Abstract
Bidens pilosa L. is an easy-to-grow, widespread, and palatable perennial on earth. Hence, it has traditionally been used as foods and medicines without noticeable adverse effects. Despite significant advancement in chemical and biological studies of B. pilosa over the past few years, comprehensive and critical reviews on its anti-diabetic properties are missing. The present review is to summarize up-to-date information on the pharmacology, phytochemistry, and toxicology of B. pilosa, in regard to type 1 diabetes and type 2 diabetes from the literature. In addition to botanical studies and records of the traditional use of B. pilosa in diabetes, scientific studies investigating antidiabetic action of this species and its active phytochemicals are presented and discussed. The structure and biosynthesis of B. pilosa and its polyynes in relation to their anti-diabetic action and mechanism are emphasized. Although some progress has been made, rigorous efforts are further required to unlock the molecular basis and structure-activity relationship of the polyynes isolated from B. pilosa before their clinical applications. The present review provides preliminary information and gives guidance for further anti-diabetic research and development of this plant.
1. Introduction
Diabetes was coined by a Greek physician, Aretaeus of Cappadocia (30-90 AD), about 2 millennia ago [1]. He first described this devastating disease with symptoms, such as constant thirst (polydipsia), excessive urination (polyuria), and weight loss, which still hold true nowadays [1]. The International Diabetes Federation (IDF) estimated that diabetes afflicted 285 million people, 6.4% of the world population, who were afflicted with diabetes in 2010 and will afflict 439 million people, 7.7% of the world population by 2030 [2]. Over 90% of diabetic patients are diagnosed with type 2 diabetes (T2D) [3, 4] and the rest are diagnosed with type 1 diabetes and others.
Diabetes is a chronic metabolic disease with fatal complications such as cardiovascular diseases, retinopathy, renopathy, and foot ulcers. The cost of health care associated with diabetes continues to grow and is a huge economic burden for afflicted patients and countries. In the states, approximately 17.5 million adults were reported to be receiving treatment for diabetes, where the estimated cost of diabetes was 174 billion dollars in 2007 [5].
Despite much progress made on basic and clinical research into diabetes, this disease has not been cured since antiquity. Main reasons for this mishap are unmet efficacy and significant side effect of the drugs. So far, 1200 plants have been claimed to be remedies for diabetes [6, 7] and one-third of them have been scientifically evaluated for T2D treatment [8]. Among them, B. pilosa is commonly used as food and medicine for humans and animals [9, 10]. It is an easy-to-grow herb that is globally distributed. The folkloric use of B. pilosa to treat diabetes has been recorded in America, Africa, Asia, and Oceania [11]. Accumulating data have shown the potential of this plant and active compounds to treat diabetes. The present review focuses on recent studies on the botany, anti-diabetic action and mechanism, phytochemistry, and toxicology of B. pilosa. The information provided here highlights the possible usefulness of B. pilosa and its isolated compounds and offers insights into possible future research directions.
2. Botanical Properties
B. pilosa is believed to have originated in South America and subsequently spread everywhere on earth [13]. Bidens species and their varieties bear vernacular names based on their sticky seeds or prosperous growth [5]. B. pilosa is taxonomically assigned to the Bidens genus, up to 240 species, as shown in Table 1 [9, 10]. Different varieties are frequently found in B. pilosa. B. pilosa is an erect, perennial herb widely distributed from temperate and tropical regions. It has serrate, lobed, or dissected form of green opposite leaves, white or yellow flowers, and long narrow ribbed black seeds (Figure 1) [14]. Apart from morphological traits, chemotaxonomical (Figure 2) and molecular characterization (Table 2) is sometimes helpful in the identification of B. pilosa strains [15].
Table 1.
Taxonomy of B. pilosa [12].
| Kingdom | Plantae |
| Division | Magnoliophyta |
| Class | Magnoliopsida |
| Subclass | Asteridae |
| Order | Asterales |
| Family | Asteraceae |
| Genus | Bidens |
| Species | Bidens pilosa L. |
Figure 1.

Image of B. pilosa (a) and its flowers (b) and seeds (c).
Figure 2.
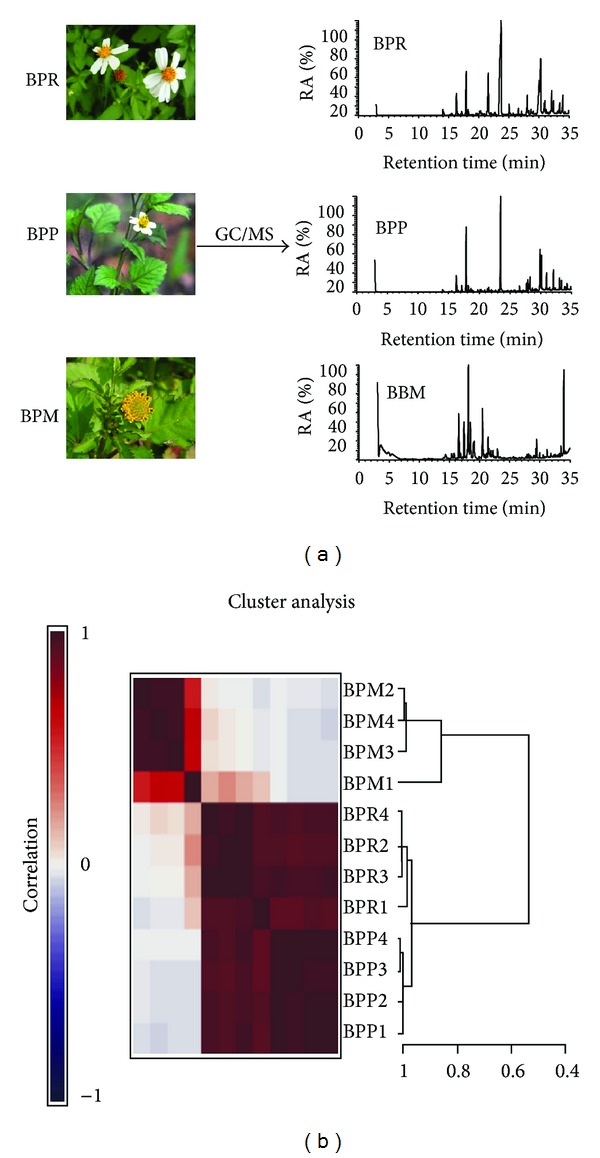
Chemotaxonomical comparison of three B. pilosa variants (BPR, B. pilosa var. radiata; BPM, B. pilosa var. Minor, and BPP, B. pilosa var. pilosa). Gas chromatography/mass spectrometry (GC/MS) and cluster analysis to assist in determining the taxonomy of 4 samples of the three Bidens variants.
Table 2.
Percent differences between the internal transcribed spacer 1 (ITS1) regions of the DNA sequences of B. pilosa variants (BPR, B. pilosa var. radiata; BPM, B. pilosa var. minor; and BPP, B. pilosa var. pilosa), B. hintonii, and B. biternata.
| ITS1 | BPM | BPP | BPR | BHa | BBb |
|---|---|---|---|---|---|
| BPM | 0 | 0.39 | 3.56 | 4.74 | 11.37 |
| BPP | 0.39 | 0 | 3.16 | 4.45 | 11.46 |
| BPR | 3.56 | 3.16 | 0 | 5.93 | 12.55 |
| BHa | 4.74 | 4.35 | 5.93 | 0 | 14.45 |
| BBb | 11.37 | 11.46 | 12.55 | 14.45 | 0 |
aITS1 obtained from GeneBank Accession Number AF330101.1.
bITS1 obtained from GeneBank Acession Number EU117248.1.
Despite its preference for full sun and semidry soil, B. pilosa can grow in arid and barren lands at different altitudes. Food and Agricultural Organization actively promoted the culture of B. pilosa in Africa in 1970s due to its fast-growing advantage [16]. B. pilosa can be propagated via seeds. After soaking, B. pilosa seeds can germinate in 3 to 4 days [17]. Minimal agricultural techniques are required for B. pilosa cultivation. B. pilosa is recognized as one of the top worst weeds worldwide because of its aggressive invasion [18].
Apart from its use as food ingredient, B. pilosa is used as herbal medicines for diabetes and 40 other diseases [5]. All parts of B. pilosa plant, the whole plant, the aerial parts (leaves, flowers, seeds, and stems), and/or the roots, fresh or dried, are used as ingredients in folk medicines. Dry powder, decoction, maceration, and tincture are usual formulations for its internal as well as external use [19]. B. pilosa can be used alone or together with other medicinal herbs.
