Abstract
Trichilia emetica and Opilia amentacea traditional Burkinabe medicinal plants were investigated to determine their therapeutic potential to inhibit key enzymes in carbohydrate metabolism, which has relevance to the management of type 2 diabetes. In vitro and in vivo antioxidant and antihypertensive potential and antilipidemia and antihyperglycemia activities in an animal model of type 2 diabetes mellitus have been studied. The antioxidant activity of the flavonoids from leaves of Trichilia emetica and Opilia amentacea has been evaluated using β-carotene-linoleic acid system, 1,1-diphenyl-2-picrylhydrazyl inhibitory activity, chelation of iron (II) ions, and lipid peroxidation which showed more pronounced antioxidant capacities of Trichilia emetica. Total cholesterol concentrations decreased in an animal model of type 2 diabetes mellitus under effects of flavonoid-rich fractions from leaves of Trichilia emetica and Opilia amentacea has been observed. Extract of flavonoid-rich fractions from Trichilia emetica shown maximum radical scavenging activity and possessed marked antiamylase activity which may be due to the presence of certain secondary metabolites. Suggested better antihyperglycemia, antilipidemia, and antihypertensive properties of flavonoid-rich fractions from Trichilia emetica compared to the extract of Opilia amentacea are demonstrating antidiabetic potential of Trichilia emetica as therapeutic targets for the management of type 2 diabetes.
1. Introduction
Diabetes mellitus is a common metabolic disorder characterized by hyperglycemia, glycosuria, polyurea, and polydipsia induced by insulin deficiency [1] and insulin resistance [2]. Diabetes mellitus is an important metabolic syndrome. The increasing worldwide incidence of diabetes mellitus in adults constitutes a global public health burden. At least, diabetes mellitus is possibly the world's fastest growing metabolic disorder, and as the knowledge of the heterogeneity of this disorder increases, so does the need for more appropriate therapy [3]. The World Health Organization (WHO) estimates that currently more than 180 million people worldwide have diabetes and it is likely to double by 2030, with India, China, and United States predicted to have the largest number of affected individuals [4, 5]. Diabetes mellitus is treated by using oral hypoglycemic agents such as sulphonylureas, biguanides, meglitinides, and α-glucosidase inhibitors. Hyperglycemia, a condition characterized by an abnormal excess of sugar in the blood, has been linked to the onset of type 2 diabetes mellitus and associated cardiovascular complications including hypertension [6, 7]. In effect, stress-related disease such as hypertension or high blood pressure has been considered as a high risk factor cardiovascular disease. Twenty percent of the world's population suffers from hypertension [8]. Experimental evidence suggests that antihypertensive or angiotensin I-converting enzyme (ACE) inhibitor treatment can offer a clinical advantage in hypertension [9]. ACE plays a key physiological role in the control of blood pressure by virtue of the rennin-angiotensin system [10]. Captopril (d-3-mercapto-2-methylpranory-l-proline), enalapril, and lisinopril have been developed and used as clinical antihypertensive drugs. Although synthetic ACE inhibitors, including captopril, are remarkably effective as antihypertensive drugs, they cause adverse side effects, such as coughing, allergic reactions, taste disturbances, and skin rashes. Therefore, research and development to find safer, innovative, and economical ACE inhibitors is necessary for the control of blood pressure.
Although a few synthetic antidiabetic drugs are available to combat the impaired insulin secretion, insulin resistance, and hyperglycemia that characterize type 2 diabetes mellitus, some of these drugs can have negative side effects at high doses [11, 12]. A major focus of current antidiabetic research is the development of antihyperglycemic agents that are safe and free of negative side effects. Many plants and their active chemical compounds have demonstrated activity in the treatment of various disorders [13]. According to ethnobotanical information, more than 800 plants are used as traditional remedies in one or other form for the treatment of diabetes [14]. The management of diabetes without any side effects is still a challenge; therefore, plants continue to play an important role in the discovery of new compounds for the treatment of this disease. The management of diabetes can be achieved by reducing postprandial hyperglycemia by delaying the activities of the enzymes α-amylase and α-glucosidase which are responsible for the digestion of carbohydrates and absorption of glucose in the digestive tract, respectively [15, 16]. Drugs derived from natural products have played a major role in the development of pharmaceutical treatments for diabetes. Metformin, the single most prescribed agent for the treatment of diabetes, originated from herbal medicine [17, 18]. A plant-derived antidiabetic agent galegine was isolated from Galega officinalis. Experimental and clinical evaluations provided the pharmacological and chemical basis for the subsequent discovery of metformin [17, 19]. 1-Deoxynojirimycin (DNJ), a potent α-glucosidase inhibitor, was isolated from the water extract of leaves of the mulberry tree (Morus alba L.) [20]. There are many cellular biochemical pathways and environmental toxins which produce reactive oxygen species (ROS) [21] and contribute to the development of diseases such as cancer, cardiovascular disorders, diabetes, cataracts, and many neurodegenerative diseases [22]. Many studies have confirmed that plants and foods rich in polyphenolic content are effective scavengers of free radicals, thus helping in the prevention of these diseases through their antioxidant activity [23]. Antioxidants which are present in plants, herbs, and dietary sources help in preventing vascular diseases in diabetic patients [24]. Tannins and flavonoids are the secondary metabolites in plants considered to be the natural source of antioxidants which prevent destruction of β cells and diabetes-induced ROS formation [25]. Thus, it is a good strategy to manage diabetes as a whole with plants which show good enzyme inhibitory and antioxidant activities [26].
Trichilia emetica and Opilia amentacea are native to sub-Saharan Africa and are essentially tropical in origin. In the western part of Burkina Faso, the leaves of these plants are used to treat cardiovascular diseases. They possess hypotensive, hypolipidemic-delite, antioxidant, antibacterial and anti-inflammatory properties [27]. But none have reported on their antioxidant, antidiabetic, and antihypertensive properties of flavonoid-rich fractions. Lack of scientific data to support these claims prompted this study which was therefore aimed at assessing the possible antioxidant, antidiabetic, and antihypertensive properties of flavonoid-rich fractions from the leaves of these plants in models using rats in order to provide a scientific basis for the traditional use of this plant for better management of type 2 diabetes.
2. Material and Methods
2.1. Plant Materials
Fresh leaves of Trichilia emetica and Opilia amentacea were collected in October 2011 in Ouagadougou, capital of Burkina Faso, with gardeners. The plants were identified in the Laboratory of Biology and Ecology, University of Ouagadougou, where a voucher specimen was deposited.
2.2. Animals Handling
Swiss NMRI mice (25–30 g) and adult albinos Wistar rats (160–200 g) of both sexes were used for this study. All animals were housed in cages under controlled conditions of 12 h light/and 12 h without light and 25°C. They received pellets of food enriched with 20% protein and water ad libitum. They were deprived of food for 15 h (but with access to drinking water) and weighed before the experiments. Experiments on the animals were performed according to the protocols already approved by the Institute of Health Sciences Research/University of Ouagadougou (Burkina Faso) and met the international standards for animal study [28].
2.3. Chemicals
Streptozotocin and alloxan monohydrate were purchased from Sigma (Germany) and all other chemicals and reagents used in this study were of analytical grade and were purchased from Sigma Chemical Co. (St. Louis, MO). Glibenclamide, rabbit lung dehydrated by acetone, and captopril were purchased from Sigma-Aldrich, USA.
2.4. Preparation of Extracts for Acute Toxicity Study
100 grams of leaves (powdered plant materials) dried in laboratory condition were extracted with 500 mL of acetone 80% (400 mL acetone + 100 mL water) for 24 h under mechanic agitation (SM 25 shaker, Edmund BÜHLER, Germany) at room temperature. After filtration, acetone was removed under reduced pressure in a rotary evaporator (BÜCHI, Rotavopor R-200, Switzerland) at approximately 40°C and freeze-dried (Telstar Cryodos 50 freeze-dryer). The extract was weighed before packing in waterproof plastic flasks and stored at 4°C until use.
2.5. Flavonoids Extraction
The fresh harvested plant materials (100 grams of leaves) were dried in the laboratory at room temperature (20–25°C); afterwards samples were ground to pass a sieve of 0.3 mm. Flavonoids were extracted with aqueous acetone (80%, v/v). The extracts were then washed with hexane to remove chlorophyll and other low molecular weight compounds. Acetone was evaporated and then the aqueous extracts with ethyl acetate is used to separate by sequential liquid-liquid extraction. The flavonoid-rich fractions were lyophilized and stored at 22°C prior to biological tests. For the tests, lyophilized sample was dissolved with 10% DMSO in water at the desired concentration.
