independent of extracellular calcium concentration (Ma et al., 2009). Calcein, an organic anion, probably is able to exit the cell by various routes, diffusing passively through open hemichannels as well as by means of MRP and p-glycoprotein efflux transporters. MRP1, MRP2 and p-glycoprotein are expressed in porcine NPE (Pelis et al., 2009) and possibly contribute to calcein efflux that was observed to persist in the presence of 18-AGA.
The evidence points to functional hemichannels in the NPE that are opened experimentally by calcium-free conditions. However, it is not possible to rule out voltage dependent hemichannel opening because depolarization is likely to occur in cells exposed to calcium-free solution. It is difficult to specify the hemichannel identity and there could be more than one mechanism. Nevertheless, apparently undocked connexin 50 was found facing the aqueous humor and it is known that both calcium-free conditions and depolarization have been reported to influence connexin 50 channel gating (Srinivas et al., 1999; Srinivas et al., 2005; Zhang et al., 2006). Connexin 5o forms hemichannels in lens fibers (Beahm and Hall, 2002; Zampighi, 2003) and hemichannels formed by connexin 50 in an exogenous expression model have been shown to open under calcium-free conditions (Zampighi et al., 1999; Zhang et al., 2006).
Connexin 43 localized to the interface of the PE and NPE likely forms the gap junctions known to couple the two layers. Using electron microprobe analysis to examine cytoplasmic ion composition, McLaughlin and coworkers (McLaughlin et al., 2004) found evidence of robust PE-NPE coupling but instances where detectable ion differences existed between adjacent cells in the same layer, suggesting zones of less effective or intermittent PE-PE and NPE-NPE coupling. From a functional standpoint, connexin gap junctions between the PE and NPE are of crucial importance to aqueous humor production by the ciliary epithelium bilayer. The ability of solutes to pass between the cell layers is a critical step in the transepithelial transport mechanism (Civan and Macknight, 2004). Nevertheless, hemichannels in the NPE that face the interior of the eye could play an important role in the defining the composition of aqueous humor. If they were to open in an intermittent fashion, functional hemichannels in the NPE could enable molecules to pass from the ciliary epithelium into the aqueous humor. Signaling substances such as cAMP and ATP do not readily cross the plasma membrane but can pass through hemichannels (Valiunas, 2013). cAMP is present in aqueous humor and recognition that its concentration changes in in a circadian manner has led to interest in its association with circadian changes of intraocular pressure. In recent studies on mice, Marmorstein and coworkers proposed modulation of aqueous humor outflow facility by soluble factors released from the ciliary processes (Lee et al., 2011; Zhang et al., 2009). These authors drew attention to the high capacity of NPE to generate cAMP by means of soluble adenylyl cyclase. The possible contribution of hemichannels to cAMP release from the NPE is a topic for future study. ATP also is known to be released cells via hemichannels (Eltzschig et al., 2006; Gomes et al., 2005; Saez et al., 2003; Shahidullah et al., 2012a; Shahidullah et al., 2012b) and ATP and its metabolite adenosine have been the focus of considerable interest by investigators interested in signaling molecules that influence aqueous humor dynamics (Li et al., 2011; Li et al., 2012). We included 1.8 mM allopurinol in the arterial perfusate to suppress oxidative damage and reperfusion injury in the intact eye preparation. In earlier studies we did not detect any appreciable effect on IOP or aqueous humor inflow (Shahidullah et al., 2005). Even though the solution used to perfuse the intact eye did not contain adenosine, we recognize the possibility that release from the ciliary epithelium might occur during the course of the experiment and ATP could also be released then converted to adenosine. We also recognize that by inhibiting xanthine oxidase, allopurinol may reduce purine degradation and this could lead to adenosine accumulation (Schmidt et al., 2009). Increasing adenosine concentration has been shown to increase fluid flow and IOP in some species, such as in rabbits and cats, by stimulating adenosine A2 receptor (Crosson and Gray, 1996). In cynomolgous monkeys, however, adenosine caused an initial 30 min of ocular hypertension mediated by A2A receptor activation followed by a prolonged hypotension by increasing outflow facility mediated by A1 receptors without any effect on fluid inflow (Tian et al., 1997).