3. Antidiabetic Properties
B. pilosa has a variety of pharmacological actions. As far as its anti-diabetic activity is concerned, B. pilosa and its anti-diabetic polyynes have been reported to effectively prevent and treat type 1 diabetes and type 2 diabetes, which are etiologically distinct [15, 20–24]. In this section, we will focus our review on the pharmacological action and mechanism of B. pilosa extract and its active phytochemicals against both types of diabetes.
3.1. Action and Mechanism of B. pilosa for Type 1 Diabetes
T1D is caused by the autoimmune destruction of pancreatic β cells, leading to insulin deficiency, hyperglycemia, and complications. Monotherapy (immune intervention and β-cell replacement/(re)generation) and their combination therapy are common approaches to treat T1D. Despite considerable advances made on these approaches, there has no cure for T1D. Helper T (Th) cell differentiation regulates T1D development. Further, Th1 cell differentiation promotes T1D, whereas Th2 cell differentiation alleviates T1D [25].
To test the immunomodulatory effect of B. pilosa, one study showed that B. pilosa extract and its butanol fraction could decrease Th1 cells and cytokines and increase Th2 cells and cytokines [23]. This study indicated that IC50 value of the butanol fraction was 200 μg/mL. This inhibition was reported to be partially attributed to cytotoxicity, because the butanol fraction at 180 μg/mL could cause 50% death of Th1 cells. Using the bioactivity-directed isolation and identification approach (Figure 3), 3 active polyynes, 3-β-D-glucopyranosyl-1-hydroxy-6(E)-tetradecene-8,10,12-triyne (17), 2-β-D-glucopyranosyloxy-1-hydroxy-5(E)-tridecene-7,9,11-triyne (16), and 2-β-D-glucopyranosyloxy-1-hydroxytrideca-5,7,9,11-tetrayne (cytopiloyne, 19), as well as 2 index compounds, 4,5-Di-O-caffeoylquinic acid, 3,5-Di-O-caffeoylquinic acid, and 3,4-Di-O-caffeoylquinic acid, were isolated from the butanol extract using high pressure liquid chromatography (HPLC) and, in turns, were structurally identified by nuclear magnetic resonance (NMR) [23, 24]. Only the first three active compounds showed similar effects on Th cell differentiation as the B. pilosa butanol fraction. Moreover, compound 19 showed greater activity than compounds 17 and 16 in terms of enhancement (by 277% compared to 34% and 8%) of differentiation of Th0 to Th2 at 10 μg/mL and inhibition (by 60% compared to 17% and 9%) of differentiation to Th1 at the same concentration (Table 3) [23, 24].
Figure 3.
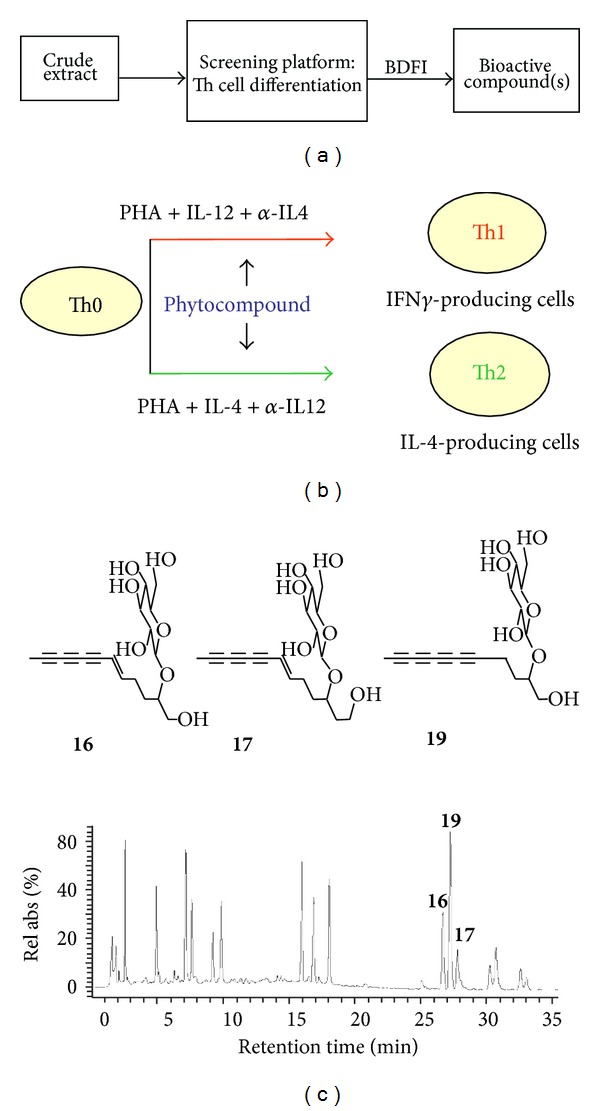
Bioactivity-directed fractionation and isolation approach to identify three active polyynes that regulate Th cell differentiation. A flowchart of the bioactivity-directed fractionation and isolation (BDFI) strategy describes the use of the screening platform and Th cell differentiation assays to determine bioactive compounds from the crude extract and fractions of B. pilosa (a). Bioassays are composed of human naïve helper T cells (Th0) which can differentiate into type 1 helper T (Th1) cells and type 2 helper T (Th2) cells in the presence of PHA plus IL-12 and anti-IL-4 antibody and PHA with IL-4 and anti-IL-12 antibody, respectively. The crude extract, fractions, and compounds of B. pilosa are added to differentiating cells to test the Th cell differentiation (b). Compounds 16, 17, and 19 are active compounds that promote Th2 cell differentiation but inhibit Th1 cell differentiation.
Table 3.
Th1 inhibition and Th2 promotion by the extract (150 μg/mL) and polyynes (10 μg/mL) of B. pilosa.
| Butanol extract | Compound 19 | Compound 17 | Compound 16 | |
|---|---|---|---|---|
| Reduction of Th1 (%) | 32% | 75% | 17% | 9% |
| Increase of Th2 (%) | 103% | 277% | 31% | 6% |
Accordingly, the butanol fraction of B. pilosa effectively prevented T1D in nonobese diabetic (NOD) mice [23]. Consistently, this prevention involved downregulation of Th1 cells or upregulation of Th2 cells. This was proven by intraperitoneal injection of the butanol fraction at a dose of 3 mg/kg body weight (BW), 3 times a week, to NOD mice from 4 to 27 weeks [23]. This dosage resulted in lower incidence of diabetes (33%). At a dose of 10 mg/kg, the butanol fraction of B. pilosa totally stopped (0%) the initiation of the disease [23]. Th1 cytokine IFNγ and Th2 cytokine IL-4 favor the production of IgG2a and IgE, respectively. To further confirm whether this butanol in vivo regulated Th cell differentiation and Th cytokine profiling, IgG2a and IgE production was measured in the serum of NOD mice. As expected, high levels of IgE and some decline in the levels of IgG2a were observed in the serum [23].
Since cytopiloyne (19) had the most potent effect on Th cell differentiation among the aforesaid polyynes [20], another study used cytopiloyne to explore the action and molecular mechanism of cytopiloyne on T1D in NOD mice [20]. NOD mice received intraperitoneal or intramuscular injection of cytopiloyne at 25 μg/kg BW, 3 times per week. Twelve-week-old NOD mice started to develop T1D, and 70% of NOD mice aged 23 weeks and over developed T1D. Remarkably, 12- to 30-week-old NOD mice treated with cytopiloyne showed normal levels of blood glucose (<200 mg/dL) and insulin (1-2 ng/mL). Consistent with T1D incidence, cytopiloyne delayed and reduced the invasion of CD4+ T cells into the pancreatic islets [20]. Albeit less effective than cytopiloyne (19), 3-β-D-glucopyranosyl-1-hydroxy-6(E)-tetradecene-8,10,12-triyne (17), and 2-β-D-glucopyranosyloxy-1-hydroxy-5(E)-tridecene-7,9,11-triyne (16) also decreased T1D development in NOD mice.
The underlying mechanism by which cytopiloyne and its derivatives inhibited T1D covered inactivation of T cells, polarization of Th cell differentiation, and Th cell depletion, leading to islet protection [20] and is illustrated in Figure 4. First, [3H] thymidine incorporation assay showed that cytopiloyne inhibited ConA/IL-2- and CD3 antibody-mediated T cell proliferation, implying that cytopiloyne could inhibit T cell activation. Second, in vitro study showed that cytopiloyne (19) inhibited the differentiation of naïve Th (Th0) cells (i.e., CD4+ T cells) into Th1 cells and promoted differentiation of Th0 cells into Th2 cells [24]. The in vitro data are consistent with the in vivo results, indicating that cytopiloyne reduced Th1 differentiation and increased Th2 differentiation as shown by intracellular cytokine staining and FACS analysis [20]. Cytopiloyne also enhanced the expression of GATA-3, a master gene for Th2 cell differentiation, but not the expression of T-bet, a master gene for Th1 cell differentiation, further supporting its role in skewing Th differentiation [20]. In line with the skewing of Th differentiation, the level of serum IFN-γ and IgG2c decreased, while that of serum IL-4 and serum IgE increased compared to the negative controls (PBS-treated mice). Third, cytopiloyne partially depleted CD4+ rather than CD8+ T cells in NOD mice [20]. Coculture assays showed that the depletion of CD4+ T cells was mediated through the induction of Fas ligand expression on pancreatic islet cells by cytopiloyne, leading to apoptosis of infiltrating CD4+ T cells in the pancreas via the Fas and Fas ligand pathway. However, cytopiloyne did not induce the expression of TNF-α in pancreatic islet cells and, thus, had no effect on CD8+ T cells [20].