2.6. Antioxidant Capacity of Flavonoids
2.6.1. β-Carotene-Linoleic Acid Assay
The antioxidant activity of the flavonoids was evaluated using β-carotene-linoleic acid system according to [29]. In short, 1 mL of β-carotene solution in chloroform (0.2 mg/mL) was pipetted into a round-bottom flask. To the solution, 20 mg of linoleic acid and 200 mg of Tween 40 were added. After removing chloroform in a rotary evaporator, 50 mL of aerated distilled water was added to the oily residue. Aliquots (5 mL) of thus obtained emulsion were transferred to a series of tubes containing 2 mg of extract or 0.5 mg of butylated hydroxyanisole (BHA) (positive control). Emulsion without antioxidant served as control. After addition of the emulsion to the tubes, they were placed in a water bath at 50°C for 2 h. During that period, the absorbance of each sample was measured at 470 nm at 15 min intervals, starting immediately after sample preparation (t = 0 min) until the end of the experiment (t = 120 min). The rate of β-carotene bleaching (R) for the extracts, BHA and water, was calculated according to first-order kinetics. The percent of antioxidant activity (ANT) was calculated as described in [30], using the equation
| (1) |
where R Control and R Sample are average bleaching rates of water control and antioxidant (flavonoids or BHA), respectively.
2.6.2. DPPH Radical-Scavenging Activity
The scavenging effect for DPPH free radical was monitored as described in [31] with minor modification. Briefly, 1.0 mL of 0.16 mM DPPH methanolic solution was added to 1.0 mL of either methanolic solution of extract (sample) or methanol (control). The mixtures were vortexed and then left to stand at room temperature in the dark. After 30 min absorbance was read at 517 nm. Radical-scavenging activity (RSA) for DPPH free radical was calculated using the following equation:
| (2) |
where A Control is the absorbance of the methanol control and A Sample is the absorbance of the flavonoids. Synthetic antioxidant, BHA, was used as positive control. DPPH radical-scavenging activity was calculated as the concentration that scavenges 50% of DPPH free radical and thus has RSA = 50% (EC50).
2.6.3. Chelating Activity (ChA)
The chelation of iron (II) ions was studied as described by [32]. An aliquot of the extract in methanol (1.3 mL) was added to 100 μL of 2 mM ferrous chloride. After 5 min, the reaction was initiated by adding 200 μL of 5 mM ferrozine. Following 10 min incubation at room temperature, the absorbance at 562 nm was recorded. For preparation of control, 1.3 mL of methanol was used instead of polyphenols solution. EDTA was used as a chelating standard. The Fe(2+)-chelating activity chelating activity (ChA) was calculated using the equation below:
| (3) |
where A Control is the absorbance of the negative control (solution to which no flavonoid was added) and A Sample is the absorbance of the extract solution. Chelating activity was expressed as ChEC50, the concentration that chelates 50% of Fe2+ ions and thus has ChA = 50%.
2.6.4. Inhibition of Lipid Peroxidation
Liver of male Wistar rats (160–180) was excised and homogenized (1% w/v) in 0.154 mol/L KCl solution. The homogenate was centrifuged at 3000 rpm at 4°C for 10 min and supernatant was used for the assay. Peroxidation of the liver homogenate was induced by FeCl2-H2O2 [33]. Briefly, 1% liver homogenate was incubated with 0.5 mmol/L of each of FeCl2 and H2O2 with or without flavonoid-rich fractions (50 μg/mL). After incubation at 37°C for 60 min, the formation of malondialdehyde (MDA) was measured at 535 nm [33]. BHT served as positive control. The equation is
| (4) |
where AC is the absorbance of the control (without any treatment) and AA is the absorbance of the antioxidants.
2.7. In Vitro Antihyperglycemia
2.7.1. Amylase Inhibition Screening Assay
The α-amylase inhibitory assay was modified from [34]. Twenty μL of porcine pancreatic α-amylase solution (EC 3.2.1.1; equivalent to 3000 U in 50 mM phosphate buffer, pH 6.9) was mixed with 15 μL of plant extract and incubated at 37°C for 45 minutes. After incubation, the mixture was applied to a sterile paper disc and placed onto the center of Petri plates containing medium consisting of 1% (w/v) agar and 1% (w/v) starch in distilled water. Plates were allowed to stand for 3 days at 25°C then stained with iodine and allowed to stand for 15 min. The diameter of the clear zone was measured and used to calculate the amylase inhibitory activity. As a control, the enzyme was mixed with the solvent in which the plants were extracted (ethanol) and applied onto the sterile disc. Results were expressed as
| (5) |
2.7.2. Amylase Inhibition Assay by Quantitative Starch Hydrolysis
The α-amylase inhibitory activity was determined [35] using porcine pancreatic α-amylase solution (EC 3.2.1.1) type VI B. To 125 μL of different plant extract concentrations (range 1.56 μg/mL to 500 μg/mL), α-amylase solution (0.5 mg/mL in 0.02 M sodium phosphate buffer) was mixed and the reaction mixture was preincubated for 10 minutes at room temperature. The reaction mixture was diluted by adding 5000 μL of distilled water. The generation of maltose was quantified by measuring the absorbance at 540 nm of 3-amino-5-nitrosalicylic acid (from reduction of 3,5-dinitrosalicylic acid) [36] using a UV-visible spectrophotometer. The control was buffer-treated in the same way as plant samples. The standard used was acarbose (concentration range 1.56 μg/mL to 500 μg/mL). Results were expressed as
| (6) |
2.7.3. Glucosidase Inhibition Assay
The α-glucosidase inhibition assay has been modified from [37] using yeast α-glucosidase (EC 2328898). A volume of 25 μL of plant extract (range 0.35 μg/mL to 100 μg/mL) was mixed with 50 μL of α-glucosidase enzyme 0.1 U/mL in 0.1 M potassium in 96 well plates and incubated at 37°C for 30 minutes. After preincubation, 25 μL of 5 mM pNPG in 0.1 M phosphate buffer was added to each well and the reaction mixture was incubated again at 37°C for 30 minutes. Thirty μL of 0.1 M sodium carbonate solution was added to the previous reaction mixture and incubated again for 20 minutes at 37°C. Before and after incubation, the absorbance was measured at 405 nm and compared to the control that contained 25 μL of buffer solution instead of polyphenols solution. The standard used was acarbose (concentration range 0.35 μg/mL to 100 μg/mL). The α-glucosidase activity was determined by measuring release of p-nitrophenol from p-nitrophenyl α-D-glucopyranoside [38]. The α-glucosidase inhibitory activity was expressed as
| (7) |
2.8. In Vitro Antihypertensive Profile by Measurement of ACE Inhibitory Activity
2.8.1. Assay Buffer
HEPES (297.5 mg, 50 mmol/L), NaCl (438.75 mg, 300 mmol/L), and Na2SO4 (1420 mg, 400 mmol/L) were added to a 25 mL volumetric flask (amount and final concentration given in parentheses). After dissolving in 20 mL of distilled water containing 50 mL of saturated NaOH solution, the pH was made up with distilled water. Phosphate buffer (100 mmol/L) was prepared by dissolving 340.2 mg of anhydrous potassium phosphate in 20 mL of distilled water, adjusted to pH 8.5 with 10% NaOH solution, and made up to 25 mL.
2.8.2. Stock and Working Solution of Rabbit Lung Dehydrated by Acetone
The stock solution was prepared as described previously [39] by dissolving 2 g of rabbit lung dehydrated powder in 10 mL of 50 mmol/L phosphate buffer (pH 8.3). The stock solution was highly active and stable for at least 3 months under refrigeration (2–6°C). Working solution (1 g/10 mL) was freshly prepared by diluting the stock solution in the phosphate buffer before performing the assays.
2.8.3. Substrate Solution
200 mg of hippuryl-glycyl-glycine was dissolved in 4 mL of 1 mol/L ammonium hydroxide solution. After complete dissolution, the volume was increased to 6.8 mL with distilled water.
2.8.4. TNBS Solution
TNBS (2030 μL) was added to a 5 mL volumetric flask and the volume was made up with distilled water to obtain a final concentration of 60 mmol/L. The solution was stored at −20°C and used within 3 months.
2.8.5. Preparation of Flavonoid-Rich Fractions
For antihypertensive screening, the flavonoid-rich fractions were dissolved in 20% methanol and 80% HEPES to a concentration of 5 mg/mL.
2.8.6. Colorimetric Methanol for ACE Inhibition Assay [40]
Ten microlitres of rabbit lung solution (1 g/10 mL) were added to an Eppendorf tube containing 10 μL of flavonoid-rich fractions solution (5 mg/mL) to be tested, or 10 μL of 50 mmol/L phosphate buffer (pH 8.3) (negative control), or 10 μL of captopril solution (5 mg/mL) (positive control). The mixture was homogenized and preincubated for 5 min at 37°C. The enzyme reaction was initiated by adding 60 μL of the assay buffer and 30 μL of the substrate solution. After homogenization, the mixture was incubated for 35 min at 37°C. The reaction was stopped by the addition of 100 μL of sulfuric acid (0.33 mmol/L); the Eppendorf tube was shaken 10 s after the addition of 1000 μL of distilled water. In the sequence, the mixture was centrifuged at 2000 rpm for 10 min. An aliquot of the supernatant (75 μL) was placed on a microtitre plate and mixed with 100 μL of phosphate buffer (100 mmol/L, pH 8.5) and 5 μL of TNBS solution. The plate was kept in the dark at room temperature for 20 min. Its absorbance was read in a microtitre plate reader (Spectramax 340 PC tunable microplate reader) at 415 nm against a blank solution prepared similarly but without adding sodium tungstate and sulfuric acid solutions to the mixture. Assays were performed in triplicate. Calculation of ACE inhibition on a percentage basis was done using the following equation:
| (8) |
where AI is the measured absorbance at 415 nm in the presence of an inhibitor and AC is the absorbance of the blank solution.
