Abstract
BACKGROUND
One proposed mechanism of extracorporeal photopheresis (ECP) in reducing chronic graft-versus-host disease (cGVHD) is alteration in numbers of circulating dendritic cells (DCs). This hypothesis was tested by correlating numbers of DC precursors and T cells in the blood before and during ECP therapy with response of cGVHD.
STUDY DESIGN AND METHODS
Twenty-five patients with cGVHD were treated with ECP. Data were collected with emphasis on blood cellular markers, clinical response to ECP, and overall survival.
RESULTS
Fourteen patients (56%) responded and had better 2-year survival than nonresponders (88% vs. 18%, p = 0.003). Responders had higher baseline circulating myeloid DC (mDC) and plasmacytoid DC precursors and CD4+ and CD8+ T cells compared with nonresponders. Receiver operating characteristic curve analyses showed that the best baseline cutoff values to predict response to ECP were mDC counts of 3.7 cells/µL (79% sensitivity, 82% specificity) and CD4+ T-cell counts of 104 cells/µL (71% sensitivity, 82% specificity). CD4+ T cells declined in responders over time, but not in nonresponders, and no significant changes were seen in CD8 T-cell or DC numbers over a 12-month period in responder or nonresponder groups.
CONCLUSIONS
Higher baseline numbers of circulating DCs and T cells may predict clinical response to ECP in patients with cGVHD.
Chronic graft-versus-host disease (cGVHD) occurs in more than 50% of patients after allogeneic hematopoietic progenitor cell transplantation (HPCT) and is a major contributor to transplant-related morbidity and mortality. The major targets of cGVHD are skin, liver, gastrointestinal tract, mucus membranes, lung, and the immune system.1, 2 Available therapeutic modalities include corticosteroids, calcineurin inhibitors, and mycophenylate mofetil, each of which can have significant toxicity. Extracorporeal photopheresis (ECP) is an apheresis technology that separates and concentrates white blood cells (WBCs) and exposes cells to 8-methoxypsoralen and ultraviolet A (UVA) irradiation before reinfusion and was first developed for treatment of cutaneous T-cell lymphoma.3 Several retrospective reports and prospective studies have demonstrated the efficacy of ECP therapy in some patients with cGVHD.4, 5
During ECP, photoactivated 8-methoxypsoralen intercalates into the DNA of nucleated cells and forms covalent cross-links preventing cell proliferation and initiating apoptosis, and one current theory for the mechanism of action of ECP is based on immunologic changes induced by apoptosis induction in lymphocytes.6 However, the effects of ECP on antigen-presenting dendritic cells (DCs) remain unresolved. A study of 10 patients with cGVHD showed that ECP treatment decreased the frequencies of circulating CD80+ and CD123+ cells representing DCs.7 Additional analyses for these patients using in vitro methods demonstrated a concurrent increase in the frequencies of CD83+ and CD86+ DC populations and a shift in helper T-cell (Th) differentiation from Th1 to Th2.8 To evaluate the hypothesis that ECP modulates the numbers of cells in circulating DC populations in patients with cGVHD, we analyzed the clinical and immunologic effects of ECP in 25 patients with steroid-dependent/steroid-refractory cGVHD or who were intolerant of steroids. Interestingly, responses correlated with higher baseline numbers of circulating pDCs, mDCs, and CD4+ and CD8+ T cells before initiating ECP therapy. CD4+ T-cell counts declined over time in responders, but no significant changes in peripheral blood DCs were observed over a 12-month period, suggesting an alternative mechanism of action.
MATERIALS AND METHODS
Study design
An institutional review board–approved retrospective analysis was performed on 25 consecutive adult recipients of HLA-matched blood HPCT with cGVHD who were steroid-dependent, steroid-refractory, or steroid-intolerant and who received ECP treatment for GVHD at Emory initiated between August 2004 and June 2008. These patients were selected from a total of 28 patients with cGVHD treated with ECP at Emory in this period and excluded one patient who began ECP at another institution and two patients who began ECP and then transferred their cGVHD care to other institutions and were lost to follow-up. All patients received initial treatment with corticosteroids, which was discontinued in four patients who demonstrated steroid intolerance as defined as development of avascular necrosis, severe myopathy, uncontrolled diabetes mellitus, psychosis, or systemic opportunistic infections during steroid treatment. All patients referred for ECP were receiving one or more forms of systemic immunosuppressive therapy when ECP was initiated (Table 1). ECP was administered 2 consecutive days every week for the first 2 months, two times a week every other week for 2 months, and then two times a week once a month, using a photopheresis system (UVAR XTS, Therakos, Exton, PA), as previously described.9 Clinical response of GVHD to ECP therapy was assessed monthly and immunosuppressive regimens were adjusted accordingly. Blood immune cells were monitored before starting ECP and at 2-month intervals thereafter as part of the standard care for cGVHD/ECP patients at this institution. Fresh blood samples were analyzed immediately after collection, with processing, antibody staining, and flow cytometry performed by the clinical lab.
