Abstract
Derivatives of furo[3,2-g]coumarin (psoralen) can bind to the DNA double helix and, in the presence of long-wavelength UV light, the bound psoralen may react covalently with pyrimidine residues on one or both strands of the helix. By using agarose gel electrophoresis, we have determined the unwinding angle associated with each of four different psoralen derivatives to be 28° ± 4°. For 4,5′,8-trimethylpsoralen (trioxsalen) the unwinding angle was found to be independent of the initial DNA superhelix density in the range that is accessible to agarose gel electrophoresis.
Also by using agarose gel electrophoresis, we have determined the unwinding angle for ethidium intercalation. This was done by the total relaxation of supercoiled DNA in the presence of a series of ethidium concentrations. By using published values for the association constant for ethidium binding to DNA and evaluating the final superhelix density (after removal of ethidium) of the DNA on gels, we calculated an unwinding angle of 29° ± 3°.
Assuming an unwinding angle of 28° for the noncovalent intercalation of psoralen derivatives, we used the same procedure to determine intercalation binding constants. The association constants for 4′-aminomethyltrioxsalen were 300-1400 M-1 in NaCl at 0.2-0.05 M and 300-2500 M-1 in Mg2+ at 4-0.5 mM. The association constant for 4′-hydroxymethyltrioxsalen in 0.5 mM Mg2+ was determined to be 70 M-1.
Keywords: nicking-closing enzyme, ethidium bromide unwinding angle, agarose
Full text
PDF
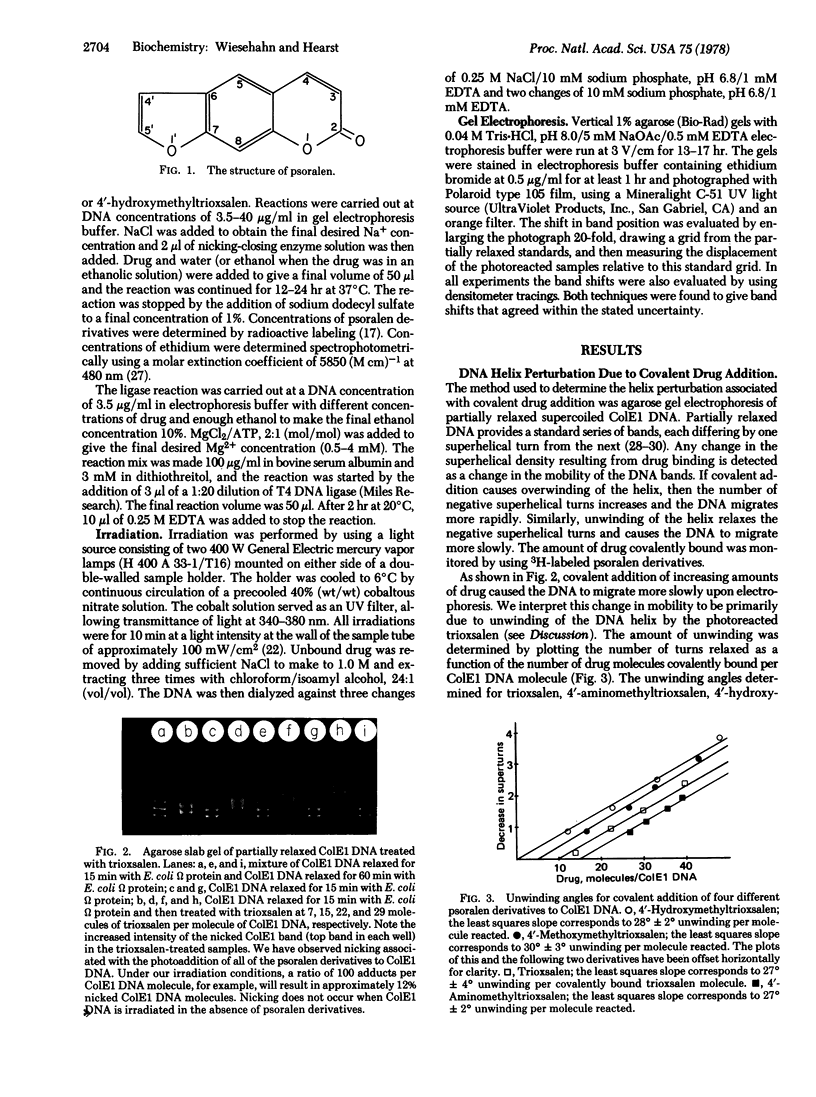
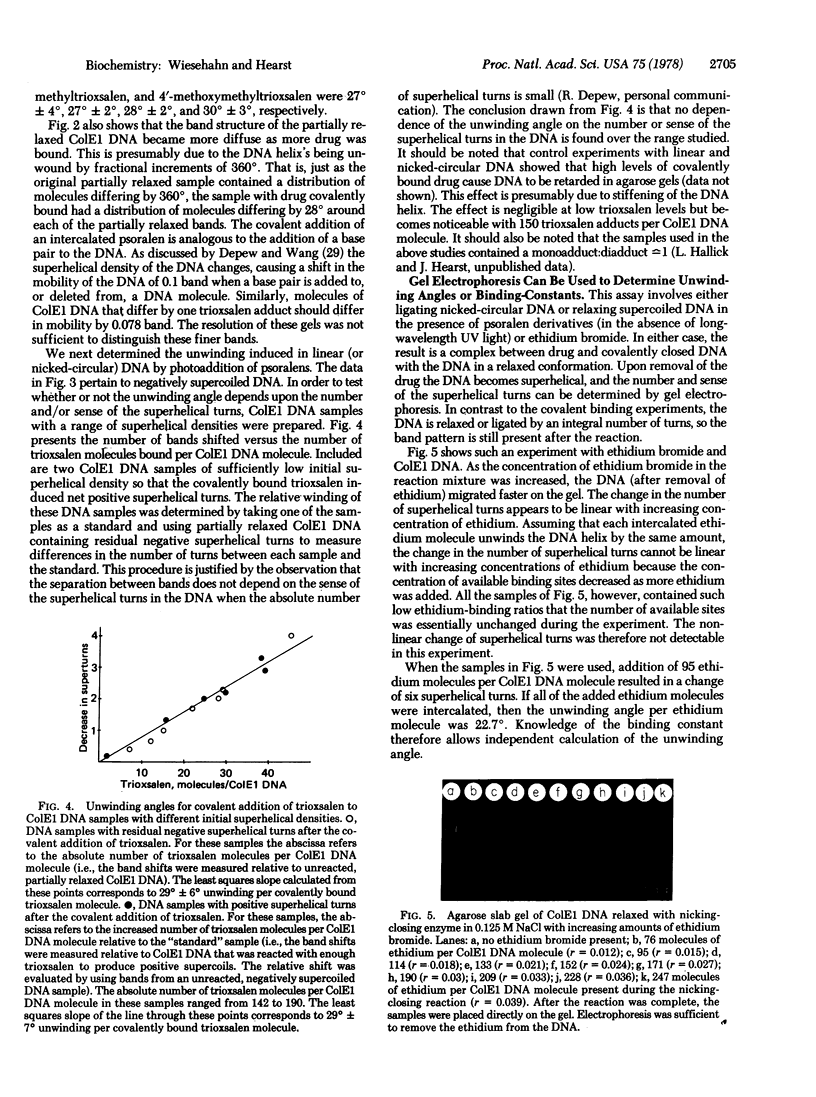


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averbeck D., Moustacchi E. 8-Methoxypsoralen plus 365 nm light effects and repair in yeast. Biochim Biophys Acta. 1975 Jul 23;395(4):393–404. doi: 10.1016/0005-2787(75)90063-5. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Bordin F., Baccichetti F. The furocoumarin photosensitizing effect on the virus-producing Graffi leukaemia cells. Z Naturforsch C. 1974 Sep-Oct;29C(9-10):630–632. doi: 10.1515/znc-1974-9-1029. [DOI] [PubMed] [Google Scholar]