2.9. Acute Toxicity Study of Aqueous Acetone Extracts
Swiss mice (male and female) were randomly divided into 7 groups (1 control group and 6 treated groups) of 6 animals (3 males and 3 females). The control group received water containing 10% dimethylsulfoxide (DMSO) administered intraperitoneally. The aqueous acetone extracts of Trichilia emetica and Opilia amentacea suspended in 10% DMSO were administered intraperitoneally at doses of 1, 2, 2.5, 3, 4, 5, and 6 g/kg [35]. The general behaviour of the mice was observed for 120 min after the treatment. The animals were observed for morbidity and mortality once a day for 14 days. The number of survivors after the 14 days period was noted. The toxicological effect was assessed on the basis of mortality for 14 days, which was expressed by the median lethal dose value (Lethal Dose 50 or LD50) estimated from the regression of log-probit mortality rate [41].
2.10. In Vivo Antihyperglycemia and Hypolipidaemia Potential of Flavonoids
2.10.1. In Vivo Antihyperglycemia
(1) Induction of Diabetes. Alloxan monohydrate was first weighed individually for each animal according to its weight and then solubilized with 0.2 mL saline just prior to injection. Diabetes was induced by injecting it at a dose of 100 mg/kg body weight intraperitoneally. After 1 hr of alloxan administration, the animals were got food and 5% dextrose solution was also given in feeding bottle for a day to overcome the early hypoglycemic phase. The animals were kept under observation and after 48 hr, blood glucose was measured. One group served as a control which received vehicle alone. The diabetic rats (glucose level >150 mg/dL) were separated and divided into four different groups for experimental study [42].
(2) Experimental Design
(i) Acute Treatment and Subacute Treatment. Normal rats are kept into group 1; diabetic induced rats are grouped into groups 2, 3, 4, and 5. Each group contains six rats: group I: normal control (saline); group II: diabetic control (saline); group III: standard (glibenclamide 10 mg/kg); group IV: test-dose (100 mg/kg of flavonoid-rich fractions from Trichilia emetica and Opilia amentacea); group V: test-dose (300 mg/kg of flavonoid-rich fractions from Trichilia emetica and Opilia amentacea). Drugs are administered via oral route. Treatment continued for seven days [42].
(ii) Acute Study (Single Day Study). Blood samples were collected from rat caudal vein and serum glucose levels were estimated at 0, 1, 3, and 5 h after the extract administration.
(iii) Subacute Stud (Seven Day Study). Blood samples were collected from rat caudal vein and serum glucose levels were estimated at 1, 3, 5, and 7 days. Blood glucose levels were determined by god-pod method.
2.10.2. In Vivo Hypolipidaemic Potential
(1) Induction of Experimental Diabetes. Diabetes was induced by a single intraperitoneal injection of a freshly prepared streptozotocin (STZ) solution (50 mg/kg in citrate buffer 0.01 M, pH 4.5) to overnight-fasted rats [43]. Control rats received normal water alone. Diabetes was identified by polydipsia, polyurea, and measuring nonfasting blood glucose levels 48 h after injection of STZ. Animals which show blood glucose levels more than 250 mg/dL were considered as diabetic rats and used as the experimental animals.
(2) Experimental Design. Adult, male rats of Wistar strain weighing 160–180 g were chosen as animal for present study. They were housed individually in clean, sterile, polypropylene cages under standard conditions and water ad libitum. The animals were acclimatized to the laboratory for one week prior to the start of experiments. The rats were divided into 7 groups comprising of 6 animals in each group as follows: group I: normal rats (controls); group II: diabetic untreated rats; group III: diabetic + glibenclamide treated rats; group IV: normal + Trichilia emetica and Opilia amentacea treated rats (100 mg/kg of flavonoid-rich fractions from leaves of Trichilia emetica and Opilia amentacea/day); group V: normal + Trichilia emetica and Opilia amentacea treated rats (500 mg/kg of flavonoid-rich fractions from leaves of Trichilia emetica and Opilia amentacea/day); group VI: diabetic + Trichilia emetica and Opilia amentacea treated rats (100 mg/kg of flavonoid-rich fractions from leaves of Trichilia emetica and Opilia amentacea/day); and group VII: diabetic + Trichilia emetica and Opilia amentacea treated rats (500 mg/kg of flavonoid-rich fractions from leaves of Trichilia emetica and Opilia amentacea/day). Drugs are administered via oral route; treatment continued for 28 days.
(3) Measurement of Serum Biochemical Parameters. Blood samples were collected by cardiac puncture. The blood samples without anticoagulant were centrifuged at 3000 rpm for 5 min to obtain plasma or serum. Estimation of serum cholesterol was carried out by the method of [44]. Serum triglycerides were estimated by the method of [45] and HDL cholesterol was estimated by the method of [46]. The VLDL cholesterol was calculated using the formula, TG/5 mg/dL. The serum LDL cholesterol was estimated by the method of [47].
(4) Estimation of Triglycerides (TG). Triglycerides in the liver tissue were estimated by modified version method of [48] with slight modifications as given below. Triglycerides were assayed by hydrolyzing them to glycerol and the liberated glycerol was determined. Tissue homogenates were taken and 0.5 mL of 1 N H2SO4 and 4 mL of chloroform were added. The contents were centrifuged at 1000 rpm for 15 min. The 0.5 of chloroform layer was taken and to 0.4 mL of methanol and 0.1 mL of alkaline barium solutions was added and the contents were heated for 30 min at 80°C; the total volume was made up to 1 mL with 2 N H2SO4 and centrifuged for 10 min at 1000 rpm. The 0.5 mL of this supernatant was taken and 0.1 mL of sodium periodate was added and shaken well for 1 min; 0.1 mL of sodium arsenate and 5 mL of chromotropic acid reagent were added and heated for 30 min and cooled. The samples were evaluated under wavelength 575 nm spectrophotometrically. The results were finally expressed in mg of triglycerides/gram wet weight of the tissue.
(5) Estimation of Total Cholesterol. The total cholesterol content of liver tissue was estimated using Liebermann Burchard reaction as described by [48].
(6) Estimation of Phospholipids. Phospholipids (PL) in the liver tissue were estimated by the method of [49].
2.10.3. Statistical Analyses
Data were expressed as mean ± standard deviation (SD) of six experiments (n = 6). Results were analyzed by one-way ANOVA followed by Dunnett's t-test using Prism 4 software. The level of significance was considered at P ≤ 0.05.
3. Results
3.1. Antioxidant Potential
3.1.1. Antioxidant Capacity of β-Carotene-Linoleic Acid Assay
The basis of β-carotene-linoleic acid assay is degradation of β-carotene in reaction with linoleic acid free radical. Antioxidants present in the solution can hinder this reaction and consequently prevent discoloration of β-carotene solution. The reduction of absorbance of β-carotene-linoleic acid emulsion was shown in presence of the flavonoid-rich fractions. Comparison of the ANT values of the samples (Figure 1) indicates that the flavonoid-rich fractions were less successful at inhibition of bleaching of β-carotene emulsion comparatively to BHA (P < 0.05 and P < 0.001).
Figure 1.
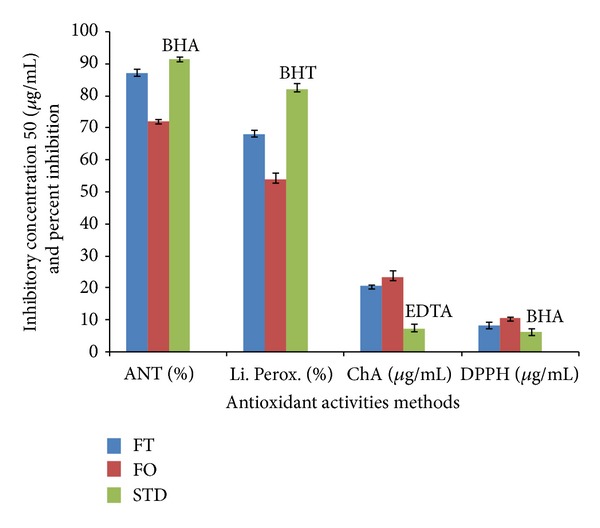
Antioxidant activity in β-carotene-linoleate test (ANT), DPPH radical scavenging activity (EC50), and metal chelating activity (ChEC50) of flavonoid-rich fractions from Trichilia emetica (FT) and Opilia amentacea (FO) and standards (STD).
3.1.2. Antioxidant of DPPH Radical-Scavenging Activity
The basis of DPPH assay is the discoloration of DPPH∙ solution in presence of an antioxidant. In its radical form, DPPH absorbs with maximum at 517 nm, but upon reduction with an antioxidant. In this study, flavonoid-rich fractions demonstrated notable antiradical activities albeit lower than the activity of BHA (P < 0.001). Results are consigned in Figure 1.