Table 1.
Patient characteristics (n = 25)
| Characteristics | Responders (n = 14) |
Nonresponders (n = 11) | p value |
|---|---|---|---|
| Median age, years (range) | 46.5 (26–71) | 34 (23–50) | 0.069 |
| Male/female | 6/8 | 6/5 | 0.695 |
| Hematologic malignancy | 0.075 | ||
| Non-Hodgkin's lymphoma | 5 | 1 | |
| Chronic myeloid leukemia | 4 | 0 | |
| Acute myeloid leukemia | 1 | 5 | |
| Acute lymphoblastic leukemia | 2 | 2 | |
| Myelodysplastic syndrome | 2 | 1 | |
| Chronic lymphocytic leukemia | 0 | 1 | |
| Hodgkin's lymphoma | 0 | 1 | |
| Donor | 0.656 | ||
| Matched related | 11 | 7 | |
| Matched unrelated | 3 | 4 | |
| cGVHD | 0.095 | ||
| Progressive | 2 | 6 | |
| De novo | 6 | 3 | |
| Interrupted | 6 | 2 | |
| Severity of cGVHD | 1.0 | ||
| Extensive | 11 | 9 | |
| Limited | 3 | 2 | |
| GVHD sites | |||
| Skin | 14 | 11 | 1.0 |
| Mouth/oropharynx | 5 | 2 | 0.407 |
| Liver | 5 | 3 | 0.695 |
| Gut | 2 | 1 | 1.0 |
| Lung | 1 | 0 | 1.0 |
| Eye | 1 | 0 | 1.0 |
| Systemic immunosuppressive therapy | |||
| Prednisone/methylprednisolone | 10 | 11 | 0.105 |
| FK506 | 6 | 6 | 0.695 |
| Mycophenolate mofetil | 7 | 5 | 1.0 |
| Methotrexate | 1 | 2 | 0.565 |
| Cyclosporine | 3 | 3 | 1.0 |
| Rapamycin | 1 | 1 | 1.0 |
| Rituximab | 1 | 0 | 1.0 |
| Pentostatin | 0 | 1 | 0.440 |
| Budesonide | 1 | 0 | 1.0 |
Clinical assessment
Data from electronic medical records containing each patient's GVHD status history and laboratory test results were compiled. Transplants for 18 of the 25 patients were performed at Emory University Hospital, and records for these patients were cross-referenced and corroborated with adjudicated GVHD records maintained in a database (Stemsoft, Vancouver, British Columbia, Canada) by the BMT Data Management Office. A good clinical response of cGVHD to ECP was chiefly defined as successful reduction in the corticosteroid dose by 50% or more within 4 months of starting ECP, with improved skin lesions (reduced area of skin involvement). The steroid tapering schedule was generally a reduction of 10 mg every 2 weeks, with adjustments based on each patient's clinical condition. If a patient had two or more sites involved with cGVHD and showed good response in at least one site, and with more than 50% reduction in corticosteroid doses at 4 months, that patient was considered a responder. Liver function tests (LFTs) including total bilirubin, alkaline phosphatase, and alanine aminotransferase and aspartase aminotransferase transaminases were assessed. Levels of more than two times the upper limit of normal were considered consistent with liver cGVHD,10 and reduction to levels less than two times the upper limit of normal indicated improvement. For cGVHD involving the mouth and oropharynx, lichen-type changes and ulcers were indicative of cGVHD. For steroid-intolerant patients, clinical parameters such as improvement in skin condition and cGVHD affecting the mouth were used as the main criteria to determine response to ECP. The number of new infections after initiating ECP was determined for each patient (viral, bacterial, and fungal infections combined).
