Abstract
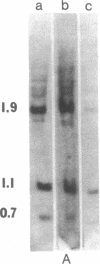
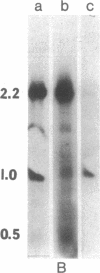
Labeled, purified 30S RNA from Moloney murine sarcoma virus was annealed to an excess of Moloney murine leukemia virus complementary DNA. Upon treatment of the resulting DNA.RNA hybrids with RNase H followed by sucrose gradient sedimentation, and undigested 18S RNA molecule was recovered. This RNA molecule was shown to represent the "sarcoma-specific" region of the virus. The unintegrated linear DNA provirus of murine sarcoma virus 124 was isolated from newly infected cells and a physical map of the sarcoma-specific region was obtained. First, unintegrated full-length linear proviral DNA molecules were cleaved by several restriction endonucleases. The reciprocal position and orientation with respect to the viral RNA of the resulting fragments were established. The location of the sarcoma-specific region was determined by competition-hybridization with 125I-labeled viral genomic RNAs and proviral DNA fragments. A 1500-base-pair fragment was obtained by cleavage with HindIII + Bgl II. This fragment mapped between 750 and 2250 base pairs from the right end of the proviral DNA (corresponding th the 3' terminus of the viral RNA) and contained the whole set of the sarcoma-specific information. This murine sarcoma virus proviral restriction fragment is approximately of the same size and map position as the isolated 18S sarcoma-specific RNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball J., McCarter J. A., Sunderland S. M. Evidence for helper independent murine sarcoma virus. I. Segregation of replication-defective and transformation-defective viruses. Virology. 1973 Nov;56(1):268–284. doi: 10.1016/0042-6822(73)90305-x. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Phillips L. A., Kramer M. J., Haapala D. K., Peebles P. T., Nomura S., Fischinger P. J. Transformation of mouse 3T3 cells by murine sarcoma virus: release of virus-like particles in the absence of replicating murine leukemia helper virus. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1520–1524. doi: 10.1073/pnas.68.7.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Duesberg P., Dina D. Cleavage map of linear mouse sarcoma virus DNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):29–33. doi: 10.1073/pnas.74.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Dina D., Beemon K., Duesberg P. The 30S Moloney sarcoma virus RNA contains leukemia virus nucleotide sequences. Cell. 1976 Oct;9(2):299–309. doi: 10.1016/0092-8674(76)90120-3. [DOI] [PubMed] [Google Scholar]
- Dina D., Beemon K. Relationship between Moloney murine leukemia and sarcoma virus RNAs: purification and hybridization map of complementary DNAs from defined regions of Moloney murine sarcoma virus 124. J Virol. 1977 Sep;23(3):524–532. doi: 10.1128/jvi.23.3.524-532.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. E., Neubauer R. L., Fischinger P. J. Fractionation of DNA nucleotide transcripts from Moloney sarcoma virus and isolation of sarcoma virus-specific complementary DNA. J Virol. 1976 May;18(2):481–490. doi: 10.1128/jvi.18.2.481-490.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J., Heijneker H. L. Size and location of the transforming region in human adenovirus type 5 DNA. Nature. 1974 Oct 25;251(5477):687–691. doi: 10.1038/251687a0. [DOI] [PubMed] [Google Scholar]
- Hu S., Davidson N. A heteroduplex study of the sequence relationships between the RNAs of M-MSV and M-MLV. Cell. 1977 Mar;10(3):469–477. doi: 10.1016/0092-8674(77)90034-4. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Berkower I., Hurwitz J. Mechanism of action of ribonuclease H isolated from avian myeloblastosis virus and Escherichia coli. Proc Natl Acad Sci U S A. 1973 Feb;70(2):466–470. doi: 10.1073/pnas.70.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLONEY J. B. Biological studies on a lymphoid-leukemia virus extracted from sarcoma 37. I. Origin and introductory investigations. J Natl Cancer Inst. 1960 Apr;24:933–951. [PubMed] [Google Scholar]
- Maisel J., Dina D., Duesberg P. Murine sarcoma viruses: the helper-independence reported for a Moloney variant is unconfirmed; distinct strains differ in the size of their RNAs. Virology. 1977 Jan;76(1):295–312. doi: 10.1016/0042-6822(77)90304-x. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Oskarsson M. K., Robey W. G., Harris C. L., Fischinger P. J., Haapala D. K., Vande Woude G. F. A p60 polypeptide in the feline leukemia virus pseudotype of Moloney sarcoma virus with murine leukemia virus p30 antigenic determinants. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2380–2384. doi: 10.1073/pnas.72.6.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Howk R. S., Anisowicz A., Scolnick E. M. Deletion mapping of moloney type C virus: polypeptide and nucleic acid expression in different transforming virus isolates. J Virol. 1976 May;18(2):491–503. doi: 10.1128/jvi.18.2.491-503.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles P. T., Gerwin B. I., Scolnick E. M. Murine sarcoma virus defectiveness: serological detection of only helper virus reverse transcriptase in sarcoma virus rescued from nonmurine S + L-cells. Virology. 1976 Apr;70(2):313–323. doi: 10.1016/0042-6822(76)90274-9. [DOI] [PubMed] [Google Scholar]
- Peebles P. T., Haapala D. K., Gazdar A. F. Deficiency of viral ribonucleic acid-dependent deoxyribonucleic acid polymerase in noninfectious virus-like particles released from murine sarcoma virus-transformed hamster cells. J Virol. 1972 Mar;9(3):488–493. doi: 10.1128/jvi.9.3.488-493.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey W. G., Oskarsson M. K., Vande Woude G. F., Naso R. B., Arlinghaus R. B., Haapala D. K., Fischinger P. J. Cells transformed by certain strains of Moloney sarcoma virus contain murine p60. Cell. 1977 Jan;10(1):79–89. doi: 10.1016/0092-8674(77)90142-8. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Howk R. S., Anisowicz A., Peebles P. T., Scher C. D., Parks W. P. Separation of sarcoma virus-specific and leukemia virus-specific genetic sequences of Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4650–4654. doi: 10.1073/pnas.72.11.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Temperature-sensitive mutants of murine leukemia virus. 3. Mutants defective in helper functions for sarcoma virus fixation. Virology. 1974 Mar;58(1):294–297. doi: 10.1016/0042-6822(74)90163-9. [DOI] [PubMed] [Google Scholar]
- Tronick S. R., Stephenson J. R., Verma I. M., Aaronson S. A. Thermolabile reverse transcriptase of a mammalian leukemia virus mutant temperature sensitive in its replication and sarcoma virus helper functions. J Virol. 1975 Dec;16(6):1476–1482. doi: 10.1128/jvi.16.6.1476-1482.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. Properties and location of poly(A) in Rous sarcoma virus RNA. J Virol. 1974 Dec;14(6):1515–1529. doi: 10.1128/jvi.14.6.1515-1529.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]