Figure 4.
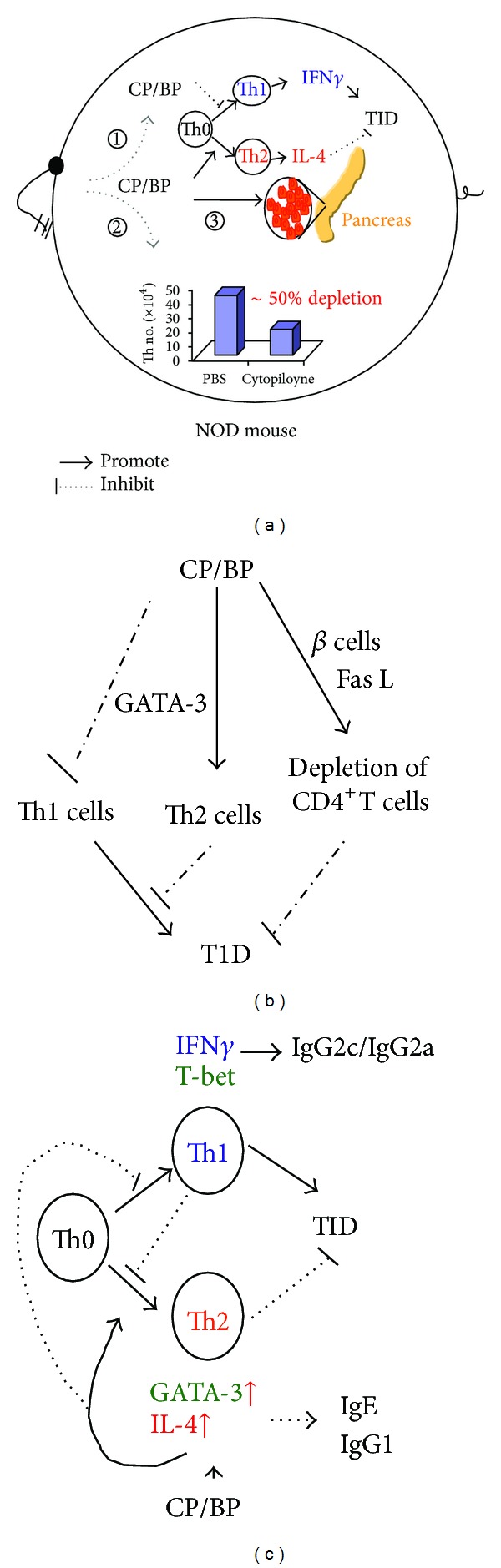
The underlying mechanism of the crude extract of B. pilosa (BP) and its active compound, compound 19 (CP), in T1D. BP and/or CP can suppress T1D development via regulation of T cells (① and ②) and β cells (③) in NOD mice (a). Their regulation of T cells involves Th cell activation and differentiation (①) and partial depletion of Th0 cells (②) as depicted (b). CP and/or BP augment the expression of GATA-3 gene and, in turn, promote the expression of IL-4 and Th2 cell differentiation. In contrast, CP and/or BP do not affect the expression of T-bet (c).
Due to the antidiabetic mechanisms of action, it was hypothesized that cytopiloyne protects NOD mice from diabetes by a generalized suppression of adaptive immunity. To evaluate this hypothesis, ovalbumin (Ova) was used as a T-cell dependent antigen to prime NOD mice, which had already received cytopiloyne or PBS vehicle. Ova priming enhanced similar anti-Ova titers in cytopiloyne-treated mice and PBS-treated mice, but a different profile of immunoglobulin isotype was observed in the two groups. Therefore, it was concluded that cytopiloyne is an immunomodulatory compound rather than an immunosuppressive compound [20, 24].
Collectively speaking, the mechanism of action of cytopiloyne and, probably, its polyyne derivatives in T1D includes inhibition of T-cell proliferation, skewing of Th cell differentiation and partial depletion of Th cells, and protection of β pancreatic islets.
3.2. Action and Mechanism of B. pilosa for Type 2 Diabetes
Mounting evidence from epidemiological studies proposes environmental and genetic factors as the primary causes of T2D. Both factors contribute to insulin resistance and loss of β-cell function, leading to impairment in insulin action, insulin production, or both. As a result, this impairment accompanies the development of hyperglycemia, a major pathological feature of T2D [26]. Hyperglycemia can cause damage to β cells and other peripheral tissues, named glucotoxicity. As a consequence, cardiovascular disease, nephropathy, retinal blindness, neuropathy, and peripheral gangrene develop and contribute to mortality [27]. Therefore, maintenance of glycemic homeostasis has been a golden standard for T2D therapy. Moreover, aberrant lipid metabolism in adipose and other tissues can cause lipotoxicity, which can further worsen diabetic complications. The β cells in the pancreas are the key players in glycemic homeostasis [28].
Plants are an extraordinary source for anti-diabetic agents. Over 1200 plant species have be claimed to treat diabetes [6, 7]. One of them, B. pilosa, has traditionally been used as an anti-diabetic herb in America, Africa, and Asia [7, 29, 30]. More recently, B. pilosa has scientifically been investigated for anti-diabetic activity. One seminal study by Ubillas et al. showed that the aqueous ethanol extract of the aerial part of B. pilosa at 1 g/kg BW lowered blood glucose in db/db mice, a T2D mouse model [30]. They also used a bioactivity-guided identification strategy to identify two polyynes, compounds 17 and 16. Moreover, the mixture of the compounds (17 : 16) in a 2 : 3 ratio significantly reduced blood glucose level and food intake on the second day when administered at doses of 0.25 g/kg twice a day to C5BL/Ks-db/db mice. When evaluated at 0.5 g/kg, a more substantial decrease in blood glucose level as well as the stronger anorexia (food intake reduced from 5.8 g/mouse/day to 2.5 g/mouse/day) was noticed [30]. This study suggested that compounds 17 and 16 were active ingredients of B. pilosa for diabetes [30]. The anti-diabetic effect of both polyynes was partially caused by the hunger suppressing effect. However, the anoxic effect of the ethanol extract of B. pilosa was not observed in the studies described below. In another study [31], water extracts of B. pilosa (BPWE) were tested in diabetic db/db mice, aged 6–8 weeks, with postmeal blood glucose levels of 350 to 400 mg/dL. Like oral anti-diabetic glimepiride, which stimulates insulin release, one-single dose of BPWE reduced blood glucose levels from 374 to 144 mg/dL. The antihyperglycemic effect of BPWE was relevant to an increase in serum insulin levels, implying that BPWE drops blood glucose concentration through an upregulation of insulin production. However, BPWE showed different insulin secretion kinetics from glimepiride [31]. One drawback in current anti-diabetic agents is their decreasing efficacy over time. Therefore, the authors investigated the long-term anti-diabetic effect of BPWE in db/db mice. BPWE lowered blood glucose, boosted blood insulin, improved glucose tolerance, and reduced the percentage of glycosylated hemoglobulin (HbA1c). Both one-time and long-term experiments strongly support the superior action of BPWE on diabetes [31]. Unlike glimepiride, which failed to preserve pancreatic islets, BPWE was significantly protected against islet atrophy in mouse pancreas. The group also evaluated anti-diabetic effect of 3 B. pilosa varieties, B. pilosa var. radiate (BPR), B. pilosa var. pilosa (BPP), and B. pilosa var. minor (BPM) in db/db mice [15]. A single oral dose (10, 50, and 250 mg/kg BW) of BPR, BPP, or BPM water extracts decreased postprandial blood glucose levels in db/db mice for up to four hours and this reduction was dose-dependent. Of note, BPR extract resulted in a higher reduction in blood glucose levels when administered at the same dose as the other two varieties. Further, the BPR extract increased serum insulin levels in db/db mice to a greater extent than the other varieties at the same dose, 50 mg/kg. Three polyynes, compounds 16, 17, and 19, were identified from the three Bidens strains though their varied contents. Compound 19 at 0.5 mg/kg exerted a better stimulation for insulin production in db/db mice than compounds 17 and 16. On the contrary, 28-day treatment with the Bidens extracts and three polyynes were then performed using diabetic mice with postprandial glucose levels from 370 to 420 mg/dL and glimepiride was used as positive control. The applied dosages ranged from 10 mg/kg to 250 mg/kg BW. Results showed that the positive control as well as the crude extracts of the three varieties lowered the blood glucose levels in db/db mice. However, only BPR extract, containing a higher content of cytopiloyne (19), reduced blood glucose levels and augmented blood insulin levels more than BPP and BPM. The percentage of glycosylated hemoglobin A1c (HbA1c), a long-term indicator of blood homeostasis, was also monitored. HbA1c in the blood of 10- to 12-week-old diabetic mice was 7.9 ± 0.5%. However, treatment with BPR crude extract (50 mg/kg), glimepiride (1 mg/kg), and compound 19 (0.5 mg/kg) led to the HbA1c of 6.6 ± 0.2%, 6.1 ± 0.3%, and 6.2 ± 0.3% in the blood of age-matched mice, respectively [15]. Since cytopiloyne (19) was the most effective polyyne found in B. pilosa, against T2D, it was used for further study on anti-diabetic action and mechanism in another study [22]. The data confirmed that cytopiloyne reduced postmeal blood glucose levels, increased blood insulin, improved glucose tolerance, suppressed HbA1c level, and protected pancreatic islets in db/db mice. Nevertheless, cytopiloyne never managed to decrease blood glucose in streptozocin- (STZ-) treated mice whose β cells were already destroyed. In addition, cytopiloyne dose-dependently promoted insulin secretion and expression in β cells as well as calcium influx, diacylglycerol, and activation of protein kinase Cα. Taken together, the mechanistic studies suggest that cytopiloyne acts to treat T2D via regulation of β cell functions (insulin production and β cell preservation) involving the calcium/diacylglycerol/PKCα cascade (Figure 5).