As mentioned above, signaling molecules delivered to the aqueous humor may be carried in the aqueous flow to elicit responses in the trabecular meshwork that affect aqueous outflow. Soluble factors released by the NPE also may act in an autocrine manner to affect ciliary body itself, or perhaps signal to the lens which is situated with its most metabolically active cells, the equatorial epithelium, in very close proximity to the source of aqueous humor production. Previously, Coca-Prados and Escribano (Coca-Prados and Escribano, 2007) proposed alteration of outflow facility due to neuropeptides synthesized by the ciliary processes that are released into the aqueous humor. However, neuropeptides and other peptides like endothelin-1 are likely to be contained in subcellular vesicles (Xu and Xu, 2008) making it unlikely that hemichannels contribute to their release from the NPE. On the other hand functional micro RNAs are known to be capable of passing through connexin gap junction channels (Katakowski et al., 2010; Valiunas et al., 2005) and thus may be able to leave cells via hemichannels. Micro RNAs have recently been detected in aqueous humor (Dunmire et al., 2013) and more study is needed before we understand their origin and function. It would be useful to know whether micro RNAs in the aqueous humor altered the physiology of the lens or trabecular meshwork and Schlemm's canal.
5. Conclusions
In conclusion, the findings point to the presence of functional connexin hemichannels in porcine NPE. The location of connexin 50 at the NPE basolateral surface, where the NPE faces the aqueous humor, and the ability of propidium iodide to enter the NPE, under calcium-free conditions and in an 18-AGA-sensitive manner, suggests that hemichannels might be a functional conduit for the transfer of substances from NPE cytoplasm into aqueous humor. Further study is needed to understand what causes the hemichannels to open, and what substances pass through them, under physiological conditions.
Highlights.
Hemichannel function was confirmed in the native porcine nonpigmented epithelium
Connexin (Cx) 43 and 50 were localized in the nonpigmented ciliary epithelium (NPE)
Cx 50 was localized on the basolateral surface of NPE
Cx 43 was localized at the apical junction of NPE and pigmented epithelium (PE)
Cx 43 was localized at the basolateral PE surface, suggesting hemichannel function
Acknowledgement
The authors are grateful to the University of Arizona Meat Science laboratory and Hatfield Quality Meat, Pennsylvania for the supply of porcine eyes. The corresponding author and the co-authors have no conflict of interest to declare. This research was supported by a grant from the National Institute of Health (Grant EY006915).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beahm DL, Hall JE. Hemichannel and junctional properties of connexin 50. Biophysical Journal. 2002;82:2016–2031. doi: 10.1016/S0006-3495(02)75550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calera MR, Wang Z, Sanchez-Olea R, Paul DL, Civan MM, Goodenough DA. Depression of Intraocular Pressure Following Inactivation of Connexin43 in the Nonpigmented Epithelium of the Ciliary Body. Invest. Ophthalmol. Vis. Sci. 2009;50:2185–2193. doi: 10.1167/iovs.08-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candia OA, Alvarez LJ. Fluid transport phenomena in ocular epithelia. Progress in Retinal & Eye Research. 2008;27:197–212. doi: 10.1016/j.preteyeres.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan MM, Macknight AD. The ins and outs of aqueous humour secretion. Experimental Eye Research. 2004;78:625–631. doi: 10.1016/j.exer.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Coca-Prados M, Escribano J. New perspectives in aqueous humor secretion and in glaucoma: the ciliary body as a multifunctional neuroendocrine gland. Progress in Retinal & Eye Research. 2007;26:239–262. doi: 10.1016/j.preteyeres.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Coca-Prados M, Ghosh S, Gilula NB, Kumar NM. Expression and cellular distribution of the alpha 1 gap junction gene product in the ocular pigmented ciliary epithelium. Current Eye Research. 1992;11:113–122. doi: 10.3109/02713689209000061. [DOI] [PubMed] [Google Scholar]