- Bresloff J. L., Crothers D. M. DNA-ethidium reaction kinetics: demonstration of direct ligand transfer between DNA binding sites. J Mol Biol. 1975 Jun 15;95(1):103–123. doi: 10.1016/0022-2836(75)90339-3. [DOI] [PubMed] [Google Scholar]
- Cech T., Pardue M. L. Cross-linking of DNA with trimethylpsoralen is a probe for chromatin structure. Cell. 1977 Jul;11(3):631–640. doi: 10.1016/0092-8674(77)90080-0. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S., Levitan D., Sinden R. R. Removal of psoralen interstrand cross-links from DNA of Escherichia coli: mechanism and genetic control. J Mol Biol. 1976 May 5;103(1):39–59. doi: 10.1016/0022-2836(76)90051-6. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim Biophys Acta. 1970 Sep 17;217(1):30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Psoralen monoadducts and interstrand cross-links in DNA. Biochim Biophys Acta. 1971 Nov 29;254(1):30–39. doi: 10.1016/0005-2787(71)90111-0. [DOI] [PubMed] [Google Scholar]
- Dall'Acqua F., Marciani S., Ciavatta L., Rodighiero G. Formation of inter-strand cross-linkings in the photoreactions between furocoumarins and DNA. Z Naturforsch B. 1971 Jun;26(6):561–569. doi: 10.1515/znb-1971-0613. [DOI] [PubMed] [Google Scholar]
- Depew D. E., Wang J. C. Conformational fluctuations of DNA helix. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4275–4279. doi: 10.1073/pnas.72.11.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearst J. E., Thiry L. The photoinactivation of an RNA animal virus, vesicular stomatitis virus, with the aid of newly synthesized psoralen derivatives. Nucleic Acids Res. 1977;4(5):1339–1347. doi: 10.1093/nar/4.5.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T. S., Wang J. C. Thermodynamic properties of superhelical DNAs. Biochemistry. 1975 Feb 11;14(3):527–535. doi: 10.1021/bi00674a011. [DOI] [PubMed] [Google Scholar]
- Hyde J. E., Hearst J. E. Binding of psoralen derivatives to DNA and chromatin: influence of the ionic environment on dark binding and photoreactivity. Biochemistry. 1978 Apr 4;17(7):1251–1257. doi: 10.1021/bi00600a019. [DOI] [PubMed] [Google Scholar]
- Isaacs S. T., Shen C. K., Hearst J. E., Rapoport H. Synthesis and characterization of new psoralen derivatives with superior photoreactivity with DNA and RNA. Biochemistry. 1977 Mar 22;16(6):1058–1064. doi: 10.1021/bi00625a005. [DOI] [PubMed] [Google Scholar]