Figure 5.
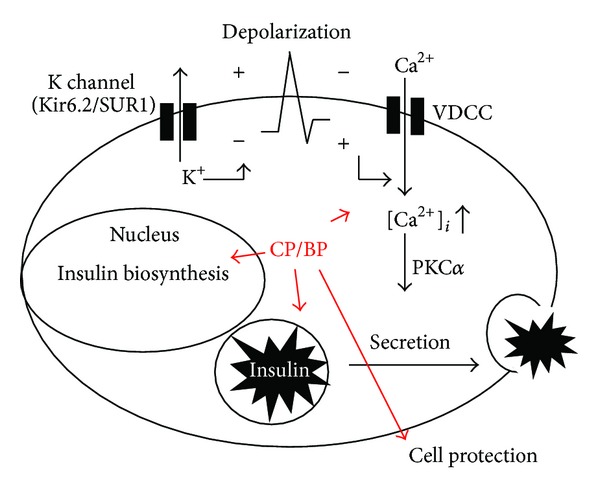
The underlying mechanism of the crude extract of B. pilosa (BP) and its active compound, compound 19 (CP), in T2D. BP and/or CP can treat T2D development via control of β cell function in db/db mice. Their anti-diabetic actions are through upregulation of insulin expression/secretion and protection of β cells involving secondary messengers (calcium and diacylglycerol) and their downstream PKCα pathway.
The above studies point to the conclusion that cytopiloyne (19) and related polyynes (compounds 16 and 17) are anti-diabetic agents in animal models. The data unfold a new biological action of polyynes. However, like all drugs developed for diabetes, cytopiloyne could neither prevent nor stop diabetes completely but reduced diabetic complications [22]. Intriguingly, 36 polyynes have been found in B. pilosa so far. It remains to be investigated whether all the polyynes present in this plant have anti-diabetic activities.
4. Phytochemistry
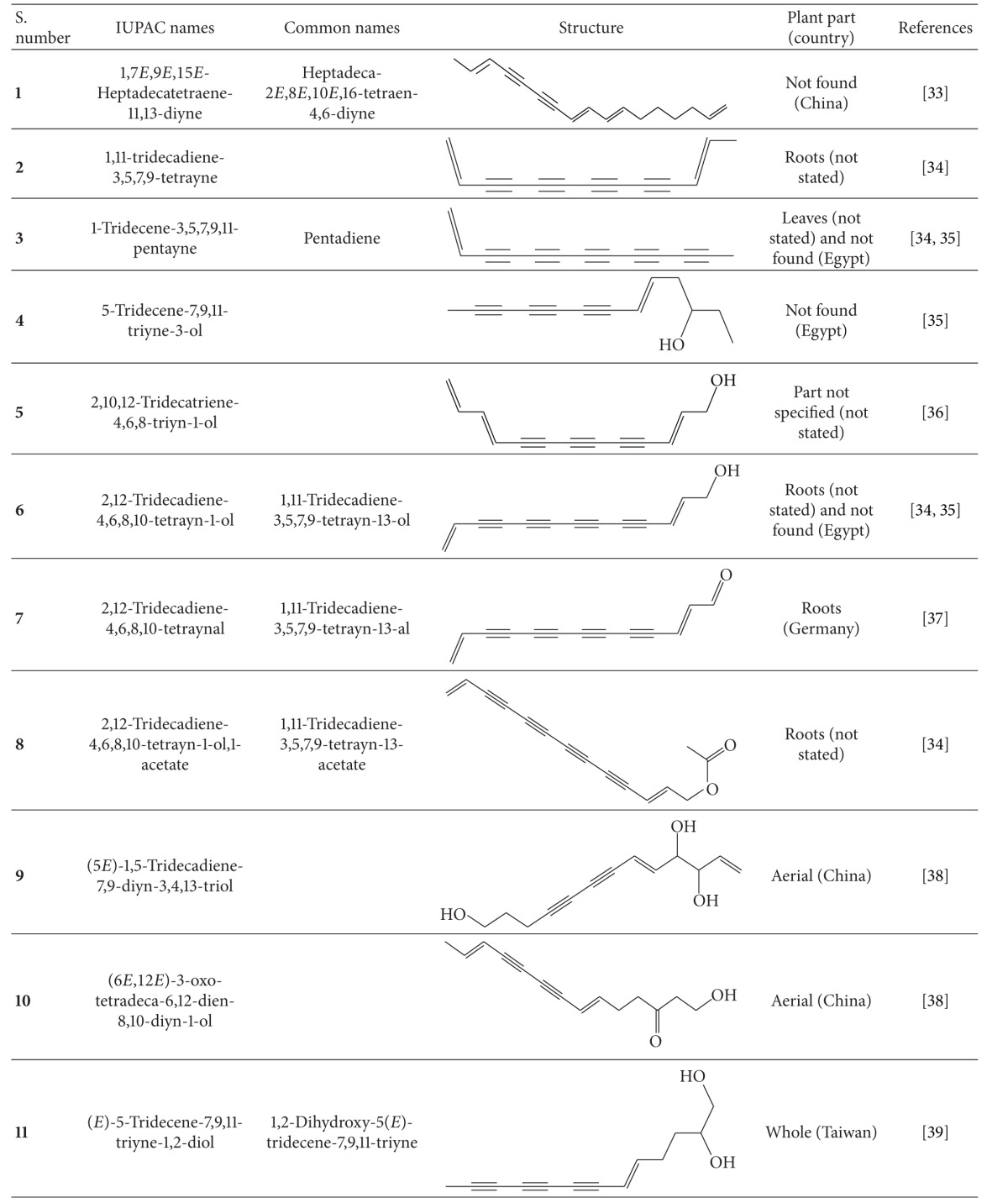
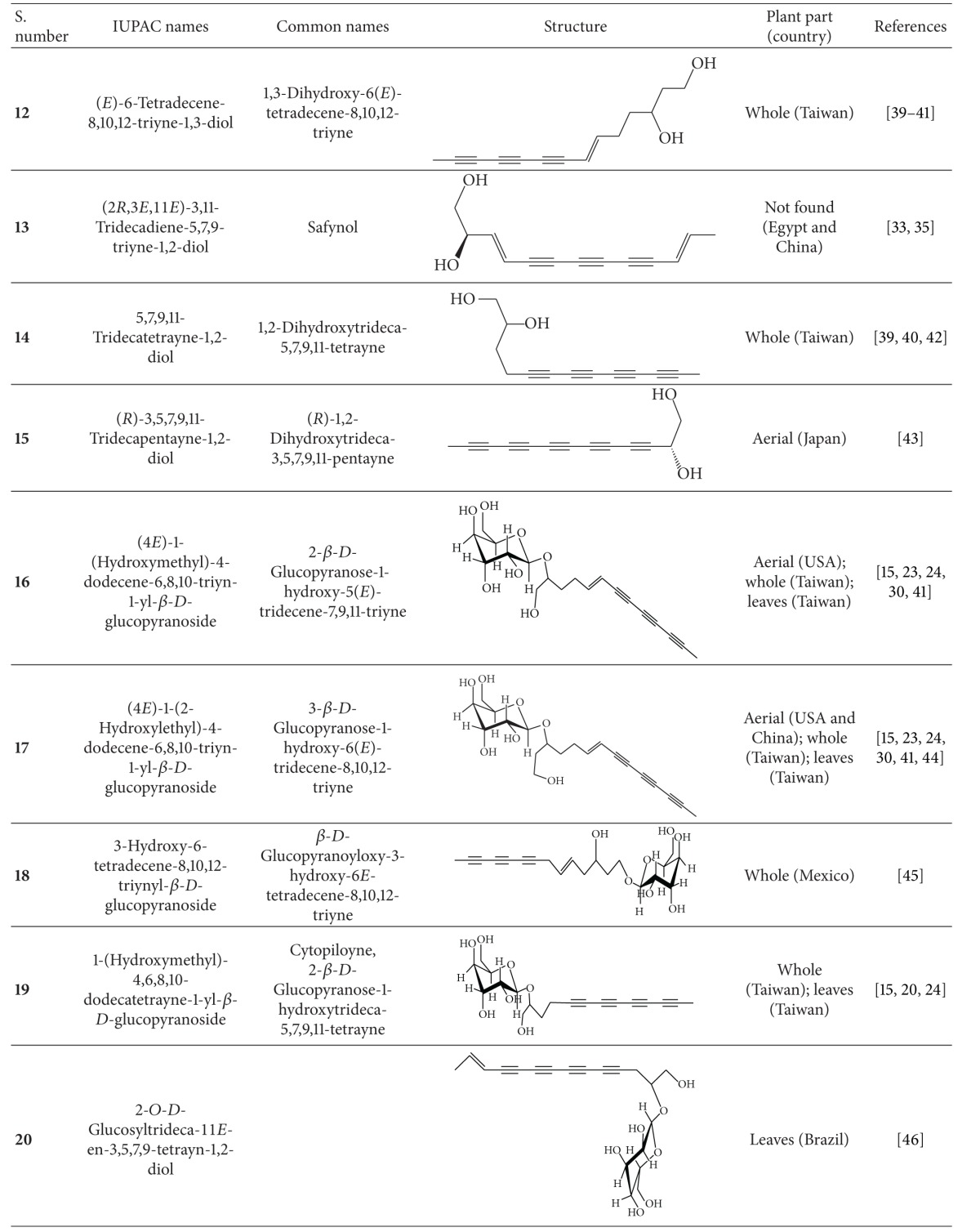
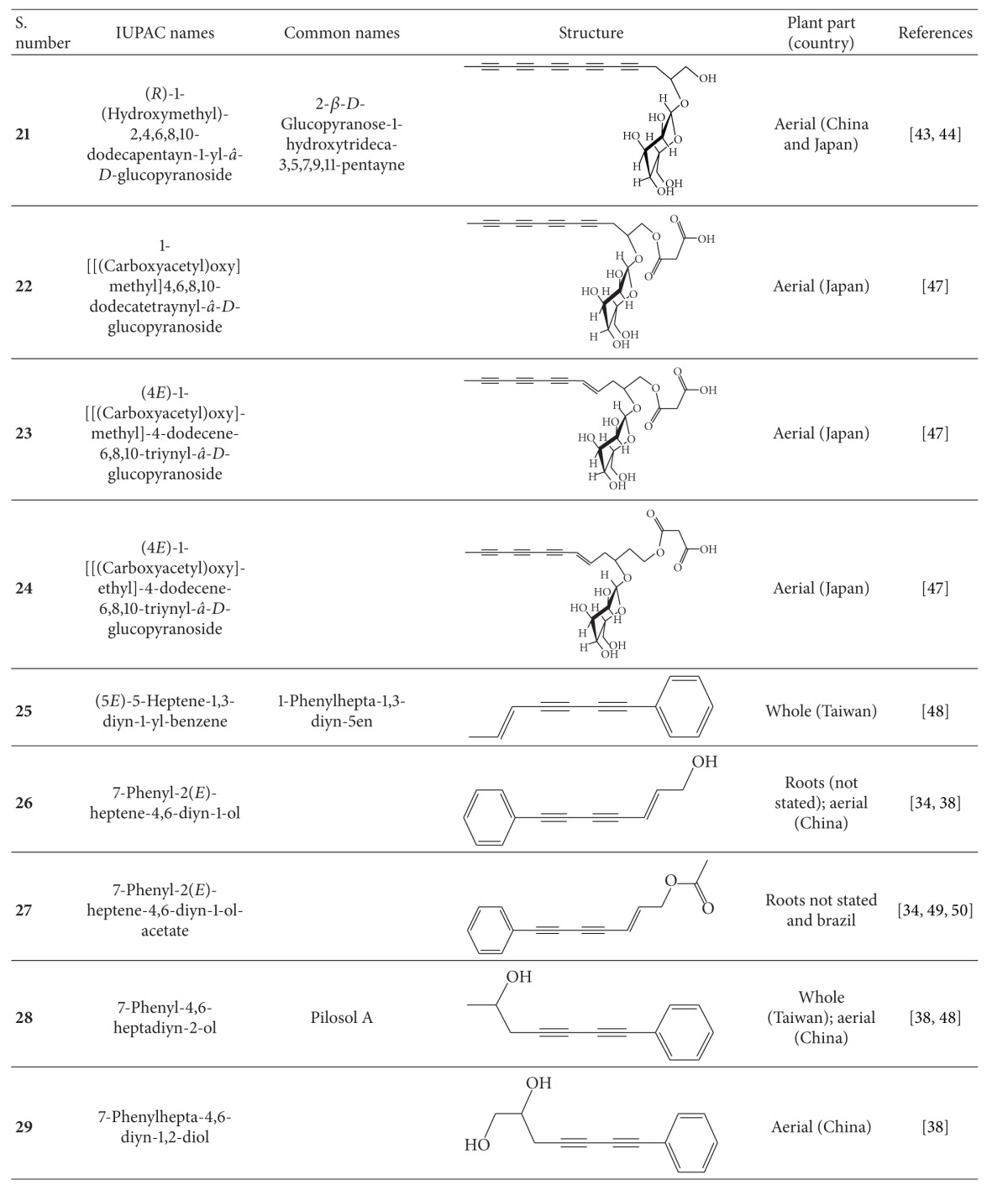
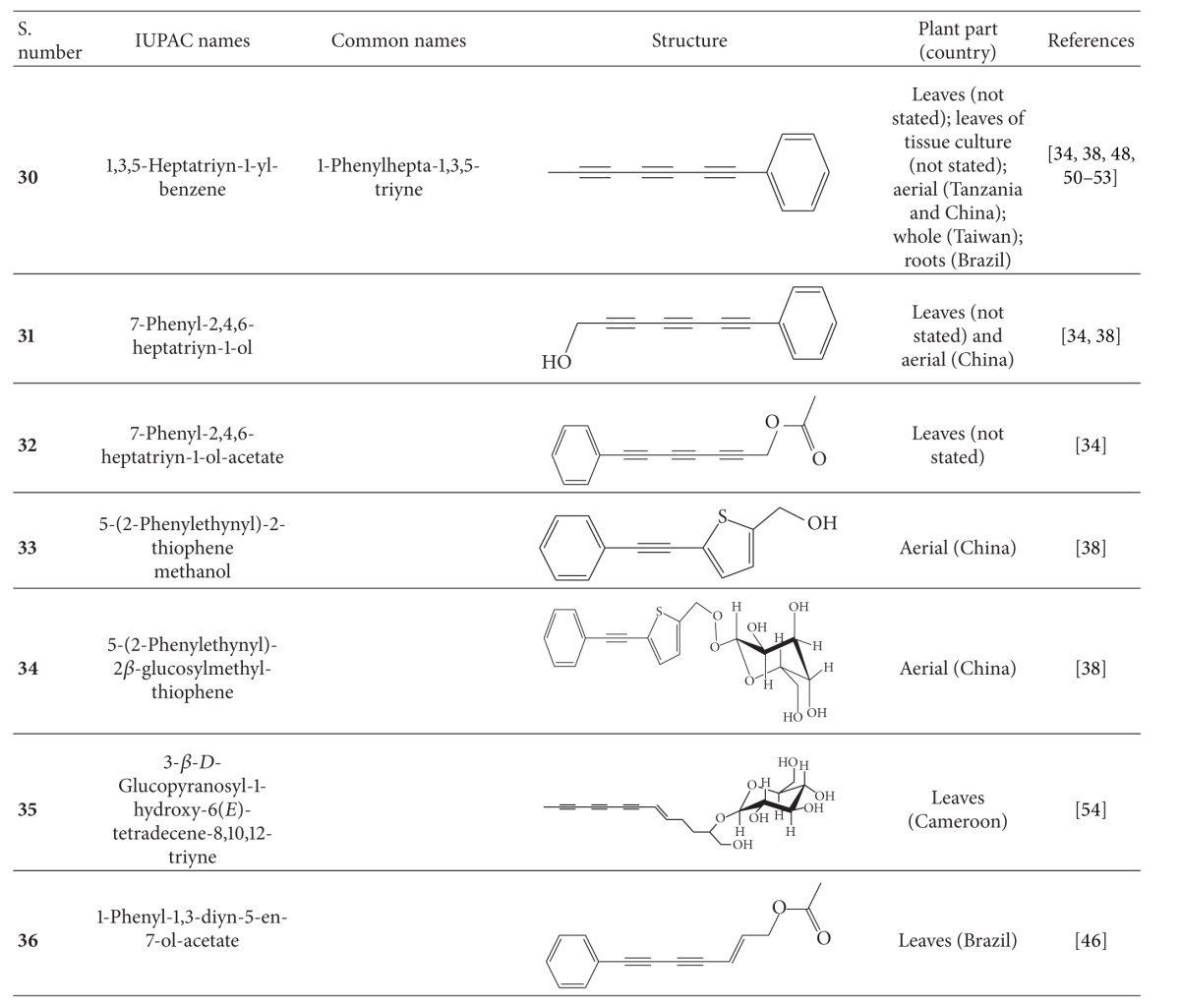
Broad application of B. pilosa all over the world has led to over 120 publications on its exploitation and use in medicines, foods, and drinks. B. pilosa is an extraordinary source of phytochemicals and 201 compounds have so far been identified from this plant, including 70 aliphatics (36 polyynes), 60 flavonoids, 25 terpenoids, 19 phenylpropanoids, 13 aromatics, 8 porphyrins, and 6 other compounds [32]. Mounting evidence suggests that phytochemical complexity of B. pilosa can account for its diverse bioactivities. The structures and likely bioactivities of these compounds were recently reviewed in the previous publications [5, 32], which are out of our scope. In this section, we focus on the chemical structures of 36 polyynes found in B. pilosa (Table 4) and their bioactivities (Table 5). We also discuss the likely biosynthesis of the polyynes in B. pilosa. Although their biosynthesis is not well defined, those polyynes are thought to derive from desaturation and acetylenation of fatty acids (Figure 6) in B. pilosa and other plants.