3.1.3. Chelating Activity (ChA)
The chelating ability of the extracts toward ferrous ions was investigated (Figure 1) in presence of ferrozine, Fe2+ ion chelator, which upon binding of the metal ion absorbs with maximum at 562 nm. The investigated flavonoid-rich fractions demonstrated significant chelating ability in the present research, although has been found lower than the ability of EDTA (P < 0.0001).
3.1.4. Lipid Peroxidation
The endogenous basal of malondialdehyde in the rat liver homogenate was 40.02 mmol/g tissue. After 30 min of incubation FeCl2-H2O2, the incubation of malondialdehyde increase was measured at 535 nm. The inhibition of lipid peroxidation activity by the flavonoid-rich fractions is presented in Figure 1. We noticed that flavonoid-rich fractions showed different statistically significant percentages of inhibition as compared to BHT (P < 0.001 and P < 0.0001).
3.2. In Vitro Antihyperglycemia
In the amylase assay, the positive control acarbose showed an IC50 of 5.70 μg/mL and flavonoid-rich fractions exerted an IC50 of 6.12 μg/mL for amylase inhibitory activity comparatively to the amylase inhibition assay by quantitative starch hydrolysis where flavonoid-rich fractions exerted an IC50 of 6.23 for glucose inhibition activity (P < 0.001). In the investigated flavonoid-rich fractions no statistical significance has been observed (IC50 of 4.70 μg/mL and IC50 of 4.61 μg/mL) compared to the control, although was lower than IC50 of acarbose (4.08 μg/mL) for glucosidase inhibition (P > 0.05) (Figure 2).
Figure 2.
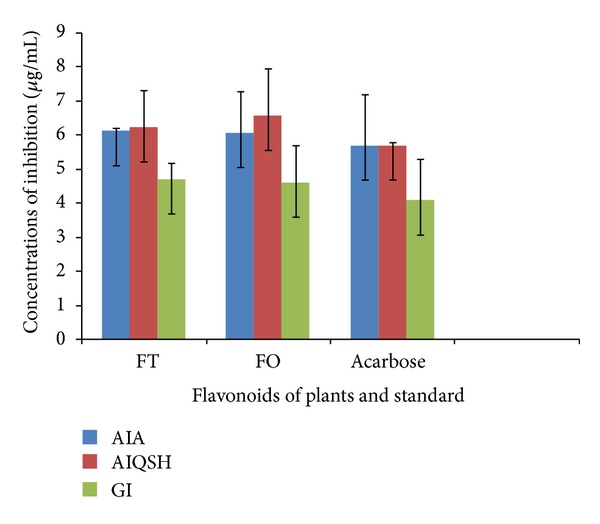
Flavonoid-rich fractions from Trichilia emetica (FT) and Opilia amentacea (OP) and acarbose (standard) on inhibition of key enzymes (AIA = amylase inhibition screening assay, AIQSH = amylase inhibition assay by quantitative starch hydrolysis, and GI = glucosidase inhibition assay) in carbohydrate metabolism.
3.3. In Vitro Antihypertension
Flavonoid-rich fractions which were tested for antihypertension assay showed >65% inhibition of ACE compared to the control Figure 3. Captopril, which was used as a positive control, gave >70%. Comparison of the flavonoid-rich fractions from Trichilia emetica and Opilia amentacea values to the control, (Figure 3) showed statistically significant percentages of inhibition in both variants (P < 0.05).
Figure 3.
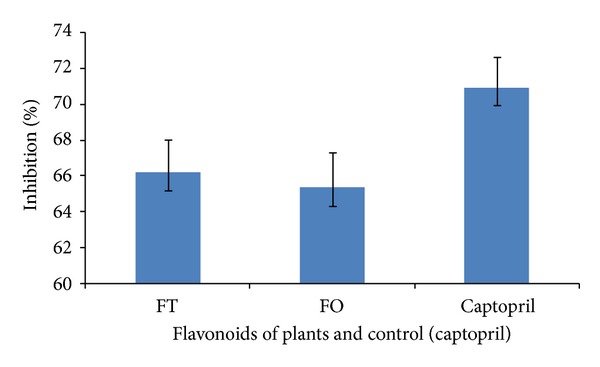
Percent antihypertensive activity upon treatment with flavonoid-rich fractions from Trichilia emetica (FT) and Opilia amentacea (FO). Captopril was the positive control.
3.4. Acute Toxicity Study in Mice
It was confirmed the efect of intraperitoneal treatment of aqueous acetone extracts from Trichilia emetica and Opilia amentacea on mortality, LD50 values. The value of LD50 is 568.5 mg/kg body weight for intraperitoneal administration for Trichilia emetica and 636.2 mg/kg body weight. During 14-day period of acute toxicity evaluation, some signs of toxicity have been observed but were quickly reversible.
3.5. In Vivo Antihyperglycemia Profile of Flavonoid-Rich Fractions
The results of in vivo antihyperglycemia of flavonoid-rich fraction are presented in Tables 1 and 2. We noticed that Trichilia emetica (300 mg/kg) possess higher antihyperglycimia parameters results than Opilia amentacea and comparatively to the control (P < 0.05, P < 0.001, and P < 0.0001).
Table 1.
Effect of flavonoid-rich fractions of Trichilia emetica (FT) and Opilia amentacea (FO) on serum glucose level for acute study (single day study).
| 0 h | 1 h | 3 h | 5 h | |
|---|---|---|---|---|
| Normal control | 84.12 ± 1.31 | 85.2 ± 2.18 | 85.30 ± 1.10 | 85.10 ± 1.99 |
| Diabetic control | 177.1 ± 2.23 | 179.3 ± 1.30 | 183.5 ± 2.40 | 186.1 ± 2.10 |
| Standard | 174.1 ± 1.20 | 165.1 ± 2.21* | 152.1 ± 1.40*** | 144.3 ± 3.20*** |
| FT (100 mg/kg) | 176.4 ± 3.08 | 174.2 ± 2.12 | 166.4 ± 2.63** | 164.3 ± 2.39** |
| FT (300 mg/kg) | 172.5 ± 1.50 | 177.2 ± 1.30 | 153.5 ± 1.54*** | 144.2 ± 2.40*** |
| FO (100 mg/kg) | 176.9 ± 1.1 | 178.1 ± 2.10 | 166.7 ± 1.33** | 167.1 ± 2.37** |
| FO (300 mg/kg) | 173.1 ± 0.10 | 180.2 ± 3.20 | 155.2 ± 1.24*** | 146.6 ± 2.71*** |
Values are mean ± SEM; N = 6. *P < 0.05, **P < 0.001, and ***P < 0.0001 versus diabetic control.
Table 2.
Effect of flavonoid-rich fractions of Trichilia emetica (FT) and Opilia amentacea (FO) on blood glucose level for subacute study (multiday study).
| 0 day | 1 day | 3 day | 5 day | 7 day | |
|---|---|---|---|---|---|
| Normal control | 84.60 ± 1.10 | 85.68 ± 1.00 | 84.33 ± 2.10 | 84.57 ± 1.00 | 84.53 ± 1.52 |
| Diabetic control | 176.2 ± 3.30 | 183.2 ± 2.31 | 192.4 ± 2.10 | 198.2 ± 2.10 | 203.2 ± 2.04 |
| Standard | 174.1 ± 2.20 | 154.2 ± 1.37** | 145.6 ± 2.03*** | 128.2 ± 2.53*** | 107.1 ± 1.10*** |
| FT (100 mg/kg) | 176.1 ± 1.20 | 170.1 ± 2.02* | 164.2 ± 1.22** | 160.4 ± 1.00** | 156.6 ± 2.67** |
| FT (300 mg/kg) | 172.5 ± 2.57 | 155.2 ± 1.42** | 145.3 ± 1.10*** | 128.4 ± 2.17*** | 113.1 ± 3.53*** |
| FO (100 mg/kg) | 176.8 ± 3.10 | 174.3 ± 1.12* | 167.5 ± 1.20** | 163.0 ± 1.01** | 158.2 ± 2.60** |
| FO (300 mg/kg) | 173.1 ± 1.53 | 157.1 ± 1.10** | 146.9 ± 2.23*** | 128.6 ± 1.10*** | 118.7 ± 1.51*** |
Values are mean ± SEM; N = 6. *P < 0.05, **P < 0.001, and ***P < 0.0001 versus diabetic control.