Biologic studies
The percentages of blood plasmacytoid DC precursors (pDCs; Lin−, CD123+, CD11c−, HLA-DR+), myeloid DC precursors (mDCs; Lin−, CD123−, CD11c+, HLA-DR+), and CD4+ and CD8+ T-cells in peripheral blood mononuclear cells were measured by flow cytometry in the clinical laboratory. The cell phenotyping data were combined with the WBC count to calculate the total number of each cell type per microliter of blood. From 2004 through 2006, flow cytometry data were acquired using a Becton Dickinson (BD, Franklin Lakes, NJ) FACSCalibur instrument, and after 2006 data were acquired on a BD FACSCanto or FACSCanto II instrument. BD Cell Quest software was used for analysis of all flow cytometry data.
Statistical analysis
Comparisons of patient characteristics (Table 1) between groups were performed using chi-square, Fisher's exact test, or t test. The t test was used to compare mean prednisone doses between groups. Kaplan-Meier survival analysis was performed for responders and nonresponders, and differences were analyzed by log rank. Cox regression was used for univariate and multivariate survival analyses. The U test was used to compare median numbers of DCs and T cells and other variables between groups. For all analyses, a p value of 0.05 or less was considered significant. Receiver operating characteristic (ROC) curves were generated to determine the power of T-cell or DC subset quantification to predict patient response to ECP.
RESULTS
Median follow-up of the 25 patients was 50.2 months (range, 13.1–101.3 months) from the time of transplant. The median number of ECP treatments was 26 (range, 2–68). Fourteen patients (56%) had good responses to ECP, with improved cGVHD based on clinical assessment, and 11 patients were nonresponders. Table 1 shows patient clinical characteristics for the two groups, and cGVHD status at the time ECP was initiated. Responders and nonresponders showed no significant differences in age, types of hematologic malignancies, donor type, severity or type of cGVHD, or systemic immunosuppressive therapy. The median time between HPCT and onset of cGVHD was higher for responders (6.9 months; range, 3.3–15.1 months) compared with nonresponders (4.0 months; range, 3.4–8.6 months; p = 0.018), while the median time between HPCT and ECP was similar for the two groups (32.3 months; range, 13.1–60.0 months; vs. 21.9 months; range, 4.1–47.5 months, respectively; p = 0.12). Although the mean prednisone dose at the start of ECP was lower for responders (24.3 mg/day) than nonresponders (41.8 mg/day) this difference was not significant (p = 0.11) and could be attributed largely to doses of 0 mg/day in four patients of the responder group who were steroid-intolerant. Considering only those patients who were taking prednisone at the start of ECP, the mean dose was 34.0 mg/day for responders, which was similar to the mean dose for nonresponders (p = 0.49). The causes of death for the two responders and seven nonresponders who died during the analysis period are listed in Table 2. Kaplan-Meier analysis showed that survival was significantly improved for responders versus nonresponders (p = 0.004;Fig. 1A), with responders having a 2-year estimated survival of 88% compared with 18% for nonresponders. This result was confirmed by Cox regression (p = 0.012; hazard ratio, 7.783). The correlation of CD4+ T-cell counts before ECP treatment with survival was nearly significant (p = 0.052; hazard ratio, 0.996). Other variables including sex, age, time from HPCT to ECP, CD8+ T cells, pDC, mDC, and steroid levels at start of ECP did not correlate with survival in univariate analyses. In multivariate analysis with response to ECP and CD4+ T-cell counts as covariates, only response was still significantly associated with survival (p = 0.042; adjusted hazard ratio, 6.574).
Table 2.
Cause of death
| Responders (n = 2) | Nonresponders (n = 7) |
|---|---|
| Pulmonary failure, cGVHD 1. | Posttransplant lymphoproliferative disease 1. |
| Recurrent acute lymphoblastic leukemia 2. | Acute respiratory failure, cGVHD 2. |
| Sepsis, cGVHD 3. | |
| Recurrent/persistent acute myeloid leukemia 4. | |
| Pneumonia, cGVHD 5. | |
| Recurrent acute lymphoblastic leukemia 6. | |
| Sepsis, cGVHD 7. |
Figure 1.
Survival and ROC curves. (A) Kaplan-Meier survival estimate curves showing significantly better survival for patients categorized as responders (—) versus nonresponders (- - -). (B, C) ROC curves showing the association of baseline mDC (B) and CD4+ T-cell levels (C) with response to ECP. The best cutoff point to predict response to ECP (cells/µL) and area under the curve (AUC) are shown.
Eight patients had cGVHD involving the liver at the time ECP was initiated. Of these, five were responders as defined by successful steroid reduction and skin improvement, and three were nonresponders. Four of the five responders showed improvements in LFTs after ECP treatment was begun. The fifth was a steroid-intolerant patient who demonstrated persistently elevated transaminases during ECP, but clear and continuous improvements in skin condition remained the dominant factor in classifying this patient as a responder. The three nonresponders showed either no improvement or worse LFT readings after starting ECP.