Table 4.
Polyynes isolated from B. pilosa [32].
|
Table 5.
Polyynes of B. pilosa and their biological activities.
| S. number | Name | Classification | Molecular formula | Biological activities |
|---|---|---|---|---|
| 11 | 1,2-Dihydroxy-5(E)-tridecene-7,9,11-triyne [55] | Polyyne | C13H14O2 | Antiangiogenetic [55] |
| Antiproliferative [55] | ||||
| 12 | 1,3-Dihydroxy-6(E)-tetradecene-8,10,12-triyne [56] | Polyyne | C14H16O2 | Antiangiogenetic [56] |
| 14 | 1,2-Dihydroxytrideca-5,7,9,11-tetrayne [56] | Polyyne | C13H12O2 | Antiangiogenetic [56] |
| 15 | (R)-1,2-dihydroxytrideca-3,5,7,9,11-pentayne [43] | Polyyne | C13H8O2 | Antimalarial [43] |
| Antibacterial [43] | ||||
| 6 | 2-β-D-Glucopyranose-1-hydroxy-5(E)-tridecene-7,9,11-triyne [54] | Polyyne | C19H24O7 | Antidiabetic [54] |
| Anti-inflammatory [57] | ||||
| Antimalarial [43] | ||||
| Antibacterial [43] | ||||
| 30 | 1-Phenylhepta-1,3,5-triyne [58] | Polyyne | C13H8 | Antimicrobial [59] |
| Antimalarial [9] | ||||
| Cytotoxic [9] | ||||
| Antifungal [60] | ||||
| 35 | 3-β-D-Glucopyranosyl-1-hydroxy-6(E)-tetradecene-8,10,12-triyne [54] | Polyyne | C20H26O7 | Antidiabetic [54] |
| Anti-inflammatory [57] |
Figure 6.
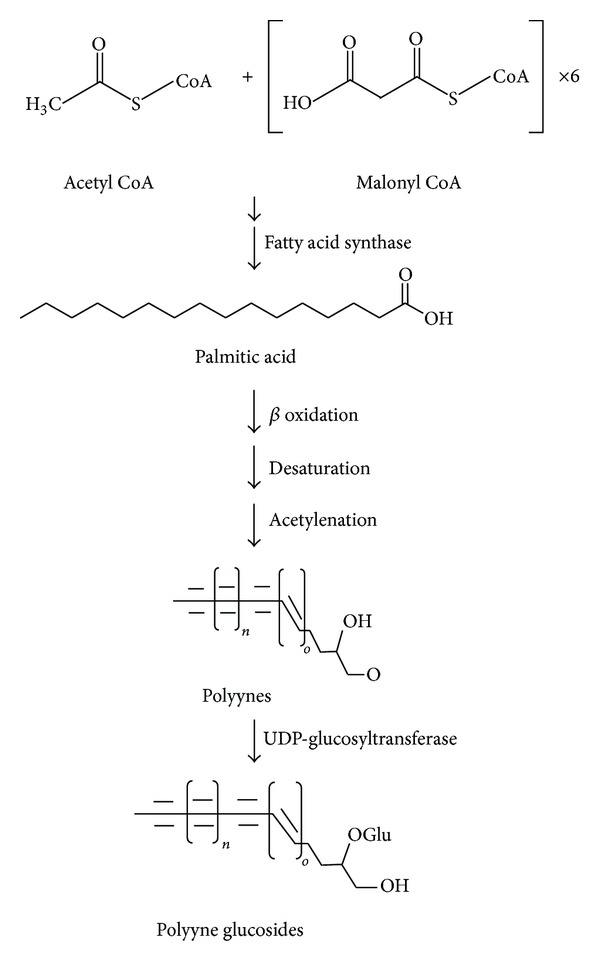
A scheme describing the biosynthesis of polyynes and its glucosides. Saturated fatty acids (e.g., palmitic acid, etc.) are synthesized from their common precursors, acetyl CoA and malonyl CoA. To generate acetylenic fatty acids (i.e., polyynes), the saturated fatty acids are proposed to undergo β oxidation, desaturation, and acetylenation. UDP-glucosyltransferase can attach a glucose moiety or more to polyynes to produce polyyne glucosides.
5. Toxicology
Food and Agricultural Organization of the United Nations has reported B. pilosa as a staple food and promoted its cultivation in Africa since 1975. Taiwanese Department of Health also allows its use as an ingredient in food for human consumption. Despite lack of systemic toxicological study of B. pilosa in humans, some information about acute and/or subchronic