- Coffey KL, Krushinsky A, Green CR, Donaldson PJ. Molecular profiling and cellular localization of connexin isoforms in the rat ciliary epithelium. Experimental Eye Research. 2002;75:9–21. doi: 10.1006/exer.2002.1187. [DOI] [PubMed] [Google Scholar]
- Crosson CE, Gray T. Characterization of ocular hypertension induced by adenosine agonists. Investigative Ophthalmology & Visual Science. 1996;37:1833–1839. [PubMed] [Google Scholar]
- Davidson JS, Baumgarten IM, Harley EH. Reversible inhibition of intercellular junctional communication by glycyrrhetinic acid. Biochemical & Biophysical Research Communications. 1986;134:29–36. doi: 10.1016/0006-291x(86)90522-x. [DOI] [PubMed] [Google Scholar]
- Dunmire JJ, Lagouros E, Bouhenni RA, Jones M, Edward DP. MicroRNA in aqueous humor from patients with cataract. Experimental Eye Research. 2013;108:68–71. doi: 10.1016/j.exer.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Ebihara L, Tong J-J, Vertel B, White TW, Chen T-L. Properties of Connexin 46 Hemichannels in Dissociated Lens Fiber Cells. Investigative Ophthalmology & Visual Science. 2011;52:882–889. doi: 10.1167/iovs.10-6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman JL, Sachs G, Adorante JS. Ion transport asymmetry and functional coupling in bovine pigmented and nonpigmented ciliary epithelial cells. American Journal of Physiology. 1994;266:C1210–1221. doi: 10.1152/ajpcell.1994.266.5.C1210. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circulation Research. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- Evans WH, Martin PEM. Gap junctions: structure and function (Review). Molecular Membrane Biology. 2002;19:121–136. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS ONE [Electronic Resource] 2008;3:e2801. doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freddo TF. Shifting the paradigm of the blood-aqueous barrier. Experimental Eye Research. 2001;73:581–592. doi: 10.1006/exer.2001.1056. [DOI] [PubMed] [Google Scholar]
- Giaume C, Leybaert L, C Naus C, C Saez J. Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles. Frontiers in Pharmacology. 2013;4:88. doi: 10.3389/fphar.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes P, Srinivas SP, Van Driessche W, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Investigative Ophthalmology & Visual Science. 2005;46:1208–1218. doi: 10.1167/iovs.04-1181. [DOI] [PubMed] [Google Scholar]
- Guan X, Wilson S, Schlender KK, Ruch RJ. Gap-junction disassembly and connexin 43 dephosphorylation induced by 18 beta-glycyrrhetinic acid. Molecular Carcinogenesis. 1996;16:157–164. doi: 10.1002/(SICI)1098-2744(199607)16:3<157::AID-MC6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Katakowski M, Buller B, Wang X, Rogers T, Chopp M. Functional microRNA is transferred between glioma cells. Cancer Research. 2010;70:8259–8263. doi: 10.1158/0008-5472.CAN-10-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Lee YS, Tresguerres M, Hess K, Marmorstein LY, Levin LR, Buck J, Marmorstein AD. Regulation of anterior chamber drainage by bicarbonate-sensitive soluble adenylyl cyclase in the ciliary body. Journal of Biological Chemistry. 2011;286:41353–41358. doi: 10.1074/jbc.M111.284679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Banerjee J, Leung CT, Peterson-Yantorno K, Stamer WD, Civan MM. Mechanisms of ATP release, the enabling step in purinergic dynamics. Cellular Physiology & Biochemistry. 2011;28:1135–1144. doi: 10.1159/000335865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Leung CT, Peterson-Yantorno K, Stamer WD, Mitchell CH, Civan MM. Mechanisms of ATP release by human trabecular meshwork cells, the enabling step in purinergic regulation of aqueous humor outflow. Journal of Cellular Physiology. 2012;227:172–182. doi: 10.1002/jcp.22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch RM, Weber CS, Nullmeyer KD, Moore EDW, Paul RJ. Clearance of store-released Ca2+ by the Na+-Ca2+ exchanger is diminished in aortic smooth muscle from Na+-K+-ATPase alpha 2-isoform gene-ablated mice. American Journal of Physiology - Heart & Circulatory Physiology. 2008;294:H1407–1416. doi: 10.1152/ajpheart.00855.2007. [DOI] [PubMed] [Google Scholar]
- Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. Journal of Pharmacology & Experimental Therapeutics. 2009;328:409–418. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin CW, Zellhuber-McMillan S, Macknight AD, Civan MM. Electron microprobe analysis of ouabain-exposed ciliary epithelium: PE-NPE cell couplets form the functional units. American Journal of Physiology Cell Physiology. 2004;286:C1376–C1389. doi: 10.1152/ajpcell.00248.2003. [DOI] [PubMed] [Google Scholar]
- Pelis RM, Shahidullah M, Ghosh S, Coca-Prados M, Wright SH, Delamere NA. Localization of Multidrug Resistance-Associated Protein 2 in the Nonpigmented Ciliary Epithelium of the Eye. Journal of Pharmacology and Experimental Therapeutics. 2009;329:479–485. doi: 10.1124/jpet.108.149625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola G, Raviola E. Intercellular junctions in the ciliary epithelium. Investigative Ophthalmology & Visual Science. 1978;17:958–981. [PubMed] [Google Scholar]
- Saez JC, Contreras JE, Bukauskas FF, Retamal MA, Bennett MVL. Gap junction hemichannels in astrocytes of the CNS. Acta Physiologica Scandinavica. 2003;179:9–22. doi: 10.1046/j.1365-201X.2003.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez JC, Schalper KA, Retamal MA, Orellana JA, Shoji KF, Bennett MVL. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Experimental Cell Research. 2010;316:2377–2389. doi: 10.1016/j.yexcr.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Scemes E. Nature of plasmalemmal functional “hemichannels”. Biochimica et Biophysica Acta. 2012;1818:1880–1883. doi: 10.1016/j.bbamem.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AP, Bohmer AE, Antunes C, Schallenberger C, Porciuncula LO, Elisabetsky E, Lara DR, Souza DO. Anti-nociceptive properties of the xanthine oxidase inhibitor allopurinol in mice: role of A1 adenosine receptors. British Journal of Pharmacology. 2009;156:163–172. doi: 10.1111/j.1476-5381.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers TJ, Visser GJ, Flik G, Theuvenet AP. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992;12:870–874. 876–879. [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Beimgraben C, Delamere NA. Hyposmotic stress causes ATP release and stimulates Na, K-ATPase activity in porcine lens. Journal of Cellular Physiology. 2012a;227:1428–1437. doi: 10.1002/jcp.22858. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Delamere NA. Responses of Sodium-Hydrogen Exchange to Nitric Oxide in Porcine Cultured Nonpigmented Ciliary Epithelium. Invest. Ophthalmol. Vis. Sci. 2009;50:5851–5858. doi: 10.1167/iovs.09-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Delamere NA. TRPV4 in porcine lens epithelium regulates hemichannel-mediated ATP release and Na-K-ATPase activity. American Journal of Physiology -Cell Physiology. 2012b;302:C1751–1761. doi: 10.1152/ajpcell.00010.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidullah M, Tamiya S, Delamere NA. Primary culture of porcine nonpigmented ciliary epithelium. Current Eye Research. 2007;32:511–522. doi: 10.1080/02713680701434899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidullah M, Wilson WS, Yap M, To CH. Effects of ion transport and channel-blocking drugs on aqueous humor formation in isolated bovine eye. Investigative Ophthalmology & Visual Science. 2003;44:1185–1191. doi: 10.1167/iovs.02-0397. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Yap MK, To CH. cGMP, sodiun nitroprusside and sodium azide reduce aqueous humour formation in the isolated arterially perfused pig eye. British Journal of Pharmacology. 2005;144:1–9. doi: 10.1038/sj.bjp.0706156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Analytical Biochemistry. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Srinivas M. Pharmacology of Connexin Channels. In: Harris AL, Locke D, editors. Connexins: A guide. Humana-Springer; New York: 2009. pp. 207–224. [Google Scholar]