3.6. In Vivo Antihyperlipidaemia Potential of Flavonoid-Rich Fractions
In this section, we noticed a significant decrease in serum HDL cholesterol levels and a significant elevation in the total cholesterol, triglycerides, and LDL-cholesterol levels in diabetic rats compared to normal rats. Oral administrations of flavonoid-rich fractions for 28 days brought back the levels of serum lipids to near normal levels in diabetic rats and they were restored after administration of flavonoids for a period of 28 days. The total cholesterol, triglycerides, and phospholipids of normal rat hepatic tissue were 48.12 ± 0.53, 1.12 ± 0.10, and 1.21 ± 0.21, respectively, whereas in the diabetic rats these levels are raised to 60.17 ± 2.31, 2.17 ± 0.32, and 10.01 ± 0.33, respectively. The changes of these investigated parameters in the plants after treatment with bioactive fractions were shown in the hepatic tissue: 52.35 ± 1.14, 1.43 ± 0.42, and 8.55 ± 1.1 for Trichilia emetica and 53.01 ± 1.10, 1.71 ± 1.10, and 9.02 ± 0.1 for Opilia amentacea, respectively (P < 0.05). The total cholesterol, triglycerides, LDL-C, and VLDL-C levels of normal rat serum were 87.61 ± 2.01, 111.1 ± 2.51, 66.52 ± 0.51, and 23.2 ± 1.1, whereas in diabetic rats these levels have been raised with the plant bioactive fraction treatment with Trichilia emetic extract to 90.27 ± 1.60, 115.28 ± 1.64, 48.21 ± 2.23, and 24.5 ± 1.10 and 118.22 ± 2.2, 93.41 ± 1.81, 70.4 ± 1.12, and 29.43 ± 1.8 for Opilia amentacea extract, respectively. The HDL-C levels of normal rats were 45.18 ± 2.26. The levels were regained with the flavonoid-rich fraction treatment to 50.61 ± 1.36. We noticed that extract of flavonoid-rich fractions from Trichilia emetica presented better effects on the stabilization of concentrations of total cholesterol, triglycerides, and phospholipids in the liver of experimental rats compared to extract of Opilia amentacea (Figures 4 and 5).
Figure 4.
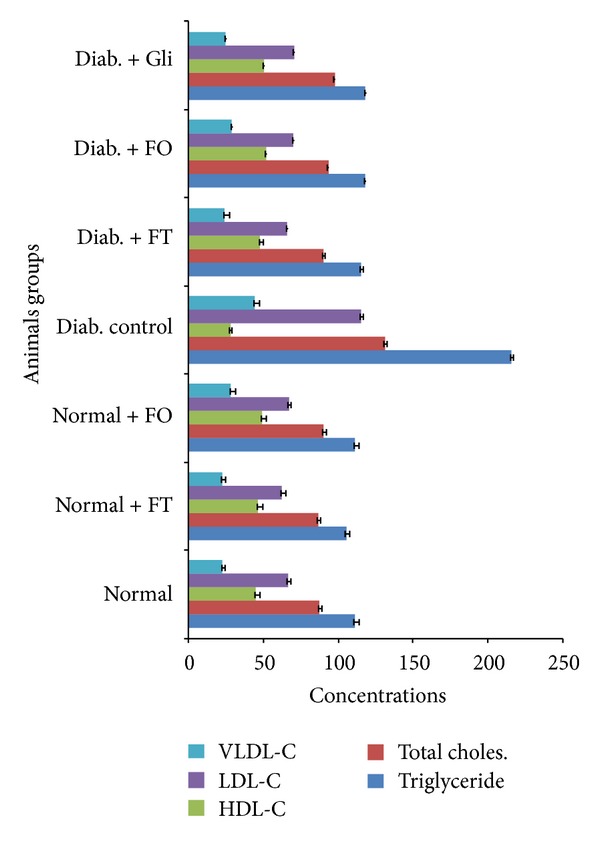
Effect of flavonoids from Trichilia emetica (FT) and Opilia amentacea (FO) on the concentrations of triglycerides, total cholesterol, HDL-C, LDL-C, and VLDL-C in serum of normal and experimental rats.
Figure 5.
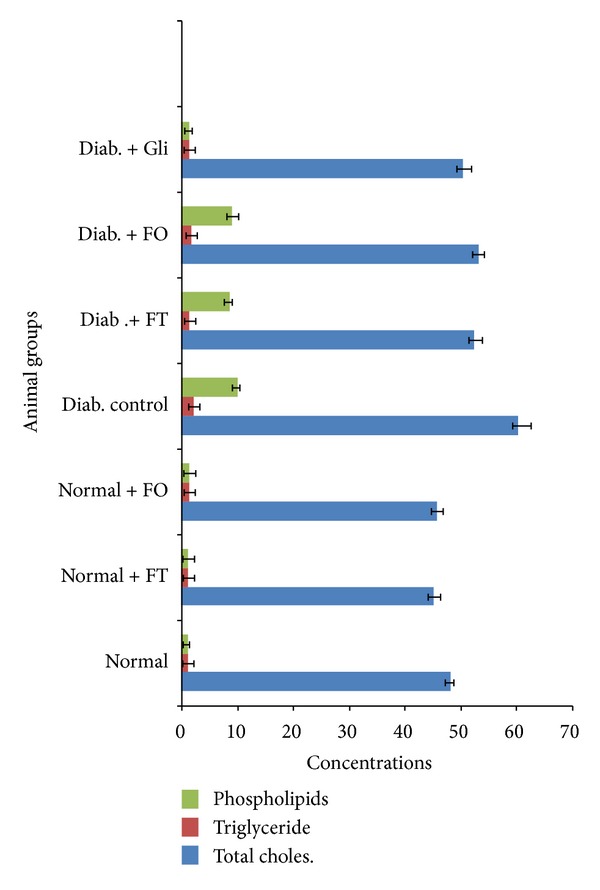
Effect of flavonoid-rich fractions from Trichilia emetica (FT) and Opilia amentacea (FO) on the concentrations of total cholesterol, triglycerides and phospholipids in the liver of normal and experimental rats.
4. Discussion
Type 2 diabetes is a global health challenge and the WHO has recommended research and use of complementary medicines for the management of this disease. Type 2 diabetes was previously considered as maturity-onset diabetes but, due to increasing rates of obesity, there is an increasing risk of developing this disease in childhood [46, 47].
In this study, flavonoid-rich fractions antioxidant activity was investigated using four assays which cover different aspects of antioxidant activity. Being a relatively stable free radical, DPPH∙ is frequently used to determine radical-scavenging activity of natural compounds. DPPH assay estimates the ability of sample to scavenge free radicals, species capable of causing damage to natural macromolecules, such as nucleic acids, polysaccharides, and lipids [50]. We noticed that the antiradical activity of flavonoid-rich fractions from Trichilia emetica was more pronounced than the activity of the same fractions from Opilia amentacea.
In β-carotene-linoleic acid assay, the degradation of β-carotene occurs in reaction with linoleic acid free radical formed at elevated temperatures. Subsequent loss of conjugation leads to a decrease in absorbance at 470 nm. Antioxidants present in the solution can prevent the degradation of β-carotene by reacting with the linoleic acid free radical or any other radical formed in the solution [29]. Thus, in this assay, the capacity of antioxidants to prevent degradation of natural lipids, such as linoleic acid, is measured. The reducing power of a compound, on the other hand, is related to its electron transfer ability and may serve as a significant indicator of its potential antioxidant activity. It estimates the ability of a substance, or an extract which contains it, to donate a proton and thus cause the transformation of free radicals into a less reactive species. In this study, also Trichilia emetica was a more successful inhibitor of β-carotene bleaching than Opilia amentacea. Among the biologically relevant ROS (H2O2, O2 ∙−, and ∙OH), hydroxyl radicals are the most reactive and dangerous species [51]. Free ferrous iron is sensitive to oxygen and gives rise to ferric iron and superoxide, thereby generating hydrogen peroxide. Thus formed hydrogen peroxide reacts with ferrous iron and generates the hydroxyl radical, which may subsequently oxidize surrounding biomolecules. The investigated flavonoid-rich fractions from Opilia amentacea constituents were incapable of chelating ferrous ions in this assay comparatively to the control. Trichilia emetica flavonoid-rich fractions, on the other hand demonstrated significant chelating ability. The differences in chemical compositions have probably caused such difference of ferrous ions. The activity of Trichilia emetica extracts could certainly be explained by phenolic compounds [27]. Some studies demonstrated that the extracts having higher phenol content also have higher DPPH radical-scavenging activity and other types of antioxidant activities [52–55]. We noticed that antioxidant activity of Trichilia emetica was more pronounced in most of the performed assays which leads to conclusion that besides polyphenols some other compounds may at least partly be responsible for the antioxidant activity of investigated extracts.
The inhibition of key enzyme linked to type 2 diabetes, such as α-amylase and α-glucosidase, has been considered to be an effective strategy to control blood glucose [56]. Agents based on natural products are particularly attractive as side effects are minimal and the therapies are well tolerated compared to the other oral hypoglycemic agents currently available [35, 57]. We noticed that extract with flavonoid-rich fractions from Trichilia emetica possessed marked antiamylase activity which may be due to the presence of certain secondary metabolites. In effect, flavonoids, alkaloids, and triterpenoids may be related to the antidiabetic activity of plants. In particular, flavonoids are responsible for variety of pharmacological activities [55]. For example, epicatechin is known to possess insulin-like properties, while epigallocatechin gallate is considered a promising hypoglycemic agent [58]. As shown in previous studies, the enzyme inhibition activity may be related to the polyphenolic content of the plant extract; however, further studies are needed to confirm this.