toxicities was revealed in rodents. Frida and colleagues reported that one-single oral dose of the water extract of B. pilosa leaves at 10 g/kg BW showed no obvious mortality or changes in the appearance of rats [61]. The same extract at 0.8 g/kg BW/day, once a day, showed no obvious sub-chronic toxicity in rats over 28 days, as shown by survival rate, body weight, and gross examination of organs [61]. They also evaluated the acute toxicity of hydroethanol extracts of B. pilosa in mice [61]. Five- to six-week-old mice, weighing between 28 and 35 g, received a peritoneal injection of both extracts at the different doses. The LD50, the dose that causes 50% lethality, of the hydroethanol extracts in mice was 12.3 g/kg BW and 6.2 g/kg BW, respectively [61]. Ezeonwumelu et al. showed that oral delivery of the water extract of the B. pilosa whole plant at 1 g/kg BW/day, once a day, seems nontoxic in rats over 28 days in Wistar rats [62], which is in line with our observations in rat [5]. Overall, these data suggest that consumption of B. pilosa aqueous extract at up to at 1 g/kg BW/day, once a day, is highly safe in rats. A complete toxicology and drug-drug interaction of B. pilosa with other drugs in humans are required prior to its further medical use.
6. Conclusions
B. pilosa is a worldwide plant and widely used as folk remedies and foods. It has long used to treat diabetes in different continents. However, a comprehensive up-to-date review of research on B. pilosa for diabetes has hitherto been not available. In this paper, scientific studies on the use of B. pilosa as an anti-diabetic remedy have been summarized and critically discussed from botanical, phytochemical, pharmacological, and toxicological aspects. Thirty-six polyynes identified from this plant were identified and three of which were showed to treat both T1D and T2D. The anti-diabetic utility of B. pilosa and its modes of action in relation to its known polyynes were discussed herein. Cautions should be taken in the anti-diabetic use of B. pilosa alone and in combination with other medicines since its overdose may cause dramatic hypoglycemia.