- Srinivas M, Costa M, Gao Y, Fort A, Fishman GI, Spray DC. Voltage dependence of macroscopic and unitary currents of gap junction channels formed by mouse connexin50 expressed in rat neuroblastoma cells. Journal of Physiology. 1999;517:673–689. doi: 10.1111/j.1469-7793.1999.0673s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M, Kronengold J, Bukauskas FF, Bargiello TA, Verselis VK. Correlative studies of gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophysical Journal. 2005;88:1725–1739. doi: 10.1529/biophysj.104.054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Gabelt BT, Crosson CE, Kaufman PL. Effects of adenosine agonists on intraocular pressure and aqueous humor dynamics in cynomolgus monkeys. Experimental Eye Research. 1997;64:979–989. doi: 10.1006/exer.1997.0296. [DOI] [PubMed] [Google Scholar]
- Vaiyapuri S, Jones CI, Sasikumar P, Moraes LA, Munger SJ, Wright JR, Ali MS, Sage T, Kaiser WJ, Tucker KL, Stain CJ, Bye AP, Jones S, Oviedo-Orta E, Simon AM, Mahaut-Smith MP, Gibbins JM. Gap Junctions and Connexin Hemichannels Underpin Hemostasis and Thrombosis. Circulation. 2012;125:2479–2491. doi: 10.1161/CIRCULATIONAHA.112.101246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiunas V. Cyclic nucleotide permeability through unopposed connexin hemichannels. Frontiers in Pharmacology. 2013;4:75. doi: 10.3389/fphar.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, Mathias RT, Robinson RB, Rosen MR, Cohen IS, Brink PR. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. Journal of Physiology. 2005;568:459–468. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez Almonacid LA, Tafuri S, Dipineto L, Matteoli G, Fiorillo E, Della Morte R, Fioretti A, Menna LF, Staiano N. Role of connexin-43 hemichannels in the pathogenesis of Yersinia enterocolitica. Veterinary Journal. 2009;182:452–457. doi: 10.1016/j.tvjl.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Wang Z, Do CW, Valiunas V, Leung CT, Cheng AKW, Clark AF, Wax MB, Chatterton JE, Civan MM. Regulation of gap junction coupling in bovine ciliary epithelium. American Journal of Physiology - Cell Physiology. 2010;298:C798–806. doi: 10.1152/ajpcell.00406.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Bruzzone R, Wolfram S, Paul DL, Goodenough DA. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins. Journal of Cell Biology. 1994;125:879–892. doi: 10.1083/jcb.125.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WS, Shahidullah M, Millar C. The bovine arterially-perfused eye: an in vitro method for the study of drug mechanisms on IOP, aqueous humour formation and uveal vasculature. Current Eye Research. 1993;12:609–620. doi: 10.3109/02713689309001840. [DOI] [PubMed] [Google Scholar]
- Wolosin JM, Schutte M, Chen S. Connexin distribution in the rabbit and rat ciliary body. A case for heterotypic epithelial gap junctions. Investigative Ophthalmology & Visual Science. 1997;38:341–348. [PubMed] [Google Scholar]
- Xu T, Xu P. Searching for molecular players differentially involved in neurotransmitter and neuropeptide release. Neurochemical Research. 2008;33:1915–1919. doi: 10.1007/s11064-008-9648-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Fukuta H, Nakahira Y, Suzuki H. Blockade by 18beta-glycyrrhetinic acid of intercellular electrical coupling in guinea-pig arterioles. Journal of Physiology. 1998;511:501–508. doi: 10.1111/j.1469-7793.1998.501bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi GA. Distribution of connexin50 channels and hemichannels in lens fibers: a structural approach. Cell Communication & Adhesion. 2003;10:265–270. doi: 10.1080/cac.10.4-6.265.270. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Loo DD, Kreman M, Eskandari S, Wright EM. Functional and morphological correlates of connexin50 expressed in Xenopus laevis oocytes. Journal of General Physiology. 1999;113:507–524. doi: 10.1085/jgp.113.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zou T, Liu Y, Qi Y. The gating effect of calmodulin and calcium on the connexin50 hemichannel. Biological Chemistry. 2006;387:595–601. doi: 10.1515/BC.2006.076. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Davidson BR, Stamer WD, Barton JK, Marmorstein LY, Marmorstein AD. Enhanced inflow and outflow rates despite lower IOP in bestrophin-2-deficient mice. Investigative Ophthalmology & Visual Science. 2009;50:765–770. doi: 10.1167/iovs.08-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]