About antihypertensive property, it is well known that, in recent years, the treatment of hypertension has achieved a breakthrough with the identification of ACE inhibitors as a modern therapeutic tool and considerable interest has focused on the action of ACE inhibitors. ACE inhibitors have potentially improved endothelial function; they are known to increase the plasma concentration of bradykinin, an endothelium-dependent vasodilator, by inhibiting degradation of the peptide [9]. Procyanidins and flavonoids are the major natural products isolated from ethnopharmacologically important plants against in vitro ACE inhibitory activity [59, 60]. In addition, other active compounds isolated from medicinal plants include phenylpropanes, xanthones, fatty acids, terpenoids, alkaloids, and peptide amino acids [61]. In the present study, flavonoid-rich fractions from Trichilia emetica have shown the best antihypertensive potential compared to the control. This may be connected with capacities of some secondary metabolites like flavonoids.
Nowadays are antihypertensive and antidiabetic effects of these plants known which makes popular a traditional medicine in the developing countries. Medicinal plants are often believed to be harmless because they are natural and commonly used for self-medication without supervision. This increase in popularity and the scarcity of scientific studies on their safety and efficacy have raised concerns regarding toxicity and adverse effects of these remedies [62]. These products of plants contain bioactive principles with the potential to cause adverse effects [63]. The results of the present study indicated that the aqueous acetone extracts of Trichilia emetica and Opilia amentacea are not toxic. During 14-day period of acute toxicity evaluation some signs of toxicity have been observed, but they were all quickly reversible. According to [64, 65], pharmacological substances where whole LD50 less than 5 mg/kg body weight are classified in the range of highly toxic substances, those with LD50 between 5 mg/kg body weight and 5000 mg/kg body weight are classified in the range of moderately toxic substances, and those with lethal dose more than 5000 mg/kg body weight not toxic. In this fact, if we refer to this classification we would say that the extracts of Trichilia emetica and Opilia amentacea are moderately toxic and would be regarded as being safe or have low toxicity.
Traditional plant remedies have been used for centuries in the treatment of diabetes mellitus [66], but only a few have been scientifically evaluated. Alloxan is known for its selective pancreatic islet β cell cytotoxicity and has been extensively used to induce diabetes mellitus in animals [67]. Generalized increase in the level of blood glucose during diabetes has been consistently reported both in animal models [68] and humans especially those suffering from insulin dependent diabetes mellitus [69]. In this study, increase in blood glucose level was observed on induction of diabetes mellitus in the rats models, which has been reduced in a dose dependent manner with the highest percentage reduction at 300 mg/kg (Tables 1 and 2). At 3rd hours of exposure in variant with dose of 100 mg/kg did not found any significant antidiabetic activity. The flavonoid-rich fractions at the dose of 500 mg/kg show very significant antidiabetic activity from the first day to seventh day; the dose of 100 mg/kg shows significant antidiabetic activity from the 5th day to 7th day. Our study showed that it is possible that extracts may act by undetermined ways apart from stimulating insulin production from the pancreatic islets since these would have been severely damaged by alloxan. The mechanism of the hypoglycaemic effects of flavonoid-rich fractions remains speculative; therefore further studies are required to unravel the pathway of its hypoglycaemic action and to shed more light on the hypoglycaemic constituents of the plants. It is, however, evident that flavonoid-rich fractions contain hypoglycaemic agents capable of lowering blood glucose level in the alloxan diabetic rats (Tables 1 and 2).
It is well known that the prevalence of hyperlipidaemia among diabetics is increasing worldwide. Alteration in serum lipids profile is known in diabetes, which are likely to increase the risk of coronary heart disease [70]. Lipid profile which is altered in serum of diabetic patients [71] appeared to be a significant factor in the development of premature atherosclerosis through increase in serum triglyceride and total cholesterol levels. The significant reduction in serum cholesterol and total lipids in a dose dependent manner as observed in this experimental work has been confirmed with results of previous reports [72]. The marked hyperlipidaemia that characterizes the diabetic state may be regarded as a consequence of the uninhibited actions of lipolytic hormones on the fat depots [73]. A reduction in lipid profile could be beneficial in preventing diabetic complications as well as improving lipid metabolism in diabetics [74].
Considering flavonoid-rich fractions effects on lipid components [75], it can be assumed a potential hypolipidaemic agent which will be a great advantage both in diabetic conditions as well as in the associated hyperlipidaemic conditions. In effect, the use of synthetic drugs for management of diabetes mellitus has certain adverse effects and therefore there is a need to develop safer and more effective antidiabetic drugs. Consequently, treatment with drugs isolated from plants has an effect on shielding β cells and leveling the oscillation in glucose levels [76]. Elevated serum or tissue lipids and lipoproteins are characteristics of uncontrolled diabetes. Type 2 diabetes mellitus is commonly associated with dyslipidemia which is a significant risk factor for the development of cardiovascular diseases [77]. This is in support with results of our present study. In the present, a marked increase in the lipid content of serum and liver was found in STZ induced diabetic rats which is mainly due to the increased mobilization of free fatty acids (FFAs) from peripheral depots [78]. Interestingly, most of the studies with different plant extracts in diabetic rats were supportive of our results [79]. The rise in serum triacylglycerols, cholesterol, and LDL-cholesterol levels in the present study indicates derangement of lipid metabolism and amplified incidence of cardiac dysfunction in diabetic rats. Rise in serum lipids indicates either the defective overproduction or removal (or both) of one or more lipoproteins [80]. An oral administration of flavonoid-rich fractions for a period of 28 days restored the altered levels of lipids (triglycerides, total cholesterol, and phospholipids) in liver tissue as well as in serum. The decreased levels, that is, restoration levels, of cholesterols and triglycerides are due to the presence of glycosides in the flavonoid-rich fractions. The elevated concentrations of cholesterol can enhance the risk of oxidative disease process due to susceptibility of cholesterol to oxidation while it is in circulation. Insulin deficiency or insulin resistance may be responsible for dyslipidemia, because insulin has an inhibitory action on HMG-coA reductase, a key rate-limiting enzyme which is responsible for the LDL particle metabolism with cholesterol-rich content [81]. A shortage of insulin is associated with rise in cholesterol levels due to the increased mobilization of lipids from the adipose tissue to the plasma. The enlarged concentration of FFAs in liver may be due to lipid catalysis which leads to enhanced generation of NADPH and activation of NADPH dependent microsomal lipid peroxidation [82]. In addition, phospholipids are vital components of biomembranes and play an essential role in the triglycerides transport [83]. In diabetic rats, the gigantic levels of PLs may be due to the high levels of FFAs and total cholesterol [84].
5. General Remarks and Conclusion
The present study assesses the antidiabetic potential of flavonoid-rich fractions from Trichilia emetica and Opilia amentacea. These plants can be promising agents with good antioxidant activity for the management of hyperglycemia and can be used also in the antihypertensive therapy. However, these results require further investigation to better understand biochemical nature of these effects as they may provide leads for the discovery of new drugs for the management of diabetes type 2 with minimal side effects. Further studies are in progress with isolating these active compounds which are responsible for the antidiabetic and antihypertensive properties.
Acknowledgments
The authors are grateful to the France Embassy in Burkina Faso/EGIDE-France for the mobility scholarship which has permitted them to do this work. The authors thank Professor Millogo Rasolodimby from the Plants Biology Department of the University of Ouagadougou for the botanical identification of plants.
Conflict of Interests
The authors declare no financial conflict of interests.