- Keller W., Wendel I. Stepwise relaxation of supercoiled SV40 DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):199–208. doi: 10.1101/sqb.1974.039.01.026. [DOI] [PubMed] [Google Scholar]
- Krauch C. H., Krämer D. M., Wacker A. Zum Wirkungsmechanismus photodynamischer Furocumarine Photoreaktion von Psoralen-(4-14C) mit DNS, RNS, Homopolynucleotiden und Nucleosiden. Photochem Photobiol. 1967 May;6(5):341–354. doi: 10.1111/j.1751-1097.1967.tb08882.x. [DOI] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- MUSAJO L., RODIGHIERO G., COLOMBO G., TORLONE V., DALLACQUA F. PHOTOSENSITIZING FUROCOUMARINS: INTERACTION WITH DNA AND PHOTO-INACTIVATION OF DNA CONTAINING VIRUSES. Experientia. 1965 Jan 15;21:22–24. doi: 10.1007/BF02136362. [DOI] [PubMed] [Google Scholar]
- Mandula B. B., Pathak M. A., Dudek G. Photochemotherapy: identification of a metabolite of 4, 5', 8-trimethylpsoralen. Science. 1976 Sep 17;193(4258):1131–1134. doi: 10.1126/science.959825. [DOI] [PubMed] [Google Scholar]
- Mickel S., Arena V., Jr, Bauer W. Physical properties and gel electrophoresis behavior of R12-derived plasmid DNAs. Nucleic Acids Res. 1977;4(5):1465–1482. doi: 10.1093/nar/4.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P., Zabel D. EcoRI endonuclease. Physical and catalytic properties of the homogenous enzyme. J Biol Chem. 1976 Oct 10;251(19):5866–5874. [PubMed] [Google Scholar]
- Musajo L., Bordin F., Caporale G., Marciani S., Rigatti G. Photoreactions at 3655 Angstrom between pyrimidine bases and skin-photosensitizing furocoumarins. Photochem Photobiol. 1967 Oct;6(10):711–719. doi: 10.1111/j.1751-1097.1967.tb08736.x. [DOI] [PubMed] [Google Scholar]
- Musajo L., Visentini P., Bacchinetti F., Razzi M. A. Photoinactivation of Ehrlich ascites tumor cells in vitro obtained with skin-photosensitizing furocoumarins. Experientia. 1967 May 15;23(5):335–336. doi: 10.1007/BF02144498. [DOI] [PubMed] [Google Scholar]
- Parrish J. A., Fitzpatrick T. B., Tanenbaum L., Pathak M. A. Photochemotherapy of psoriasis with oral methoxsalen and longwave ultraviolet light. N Engl J Med. 1974 Dec 5;291(23):1207–1211. doi: 10.1056/NEJM197412052912301. [DOI] [PubMed] [Google Scholar]