Acknowledgments
The author thanks the laboratory members for their excellent technique assistance and figure preparation and the authors whose publications are cited for their contributions. This work was supported by Grants 99-CDA-L11 from Academia Sinica, Taiwan.
Conflict of Interests
The author declares that there is no conflict of interests regarding the publication of this paper.
References
- 1.Laios K, Karamanou M, Saridaki Z, Androutsos G. Aretaeus of Cappadocia and the first description of diabetes. Hormones. 2012;11(1):109–113. doi: 10.1007/BF03401545. [DOI] [PubMed] [Google Scholar]
- 2.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Research and Clinical Practice. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Attele AS, Zhou Y-P, Xie J-T, et al. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51(6):1851–1858. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 4.Boyle JP, Engelgau MM, Thompson TJ, et al. Estimating prevalence of type 1 and type 2 diabetes in a population of African Americans with diabetes mellitus. American Journal of Epidemiology. 1999;149(1):55–63. doi: 10.1093/oxfordjournals.aje.a009728. [DOI] [PubMed] [Google Scholar]
- 5.Bartolome AP, Villasenor IM, Yang WC. Bidens pilosa L. (Asteraceae): botanical properties, traditional uses, phytochemistry, and pharmacology. Evidence-Based Complementary and Alternative Medicine. 2013;2013:51 pages. doi: 10.1155/2013/340215.340215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habeck M. Diabetes treatments get sweet help from nature. Nature Medicine. 2003;9(10):p. 1228. doi: 10.1038/nm1003-1228a. [DOI] [PubMed] [Google Scholar]
- 7.Marles RJ, Farnsworth NR. Antidiabetic plants and their active constituents. Phytomedicine. 1995;2(2):137–189. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- 8.Singh J, Cumming E, Manoharan G, Kalasz H, Adeghate E. Medicinal chemistry of the anti-diabetic effects of momordica charantia: active constituents and modes of actions. Open Medicinal Chemistry Journal. 2011;5(2, supplement):70–77. doi: 10.2174/1874104501105010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karis PO, Ryding O. Asteraceae: cladistics and classification. In: Bremer K, editor. Portland, Ore, USA: Timber press; 1994. pp. 559–569. [Google Scholar]
- 10.Pozharitskaya ON, Shikov AN, Makarova MN, et al. Anti-inflammatory activity of a HPLC-fingerprinted aqueous infusion of aerial part of Bidens tripartita L. Phytomedicine. 2010;17(6):463–468. doi: 10.1016/j.phymed.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira FQ, Andrade-Neto V, Krettli AU, Brandão MGL. New evidences of antimalarial activity of Bidens pilosa roots extract correlated with polyacetylene and flavonoids. Journal of Ethnopharmacology. 2004;93(1):39–42. doi: 10.1016/j.jep.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 12.Agriculture USDo. Natural Resources Conservation Service, United State. United States Department of Agriculture; 2012. Plants database. [Google Scholar]
- 13.Ge C. Cytologic study of Bidens bipinnata L. China Journal of Chinese Materia Medica. 1990;15(2):72–125. [PubMed] [Google Scholar]
- 14.Alcaraz MJ, Jimenez MJ. Flavonoids as anti-inflammatory agents. Fitoterapia. 1988;59(1):25–38. [Google Scholar]
- 15.Chien S-C, Young PH, Hsu Y-J, et al. Anti-diabetic properties of three common Bidens pilosa variants in Taiwan. Phytochemistry. 2009;70(10):1246–1254. doi: 10.1016/j.phytochem.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 16.FAO U. A Resource Book for Teachers of Agriculture. Rome, Italy: Publishing Management Group, FAO Information Division; 1997. Agriculture food and nutrition for Africa. [Google Scholar]
- 17.Rokaya MB, Münzbergová Z, Timsina B, Bhattarai KR. Rheum australe D. Don: a review of its botany, ethnobotany, phytochemistry and pharmacology. Journal of Ethnopharmacology. 2012;141(3):761–774. doi: 10.1016/j.jep.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 18.Young PH, Hsu YJ, Yang CW. Bidens pilosa L. and its medicinal use. In: Awaad AS, Singh VK, Govil JN, editors. Recent Progress in Medicinal Plants Drug Plant II. Standium Press LLC; 2010. [Google Scholar]
- 19.Redl K, Breu W, Davis B, Bauer R. Anti-inflammatory active polyacetylenes from Bidens campylotheca . Planta Medica. 1994;60(1):58–62. doi: 10.1055/s-2006-959409. [DOI] [PubMed] [Google Scholar]
- 20.Chang CL-T, Chang S-L, Lee Y-M, et al. Cytopiloyne, a polyacetylenic glucoside, prevents type 1 diabetes in nonobese diabetic mice. Journal of Immunology. 2007;178(11):6984–6993. doi: 10.4049/jimmunol.178.11.6984. [DOI] [PubMed] [Google Scholar]
- 21.Chang CL-T, Kuo H-K, Chang S-L, et al. The distinct effects of a butanol fraction of Bidens pilosa plant extract on the development of Th1-mediated diabetes and Th2-mediated airway inflammation in mice. Journal of Biomedical Science. 2005;12(1):79–89. doi: 10.1007/s11373-004-8172-x. [DOI] [PubMed] [Google Scholar]
- 22.Chang CLT, Liu HY, Kuo TF. Anti-diabetic effect and mode of action of cytopiloyne. Evidence-Based Complementary and Alternative Medicine. 2013;2013:13 pages. doi: 10.1155/2013/685642.685642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang S-L, Chang CL-T, Chiang Y-M, et al. Polyacetylenic compounds and butanol fraction from Bidens pilosa can modulate the differentiation of helper T cells and prevent autoimmune diabetes in non-obese diabetic mice. Planta Medica. 2004;70(11):1045–1051. doi: 10.1055/s-2004-832645. [DOI] [PubMed] [Google Scholar]
- 24.Chiang Y-M, Chang CL-T, Chang S-L, Yang W-C, Shyur L-F. Cytopiloyne, a novel polyacetylenic glucoside from Bidens pilosa, functions as a T helper cell modulator. Journal of Ethnopharmacology. 2007;110(3):532–538. doi: 10.1016/j.jep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Chang CLT, Chen YC, Chen HM, Yang NS, Yang WC. Natural cures for type 1 diabetes: a review of phytochemicals, biological actions, and clinical potential. Current Medicinal Chemistry. 2013;20(7):899–907. [PubMed] [Google Scholar]
- 26.Laakso M. Insulin resistance and its impact on the approach to therapy of Type 2 diabetes. International Journal of Clinical Practice. 2001;(121):8–12. [PubMed] [Google Scholar]
- 27.Clements RS, Jr., Bell DSH. Complications of diabetes: prevalence, detection, current treatment, and prognosis. American Journal of Medicine. 1985;79(5):2–7. doi: 10.1016/0002-9343(85)90503-0. [DOI] [PubMed] [Google Scholar]
- 28.Leahy JL, Hirsch IB, Peterson KA, Schneider D. Targeting β-cell function early in the course of therapy for type 2 diabetes mellitus. Journal of Clinical Endocrinology and Metabolism. 2010;95(9):4206–4216. doi: 10.1210/jc.2010-0668. [DOI] [PubMed] [Google Scholar]
- 29.Lin HW, Han GY, Liao SX. Studies on the active constituents of the Chinese traditional medicine Polygonatum odoratum (Mill.) Druce. Acta Pharmaceutica Sinica. 1994;29(3):215–222. [PubMed] [Google Scholar]
- 30.Ubillas RP, Mendez CD, Jolad SD, et al. Antihyperglycemic acetylenic glucosides from Bidens pilosa . Planta Medica. 2000;66(1):82–83. doi: 10.1055/s-0029-1243117. [DOI] [PubMed] [Google Scholar]