To determine whether baseline DC or T-cell counts could serve as predictors of response to ECP, univariate analyses were performed for each subset. Responders had higher baseline numbers of mDC (median, 11.8 cells/µL vs. 2.3 cells/µL; p = 0.01), pDC (median, 3.4 cells/µL vs. 0.7 cells/µL; p = 0.08), CD4+ T cells (median, 598 cells/µL vs. 62 cells/µL; p = 0.007), and CD8+ T cells (median, 580 cells/µL vs. 147 cells/µL; p = 0.03) compared with nonresponders. Baseline values of mDC and CD4+ T cells above the median value for the group of 25 cGVHD patients (3.7 mDC/µL, 251 CD4+ T-cells/µL) were both associated with an increased probability of response to ECP (p = 0.003 and p = 0.032, respectively). ROC curve analyses showed that the best baseline cutoff values to predict a good response to ECP were mDC counts of 3.7 cells/µL (79% sensitivity, 82% specificity; Fig. 1B) and CD4+ T-cell counts of 104 cells/µL (71% sensitivity, 82% specificity; Fig. 1C). The overall rates of infectious episodes in responders and nonresponders were similar, with median values of 0.1 and 0.09 infections per month after starting ECP, respectively (p = 0.54), suggesting that response to ECP is not associated with global immunosuppression.
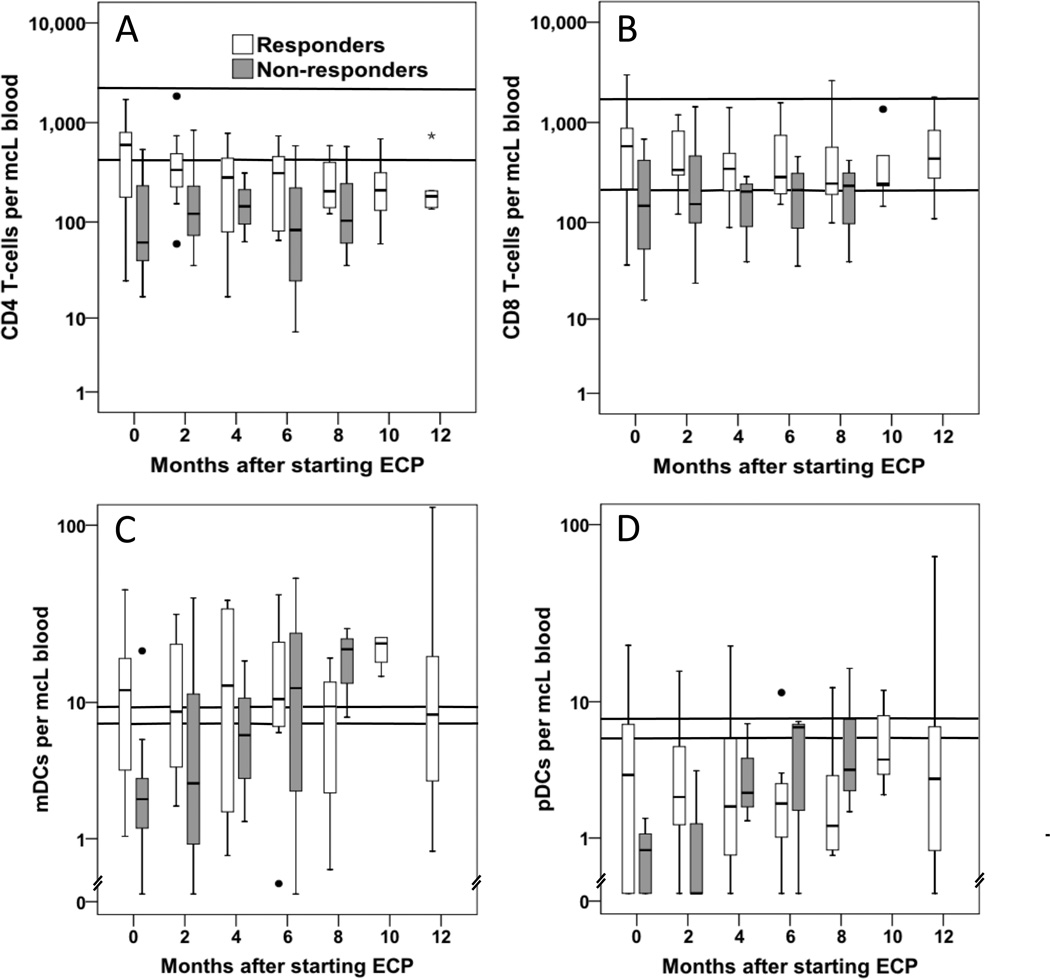
Median numbers of circulating pDC, mDC, and CD4+ and CD8+ T cells were determined by flow cytometry (Fig. 2) for all evaluable patients approximately every 2 months for a 12-month period and compared to the baseline values (Fig. 3). Overall, CD4+ T-cell counts were below the normal range, with higher median counts for responders compared with nonresponders. CD8+ T-cell counts were within or slightly below the normal range, again with slightly higher counts in responders. Counts for mDCs were also close to the normal range, but median pDC levels were well below normal,11 possibly due to sensitivity of this population to corticosteroid treatment.12 The only significant change in cell numbers over time was a decline in CD4+ T-cell counts in the responder group, with significantly reduced numbers at 6 and 8 months compared to baseline (p = 0.05 and p = 0.03, respectively). Contrary to the original hypothesis, there were no significant changes in the numbers of circulating DCs over a 12-month period when comparisons were made for the entire cohort of 25 patients or for the responding or non-responding patient groups. The numbers of patients with DC and T-cell count data declined over time due to deaths of some patients, some missed appointments, and also because some patients stopped ECP treatment, either due to response or due to lack of response, and were no longer monitored for DC and T-cell counts as part of this study.
Figure 2.