References
- 1.Department of Noncommunicable Disease Surveillance, World Health Organisation. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. 1999. [Google Scholar]
- 2.da Silva Pinto M, Kwon Y-I, Apostolidis E, Lajolo FM, Genovese MI, Shetty K. Evaluation of red currants (Ribes rubrum L.), black currants (Ribes nigrum L.), red and green gooseberries (Ribes uva-crispa) for potential management of type 2 diabetes and hypertension using in vitro models. Journal of Food Biochemistry. 2010;34(3):639–660. [Google Scholar]
- 3.Oršolić N, Bašić I. Honey bee products and their polyphenolic compounds in tretment of diabetes. In: Govil JN, Singh VK, editors. Phytopharmacology and Therapetutic Values IV. Vol. 22. Houston, Tex, USA: Studium Press; 2008. pp. 455–471. (Recent Progress in Medical Plants). [Google Scholar]
- 4.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 5.Fröde TS, Medeiros YS. Animal models to test drugs with potential antidiabetic activity. Journal of Ethnopharmacology. 2008;115(2):173–183. doi: 10.1016/j.jep.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 6.Haffner SM. The importance of hyperglycemia in the nonfasting state to the development of cardiovascular disease. Endocrine Reviews. 1998;19(5):583–592. doi: 10.1210/edrv.19.5.0343. [DOI] [PubMed] [Google Scholar]
- 7.Hayden MR, Tyagi SC. Is type 2 diabetes mellitus a vascular disease (atheroscleropathy) with hyperglycemia a late manifestation? The role of NOS, NO, and redox stress. Cardiovascular Diabetology. 2003;2, article 2 doi: 10.1186/1475-2840-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeolekar ME, Shete MM. Homocysteine and hypertension. Journal of Association of Physicians of India. 2002;50(5):29–35. [PubMed] [Google Scholar]
- 9.Ghiadoni L, Magagna A, Versari D, et al. Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension. 2003;41(6):1281–1286. doi: 10.1161/01.HYP.0000070956.57418.22. [DOI] [PubMed] [Google Scholar]
- 10.Fujita H, Yokoyama K, Yoshikawa M. Classification and antihypertensive activity of angiostensin I-converting enzyme inhibitory peptides derived from food proteins. Journal of Food Science. 2000;65(4):564–569. [Google Scholar]
- 11.Ohmura C, Tanaka Y, Mitsuhashi N, et al. Efficacy of low-dose metformin in Japanese patients with type 2 diabetes mellitus. Current Therapeutic Research. 1998;59(12):889–895. [Google Scholar]
- 12.Fonseca V. Clinical significance of targeting postprandial and fasting hyperglycemia in managing type 2 diabetes mellitus. Current Medical Research and Opinion. 2003;19(7):635–641. doi: 10.1185/030079903125002351. [DOI] [PubMed] [Google Scholar]
- 13.Palombo EA. Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phytotherapy Research. 2006;20(9):717–724. doi: 10.1002/ptr.1907. [DOI] [PubMed] [Google Scholar]
- 14.Alarcon-Aguilara FJ, Roman-Ramos R, Perez-Gutierrez S, Aguilar-Contreras A, Contreras-Weber CC, Flores-Saenz JL. Study of the anti-hyperglycemic effect of plants used as antidiabetics. Journal of Ethnopharmacology. 1998;61(2):101–110. doi: 10.1016/s0378-8741(98)00020-8. [DOI] [PubMed] [Google Scholar]
- 15.Ali H, Houghton PJ, Soumyanath A. α-amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus . Journal of Ethnopharmacology. 2006;107(3):449–455. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Bhandari MR, Jong-Anurakkun N, Hong G, Kawabata J. α-glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.) Food Chemistry. 2008;106(1):247–252. [Google Scholar]
- 17.Bailey CJ, Day C. Metformin: its botanical background. Practical Diabetes International. 2004;21(3):115–117. [Google Scholar]
- 18.Day C. Are herbal remedies of use in diabetes? Diabetic Medicine. 2005;22(1):10–12. doi: 10.1111/j.1464-5491.2005.1531e.x. [DOI] [PubMed] [Google Scholar]
- 19.Howlett HCS, Bailey CJ, editors. Galegine and Antidiabetic Plants. Vol. 22. Chichester, UK: John Wiley & Sons; 2007. [Google Scholar]
- 20.Asano N, Oseki K, Kaneko E, Matsui K. Enzymic synthesis of α- and β-D-glucosides of 1-deoxynojirimycin and their glycosidase inhibitory activities. Carbohydrate Research. 1994;258:255–266. doi: 10.1016/0008-6215(94)84091-1. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan R, Chandrasekar MJN, Nanjan MJ, Suresh B. Antioxidant activity of Caesalpinia digyna root. Journal of Ethnopharmacology. 2007;113(2):284–291. doi: 10.1016/j.jep.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Marwah RG, Fatope MO, Mahrooqi RA, Varma GB, Abadi HA, Al-Burtamani SKS. Antioxidant capacity of some edible and wound healing plants in Oman. Food Chemistry. 2007;101(2):465–470. [Google Scholar]
- 23.Nabavi SM, Ebrahimzadeh MA, Nabavi SF, Fazelian M, Eslami B. In vitro antioxidant and free radical scavenging activity of Diospyros lotus and Pyrus boissieriana growing in Iran. Pharmacognosy Magazine. 2009;4(18):122–126. [Google Scholar]
- 24.Büyükbalci A, El SN. Determination of in vitro antidiabetic effects, antioxidant activities and phenol contents of some herbal teas. Plant Foods for Human Nutrition. 2008;63(1):27–33. doi: 10.1007/s11130-007-0065-5. [DOI] [PubMed] [Google Scholar]
- 25.Aslan M, Orhan N, Orhan DD, Ergun F. Hypoglycemic activity and antioxidant potential of some medicinal plants traditionally used in Turkey for diabetes. Journal of Ethnopharmacology. 2010;128(2):384–389. doi: 10.1016/j.jep.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 26.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. The New England Journal of Medicine. 1999;341(25):1906–1912. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 27.Nacoulma OG. Medicinal plants and their traditional uses in Burkina Faso [Ph.D. thesis] Biochemistry-Microbiology Department, University of Ouagadougou; 1996. [Google Scholar]
- 28.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 29.Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chemistry. 2004;84(4):551–562. [Google Scholar]
- 30.Al-Saikhan MS, Howard LR, Miller JC. Antioxidant activity and total phenolics in different genotypes of potato (Solanum tuberosum, L.) Journal of Food Science. 1995;60(2):341–347. [Google Scholar]
- 31.Končić MZ, Kremer D, Gruz J, et al. Antioxidant and antimicrobial properties of Moltkia petraea (Tratt.) Griseb. flower, leaf and stem infusions. Food and Chemical Toxicology. 2010;48(6):1537–1542. doi: 10.1016/j.fct.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Decker EA, Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. Journal of Agricultural and Food Chemistry. 1990;38(3):674–677. [Google Scholar]
- 33.Buege JA, Aust ST. Microsomal lipid peroxidation. Methods in Enzymology. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 34.Correia RTP, Mccue P, Vattem DA, Magalhães MMA, Macêdo GR, Shetty K. Amylase and helicobacter pylori inhibition by phenolic extracts of pineapple wastes bioprocessed by rhizopus oligosporus. Journal of Food Biochemistry. 2004;28(5):419–434. [Google Scholar]
- 35.Kwon Y-I, Apostolidis E, Shetty K. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresource Technology. 2008;99(8):2981–2988. doi: 10.1016/j.biortech.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 36.Loizzo MR, Saab AM, Tundis R, et al. In vitro inhibitory activities of plants used in Lebanon traditional medicine against angiotensin converting enzyme (ACE) and digestive enzymes related to diabetes. Journal of Ethnopharmacology. 2008;119(1):109–116. doi: 10.1016/j.jep.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Apostolidis E, Kwon Y-I, Shetty K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innovative Food Science and Emerging Technologies. 2007;8(1):46–54. [Google Scholar]
- 38.Hogan S, Zhang L, Li J, Sun S, Canning C, Zhou K. Antioxidant rich grape pomace extract suppresses postprandial hyperglycemia in diabetic mice by specifically inhibiting alpha-glucosidase. Nutrition and Metabolism. 2010;7, article 71 doi: 10.1186/1743-7075-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serra CP, Côrtes SF, Lombardi JA, de Oliveira AB, Braga FC. Validation of a colorimetric assay for the in vitro screening of inhibitors of angiotensin-converting enzyme (ACE) from plant extracts. Phytomedicine. 2005;12(6-7):424–432. doi: 10.1016/j.phymed.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Vermeirssen V, van Camp J, Verstraete W. Optimisation and validation of an angiotensin-converting enzyme inhibition assay for the screening of bioactive peptides. Journal of Biochemical and Biophysical Methods. 2002;51(1):75–87. doi: 10.1016/s0165-022x(02)00006-4. [DOI] [PubMed] [Google Scholar]
- 41.Miller LC, Tainter ML. Estimation of the ED50 and its error by means of logarithmic probit graph paper. Experimental Biology and Medicine. 1944;57(2):261–264. [Google Scholar]
- 42.Venkatesh S, Thilagavathi J, Sundar DS. Anti-diabetic activity of flowers of Hibiscus rosasinensis. Fitoterapia. 2008;79(2):79–81. doi: 10.1016/j.fitote.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 43.Babu KR, Vinay K, Sameena SK, Prasad SV, Swapna S, Rao ARCA. Antihyperglycemic and antioxidant effects of Talinum portulacifolium leaf extracts in streptozotocin diabetic rats: a dose-dependent study. Pharmacognosy Magazine. 2009;5(19):1–10. [Google Scholar]
- 44.Zlatkis A, Zak B, Boyle AJ. A new method for the direct determination of serum cholesterol. Journal of Laboratory and Clinical Medicine. 1953;41(3):486–492. [PubMed] [Google Scholar]
- 45.Foster LB, Dunn RT. Stable reagents for determination of serum triglycerides by a colorimetric Hantzsch condensation method. Clinical Chemistry. 1973;19(3):338–340. [PubMed] [Google Scholar]