- 31.Hsu Y-J, Lee T-H, Chang CL-T, Huang Y-T, Yang W-C. Anti-hyperglycemic effects and mechanism of Bidens pilosa water extract. Journal of Ethnopharmacology. 2009;122(2):379–383. doi: 10.1016/j.jep.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 32.Silva FL, Fischer DCH, Tavares JF, Silva MS, De Athayde-Filho PF, Barbosa-Filho JM. Compilation of secondary metabolites from Bidens pilosa L. Molecules. 2011;16(2):1070–1102. doi: 10.3390/molecules16021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Yang B, Zhu D, He D, Wang L. Active components of Bidens pilosa L. Zhongcaoyao. 2005;36:20–21. [Google Scholar]
- 34.Bohlmann F, Burkhardt T, Zdero C. Naturally Occuring Acetylenes. New York, NY, USA: Academic Press; 1973. [Google Scholar]
- 35.Sarg TM, Ateya AM, Farrag NM, Abbas FA. Constituents and biological activity of Bidens pilosa L. grown in Egypt. Acta Pharmaceutica Hungarica. 1991;61(6):317–323. [PubMed] [Google Scholar]
- 36.Lastra Valdés HA, De León Rego HP. Bidens pilosa Linné. Revista Cubana de Plantas Medicinales. 2001;6(1):28–33. [Google Scholar]
- 37.Bohlmann F, Bornowski H, Kleine KM. New polyynes from the tribe Heliantheae. Chemische Berichte. 1964;97:2135–2138. [Google Scholar]
- 38.Wang R, Wu Q-X, Shi Y-P. Polyacetylenes and flavonoids from the aerial parts of Bidens pilosa . Planta Medica. 2010;76(9):893–896. doi: 10.1055/s-0029-1240814. [DOI] [PubMed] [Google Scholar]
- 39.Wu L-W, Chiang Y-M, Chuang H-C, et al. A novel polyacetylene significantly inhibits angiogenesis and promotes apoptosis in human endothelial cells through activation of the CDK inhibitors and caspase-7. Planta Medica. 2007;73(7):655–661. doi: 10.1055/s-2007-981527. [DOI] [PubMed] [Google Scholar]
- 40.Wu L-W, Chiang Y-M, Chuang H-C, et al. Polyacetylenes function as anti-angiogenic agents. Pharmaceutical Research. 2004;21(11):2112–2119. doi: 10.1023/b:pham.0000048204.08865.41. [DOI] [PubMed] [Google Scholar]
- 41.Yang H-L, Chen S-C, Chang N-W, et al. Protection from oxidative damage using Bidens pilosa extracts in normal human erythrocytes. Food and Chemical Toxicology. 2006;44(9):1513–1521. doi: 10.1016/j.fct.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Wang H-Q, Lu S-J, Li H, Yao Z-H. EDTA-enhanced phytoremediation of lead contaminated soil by Bidens maximowicziana . Journal of Environmental Sciences. 2007;19(12):1496–1499. doi: 10.1016/s1001-0742(07)60243-5. [DOI] [PubMed] [Google Scholar]
- 43.Tobinaga S, Sharma MK, Aalbersberg WGL, et al. Isolation and identification of a potent antimalarial and antibacterial polyacetylene from Bidens pilosa . Planta Medica. 2009;75(6):624–628. doi: 10.1055/s-0029-1185377. [DOI] [PubMed] [Google Scholar]
- 44.Zhao A, Zhao Q, Peng L. A new chalcone glycoside from Bidens pilosa . Acta Botanica Yunnanica. 2004;26(1):121–126. [Google Scholar]
- 45.Alvarez L, Marquina S, Villarreal ML, Alonso D, Aranda E, Delgado G. Bioactive polyacetylenes from Bidens pilosa . Planta Medica. 1996;62(4):355–357. doi: 10.1055/s-2006-957902. [DOI] [PubMed] [Google Scholar]
- 46.Pereira RLC, Ibrahim T, Lucchetti L, Da Silva AJR, De Moraes VLG. Immunosuppressive and anti-inflammatory effects of methanolic extract and the polyacetylene isolated from Bidens pilosa L. Immunopharmacology. 1999;43(1):31–37. doi: 10.1016/s0162-3109(99)00039-9. [DOI] [PubMed] [Google Scholar]
- 47.Kusano G, Kusano A, Seyama Y. JPO. Tokyo, Japan: 2004. Novel hypoglycemic and antiinflammatory polyacetylenic compounds, their compositions, Bidens plant extract fractions, and compositions containing the plant or fraction. [Google Scholar]
- 48.Lee C-K. The low polar constituents from Bidens pilosa L. var. minor (blume) sherff. Journal of the Chinese Chemical Society. 2000;47(5):1131–1136. [Google Scholar]
- 49.Brandão MGL, Krettli AU, Soares LSR, Nery CGC, Marinuzzi HC. Antimalarial activity of extracts and fractions from Bidens pilosa and other Bidens species (Asteraceae) correlated with the presence of acetylene and flavonoid compounds. Journal of Ethnopharmacology. 1997;57(2):131–138. doi: 10.1016/s0378-8741(97)00060-3. [DOI] [PubMed] [Google Scholar]
- 50.Krettli AU, Andrade-Neto VF, Brandão MDGL, Ferrari WMS. The search for new antimalarial drugs from plants used to treat fever and malaria or plants ramdomly selected: a review. Memorias do Instituto Oswaldo Cruz. 2001;96(8):1033–1042. doi: 10.1590/s0074-02762001000800002. [DOI] [PubMed] [Google Scholar]
- 51.Geissberger P, Sequin U. Constituents of Bidens pilosa L.: do the components found so far explain the use of this plant in traditional medicine? Acta Tropica. 1991;48(4):251–261. doi: 10.1016/0001-706x(91)90013-a. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Yang H, Lin Z-W, Sun H-D. Flavonoids from Bidens pilosa var. radiata. Phytochemistry. 1997;46(7):1275–1278. [Google Scholar]
- 53.Wat C-K, Biswas RK, Graham EA, Bohm L, Towers GHN, Waygood ER. Ultraviolet-mediated cytotoxic activity of phenylheptatriyne from Bidens pilosa L. Journal of Natural Products. 1979;42:103–111. doi: 10.1021/np50001a005. [DOI] [PubMed] [Google Scholar]
- 54.Dimo T, Azay J, Tan PV, et al. Effects of the aqueous and methylene chloride extracts of Bidens pilosa leaf on fructose-hypertensive rats. Journal of Ethnopharmacology. 2001;76(3):215–221. doi: 10.1016/s0378-8741(01)00229-x. [DOI] [PubMed] [Google Scholar]
- 55.Wright SW, Harris RR, Kerr JS, et al. Synthesis, chemical, and biological properties of vinylogous hydroxamic acids: dual inhibitors of 5-lipoxygenase and IL-1 biosynthesis. Journal of Medicinal Chemistry. 1992;35(22):4061–4068. doi: 10.1021/jm00100a011. [DOI] [PubMed] [Google Scholar]
- 56.Wu H, Chen H, Hua X, Shi Z, Zhang L, Chen J. Clinical therapeutic effect of drug-separated moxibustion on chronic diarrhea and its immunologic mechanisms. Journal of Traditional Chinese Medicine. 1997;17(4):253–258. [PubMed] [Google Scholar]
- 57.Nguelefack TB, Dimo T, Nguelefack Mbuyo EP, Tan PV, Rakotonirina SV, Kamanyi A. Relaxant effects of the neutral extract of the leaves of Bidens pilosa linn on isolated rat vascular smooth muscle. Phytotherapy Research. 2005;19(3):207–210. doi: 10.1002/ptr.1646. [DOI] [PubMed] [Google Scholar]
- 58.Almirón WR, Brewer ME. Classification of Immature Stage Habitats of Culicidae (Diptera) Collected in Córdoba, Argentina. Memorias do Instituto Oswaldo Cruz. 1996;91(1):1–9. doi: 10.1590/s0074-02761996000100001. [DOI] [PubMed] [Google Scholar]
- 59.Wang N-L, Wang J, Yao X-S, Kitanaka S. Two new monoterpene glycosides and a new (+)-jasmololone glucoside from Bidens parviflora Willd. Journal of Asian Natural Products Research. 2007;9(5):473–479. doi: 10.1080/10286020500532033. [DOI] [PubMed] [Google Scholar]
- 60.Rybalchenko NP, Prykhodko VA, Nagorna SS, et al. In vitro antifungal activity of phenylheptatriyne from Bidens cernua L. against yeasts. Fitoterapia. 2010;81(5):336–338. doi: 10.1016/j.fitote.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Frida L, Rakotonirina S, Rakotonirina A, Savineau J-P. In vivo and in vitro effects of Bidens pilosa L. (asteraceae) leaf aqueous and ethanol extracts on primed-oestrogenized rat uterine muscle. African Journal of Traditional, Complementary and Alternative Medicines. 2008;5(1):79–91. [PMC free article] [PubMed] [Google Scholar]
- 62.Ezeonwumelu JOC, Julius AK, Muhoho CN, et al. Biochemical and histological studies of aqueous extract of Bidens pilosa leaves from Ugandan Rift valley in Rats. British Journal of Pharmacology and Toxicology. 2011;2(6):302–309. [Google Scholar]