- Shen C. J., Hearst J. E. Detection of long-range sequence order in Drosophila melanogaster satellite DNA IV by a photochemical crosslinking reaction and denaturation microscopy. J Mol Biol. 1977 May 25;112(3):495–507. doi: 10.1016/s0022-2836(77)80195-2. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Hearst J. E. Mapping of sequences with 2-fold symmetry on the simian virus 40 genome: a photochemical crosslinking approach. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1363–1367. doi: 10.1073/pnas.74.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C. K., Hearst J. E. Psoralen-crosslinked secondary structure map of single-stranded virus DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2649–2653. doi: 10.1073/pnas.73.8.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shure M., Pulleyblank D. E., Vinograd J. The problems of eukaryotic and prokaryotic DNA packaging and in vivo conformation posed by superhelix density heterogeneity. Nucleic Acids Res. 1977;4(5):1183–1205. doi: 10.1093/nar/4.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. C., Jain S. C., Sobell H. M. X-ray crystallographic visualization of drug-nucleic acid intercalative binding: structure of an ethidium-dinucleoside monophosphate crystalline complex, Ethidium: 5-iodouridylyl (3'-5') adenosine. Proc Natl Acad Sci U S A. 1975 Feb;72(2):628–632. doi: 10.1073/pnas.72.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. Interactions between twisted DNAs and enzymes: the effects of superhelical turns. J Mol Biol. 1974 Aug 25;87(4):797–816. doi: 10.1016/0022-2836(74)90085-0. [DOI] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Wieshahn G. P., Hyde J. E., Hearst J. E. The photoaddition of trimethylpsoralen to Drosophila melanogaster nuclei: a probe for chromatin substructure. Biochemistry. 1977 Mar 8;16(5):925–932. doi: 10.1021/bi00624a018. [DOI] [PubMed] [Google Scholar]
- Wolff K. W., Fitzpatrick T. B., Parrish J. A., Gschnait F., Gilchrest B., Hönigsmann H., Pathak M. A., Tannenbaum L. Photochemotherapy for psoriasis with orally administered methoxsalen. Arch Dermatol. 1976 Jul;112(7):943–950. [PubMed] [Google Scholar]