- 46.Burstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. Journal of Lipid Research. 1970;11(6):583–595. [PubMed] [Google Scholar]
- 47.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 48.Haux P, Natelson S. Microprocedure for serum triglyceride estimation. Microchemical Journal. 1971;16(1):68–76. [Google Scholar]
- 49.Zilversmit DB, Davis AK. Microdetermination of plasma phospholipids by trichloroacetic acid precipitation. Journal of Laboratory and Clinical Medicine. 1950;35(1):155–160. [PubMed] [Google Scholar]
- 50.Issa AY, Volate SR, Wargovich MJ. The role of phytochemicals in inhibition of cancer and inflammation: new directions and perspectives. Journal of Food Composition and Analysis. 2006;19(5):405–419. [Google Scholar]
- 51.Peshev D, Vergauwen R, Moglia A, Hideg E, van den Ende W. Towards understanding vacuolar antioxidant mechanisms: a role for fructans. Journal of Experimental Botany. 2013;64(4):1025–1038. doi: 10.1093/jxb/ers377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orhan I, Şenol FS, Gülpinar AR, et al. Acetylcholinesterase inhibitory and antioxidant properties of Cyclotrichium niveum, Thymus praecox subsp. caucasicus var. caucasicus, Echinacea purpurea and E. pallida . Food and Chemical Toxicology. 2009;47(6):1304–1310. doi: 10.1016/j.fct.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Yam MF, Lim V, Salman IM, et al. HPLC and anti-inflammatory studies of the flavonoid rich chloroform extract fraction of Orthosiphon stamineus leaves. Molecules. 2010;15(6):4452–4466. doi: 10.3390/molecules15064452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gabr MMA, Sytar O, Abdelrahman RA, Smetanska I. Production of phenolic acid and antioxidant activity in transformed hairy root cultures of common buckwheat (Fagopyrum esculentum M.) Australian Journal of Basic and Applied Sciences. 2012;6(7):577–586. [Google Scholar]
- 55.Sytar O, Brestic M, Rai M, Shao HB. Phenolic compounds for food, pharmaceutical and cosmetics production. Journal of Medicinal Plants Research. 2012;6(13):2526–2539. [Google Scholar]
- 56.Oboh G, Akinyemi AJ, Ademiluyi AO. Inhibition of α-amylase and α-glucosidase activities by ethanolic extract of Telfairia occidentalis (fluted pumpkin) leaf. Asian Pacific Journal of Tropical Biomedicine. 2012;2(9):733–738. doi: 10.1016/S2221-1691(12)60219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ali H, Houghton PJ, Soumyanath A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus . Journal of Ethnopharmacology. 2006;107(3):449–455. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Mukherjee PK, Maiti K, Mukherjee K, Houghton PJ. Leads from Indian medicinal plants with hypoglycemic potentials. Journal of Ethnopharmacology. 2006;106(1):1–28. doi: 10.1016/j.jep.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 59.Wagner H, Elbl G, Lotter H, Guinea M. Evaluation of natural products as inhibitors of angiotensin I-converting enzyme (ACE) Pharmaceutical and Pharmacological Letters. 1991;1:15–18. [Google Scholar]
- 60.Lacaille-Dubois MA, Franck U, Wagner H. Search for potential angiotensin converting enzyme (ACE)-inhibitors from plants. Phytomedicine. 2001;8(1):47–52. doi: 10.1078/0944-7113-00003. [DOI] [PubMed] [Google Scholar]
- 61.Braga FC, Wagner H, Lombardi JA, de Oliveira AB. Screening the Brazilian flora for antihypertensive plant species for in vitro angiotensin-I-converting enzyme inhibiting activity. Phytomedicine. 2000;7(3):245–250. doi: 10.1016/s0944-7113(00)80011-2. [DOI] [PubMed] [Google Scholar]
- 62.Saad B, Azaizeh H, Abu-Hijleh G, Said O. Safety of traditional Arab herbal medicine. Evidence-Based Complementary and Alternative Medicine. 2006;3(4):433–439. doi: 10.1093/ecam/nel058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bent S, Ko R. Commonly used herbal medicines in the United States: a review. American Journal of Medicine. 2004;116(7):478–485. doi: 10.1016/j.amjmed.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 64.Kennedy GL, Ferenz RL, Burgess BA. Estimation of acute oral toxicity in rats by determination of the approximate lethal dose rather than the LD50. Journal of Applied Toxicology. 1986;6(3):145–148. doi: 10.1002/jat.2550060302. [DOI] [PubMed] [Google Scholar]
- 65.Konaté K, Bassolé IHN, Hilou A, et al. Toxicity assessment and analgesic activity investigation of aqueous acetone extracts of Sida acuta Burn f. and Sida cordifolia L., (Malvaceae), medicinal plants of Burkina Faso. BMC Complementary and Alternative Medicine. 2012;12, article 120 doi: 10.1186/1472-6882-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akhat MS, Ali MR. Study of anti diabetic effect of a compound medicinal plant prescription in normal and diabetic rabbits. Journal of the Pakistan Medical Association. 1984;34(8):239–244. [PubMed] [Google Scholar]
- 67.Fernandes NPC, Lagishetty CV, Panda VS, Naik SR. An experimental evaluation of the antidiabetic and antilipidemic properties of a standardized Momordica charantia fruit extract. BMC Complementary and Alternative Medicine. 2007;7, article 29 doi: 10.1186/1472-6882-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tütüncü NB, Bayraktar M, Varli K. Reversal of defective nerve conduction with vitamin E supplementation in type 2 diabetes: a preliminary study. Diabetes Care. 1998;21(11):1915–1918. doi: 10.2337/diacare.21.11.1915. [DOI] [PubMed] [Google Scholar]
- 69.Bell PM, Hayes JR, Stout RW. Lipoproteins, insulin and glycaemic control in diabetes. Hormone and Metabolic Research. 1984;16(5):262–263. doi: 10.1055/s-2007-1014760. [DOI] [PubMed] [Google Scholar]
- 70.Massing MW, Sueta CA, Chowdhury M, Biggs DP, Simpson RJ., Jr. Lipid management among coronary artery disease patients with diabetes mellitus or advanced age. American Journal of Cardiology. 2001;87(5):646–649. doi: 10.1016/s0002-9149(00)01447-8. [DOI] [PubMed] [Google Scholar]
- 71.Betteridge DJ. Diabetic dyslipidaemia. American Journal of Medicine. 1994;96(6, supplement 1):S25–S31. [Google Scholar]
- 72.Blumenthal M. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Austin, Tex, USA: American Botanical Council; 1998. [Google Scholar]
- 73.Hardman JG, Limberd LE. Goodman and Gilman’s: The Pharmacological Basis of Therapeutics. 10th edition. New York, NY, USA: McGraw-Hill; 2001. Insulin, oral hypoglycaemic agents and the pharmacology of the endocrine pancreas; pp. 1383–1399. [Google Scholar]
- 74.Cho S-Y, Park J-Y, Park E-M, et al. Alternation of hepatic antioxidant enzyme activities and lipid profile in streptozotocin-induced diabetic rats by supplementation of dandelion water extract. Clinica Chimica Acta. 2002;317(1-2):109–117. doi: 10.1016/s0009-8981(01)00762-8. [DOI] [PubMed] [Google Scholar]
- 75.Lecumberri E, Goya L, Mateos R, et al. A diet rich in dietary fiber from cocoa improves lipid profile and reduces malondialdehyde in hypercholesterolemic rats. Nutrition. 2007;23(4):332–341. doi: 10.1016/j.nut.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 76.Fatima SS, Rajasekhar MD, Kumar KV, Kumar MTS, Babu KR, Rao CA. Antidiabetic and antihyperlipidemic activity of ethyl acetate:Isopropanol (1 : 1) fraction of Vernonia anthelmintica seeds in Streptozotocin induced diabetic rats. Food and Chemical Toxicology. 2010;48(2):495–501. doi: 10.1016/j.fct.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 77.Karthikesan K, Pari L, Menon VP. Antihyperlipidemic effect of chlorogenic acid and tetrahydrocurcumin in rats subjected to diabetogenic agents. Chemico-Biological Interactions. 2010;188(3):643–650. doi: 10.1016/j.cbi.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 78.Krishnaveni M, Mirunalini S, Karthishwaran K, Dhamodharan G. Antidiabetic and antihyperlipidemic properties of Phyllanthus emblica Linn. (Euphorbiaceae) on streptozotocin induced diabetic rats. Pakistan Journal of Nutrition. 2010;9(1):43–51. [Google Scholar]
- 79.Nirmala A, Eliza J, Rajalakshmi M, Priya E, Daisy P. Effect of hexane extract of Cassia fistula barks on blood glucose and lipid profile in Streptozotocin diabetic rats. International Journal of Pharmacology. 2008;4(4):292–296. [Google Scholar]
- 80.Fernandes AAH, Novelli ELB, Okoshi K, et al. Influence of rutin treatment on biochemical alterations in experimental diabetes. Biomedicine and Pharmacotherapy. 2010;64(3):214–219. doi: 10.1016/j.biopha.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 81.Rydgren T, Sandler S. The protective effect of simvastatin against low dose streptozotocin induced type 1 diabetes in mice is independent of inhibition of HMG-CoA reductase. Biochemical and Biophysical Research Communications. 2009;379(4):1076–1079. doi: 10.1016/j.bbrc.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 82.Banu GS, Kumar G, Murugesan AG. Antihyperlipidemic effect of Garlip, a polyherbal formulation in streptozotocin induced diabetic rats. Food and Chemical Toxicology. 2009;47(9):2361–2365. doi: 10.1016/j.fct.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 83.Arulmozhi V, Krishnaveni M, Karthishwaran K, Dhamodharan G, Mirunalini S. Antioxidant and antihyperlipidemic effect of Solanum nigrum fruit extract on the experimental model against chronic ethanol toxicity. Pharmacognosy Magazine. 2010;6(21):42–50. doi: 10.4103/0973-1296.59965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rajagopal K, Sasikala K. Antihyperglycaemic and antihyperlipidaemic effects of Nymphaea stellata in alloxan-induced diabetic rats. Singapore Medical Journal. 2008;49(2):137–141. [PubMed] [Google Scholar]


