Abstract
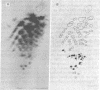
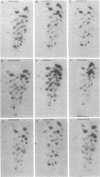
Fragments from the DNA of Chinese hamster ovary cells produced by restriction endonuclease EcoRI were cloned in Charon 16A lambda bacteriophage and examined for the ability to hybridize in situ with 32P-labeled double-stranded regions from heterogeneous nuclear RNA (hnRNA). Of 235 clones tested, 87 (37%) contained sequences that hybridized with the double-stranded hnRNA. Nine of these were examined for the presence of inverted repeat DNA structures (ir-DNA) by electron microscopy. All nine contained at least two elements of ir-DNA. Analysis of heteroduplexes formed from the DNAs of the different clones as well as T1 fingerprint analysis of the double-stranded hnRNA hybridized to each of the nine clones suggest that there is detectable nucleotide sequence homology in the various ir-DNAs. There are ca 3 X 10(5) ir-DNA pairs in the haploid Chinese hamster ovary cell genome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Hough B. R., Amenson C. S., Britten R. J. General interspersion of repetitive with non-repetitive sequence elements in the DNA of Xenopus. J Mol Biol. 1973 Jun 15;77(1):1–23. doi: 10.1016/0022-2836(73)90359-8. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., Schmid C. W. An electron microscope study of the DNA sequence organization of the human genome. J Mol Biol. 1976 Sep 25;106(3):773–790. doi: 10.1016/0022-2836(76)90264-3. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R. Specific nucleotide sequences in HeLa cell inverted repeated DNA: enrichment for sequences found in double-stranded regions of heterogeneous nuclear RNA. J Mol Biol. 1977 Oct 5;115(4):591–601. doi: 10.1016/0022-2836(77)90104-8. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Darnell J. E. Double-stranded regions in heterogeneous nuclear RNA from Hela cells. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2537–2541. doi: 10.1073/pnas.69.9.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Molloy G., Fernandez-Munoz R., Salditt M., Darnell J. E. Secondary structure in heterogeneous nuclear RNA: involvement of regions from repeated DNA sites. J Mol Biol. 1974 Jan 25;82(3):361–370. doi: 10.1016/0022-2836(74)90597-x. [DOI] [PubMed] [Google Scholar]
- Kronenberg L. H., Humphreys T. Double-stranded ribonucleic acid in sea urchin embryos. Biochemistry. 1972 May 23;11(11):2020–2026. doi: 10.1021/bi00761a005. [DOI] [PubMed] [Google Scholar]
- Montagnier L. Présence d'un acide ribonucléique en double chaîne dans des cellules animales. C R Acad Sci Hebd Seances Acad Sci D. 1968 Oct 21;267(17):1417–1420. [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Jelinek W. Determination of nucleotide sequences from double-stranded regions of HeLa cell nuclear RNA. J Mol Biol. 1977 Oct 5;115(4):571–589. doi: 10.1016/0022-2836(77)90103-6. [DOI] [PubMed] [Google Scholar]
- Ryskov A. P., Farashyan V. R., Georgiev G. P. Ribonuclease-stable base sequences specific exclusively for giant dRNA. Biochim Biophys Acta. 1972 Apr 12;262(4):568–572. doi: 10.1016/0005-2787(72)90502-3. [DOI] [PubMed] [Google Scholar]
- Ryskov A. P., Saunders G. F., Farashyan V. R., Georgiev G. P. Double-helical regions in nuclear precursor of mRNA (pre-mRNA). Biochim Biophys Acta. 1973 Jun 8;312(1):152–164. doi: 10.1016/0005-2787(73)90060-9. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Deininger P. L. Sequence organization of the human genome. Cell. 1975 Nov;6(3):345–358. doi: 10.1016/0092-8674(75)90184-1. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Manning J. E., Davidson N. Inverted repeat sequences in the Drosophila genome. Cell. 1975 Jun;5(2):159–172. doi: 10.1016/0092-8674(75)90024-0. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Thomas C. A., Jr Palindromes in chromosomes. J Mol Biol. 1974 Mar 25;84(1):115–138. doi: 10.1016/0022-2836(74)90216-2. [DOI] [PubMed] [Google Scholar]