Identification of (A) T-cell and (B) DC subsets in peripheral blood samples. Representative flow cytometric analyses at baseline and 3 months after starting ECP. Data are shown for a single patient with a positive response to ECP. In each gating strategy, cells were first selected based on low side scatter (not shown). (A) For T-cell analysis, cells were gated on the CD45+CD3+T-cell population (left), and then CD4+and CD8+T cells (right) were identified as subsets of the CD3+ T-cell gate. (B) For DC analysis, a rare population of Lin–HLA-DR+ cells was identified (left), which was then plotted to identify the CD11c+CD123– mDC and CD11c–CD123+pDC populations. All plots show 10% probability with outliers, except the right panels in B, which show 50% probability to better represent the rare DC populations. The figure was made using FlowJo (Treestar, Ashland, OR) software and shows the same results obtained from BD CellQuest software that was used for all reported data. APC = allophycocyanin; FITC = fluorescein isothiocyanate; PE = phycoerythrin; PerCP = peridininchlorophyll protein complex.
Figure 3.
DC and T-cell measurements in ECP patients (□, responders; and  , nonresponders). Log-scale box plots representing (A) CD4+ T cell, (B) CD8+ T cell, (C) mDC, and (D) pDC measurements in all evaluable patients at baseline (before starting ECP, 0 month) and approximately every 2 months for a 1-year time frame. Median values are shown, with boxes representing the 25th to 75th percentiles and bars showing minimum and maximum values. Outliers are shown as individual points. Normal ranges for the different cell types are shown with horizontal lines. Sample sizes for responder and nonresponder groups at the different time points were (responders/nonresponders): 0 months 14/11, 2 months 13/9, 4 months 8/3, 6 months 10/3, 8 months 8/3, 10 months 5/0, and 12 months 5/0. For responders, the 6- and 8-month CD4+ T-cell counts were significantly reduced compared to baseline (p = 0.05 and p = 0.03, respectively). No other significant changes were observed.
, nonresponders). Log-scale box plots representing (A) CD4+ T cell, (B) CD8+ T cell, (C) mDC, and (D) pDC measurements in all evaluable patients at baseline (before starting ECP, 0 month) and approximately every 2 months for a 1-year time frame. Median values are shown, with boxes representing the 25th to 75th percentiles and bars showing minimum and maximum values. Outliers are shown as individual points. Normal ranges for the different cell types are shown with horizontal lines. Sample sizes for responder and nonresponder groups at the different time points were (responders/nonresponders): 0 months 14/11, 2 months 13/9, 4 months 8/3, 6 months 10/3, 8 months 8/3, 10 months 5/0, and 12 months 5/0. For responders, the 6- and 8-month CD4+ T-cell counts were significantly reduced compared to baseline (p = 0.05 and p = 0.03, respectively). No other significant changes were observed.
DISCUSSION
The first line of treatment for cGVHD is a prolonged course of high-dose corticosteroids, which is associated with numerous toxicities or side effects including increased risk of serious infections, diabetes mellitus, hypothalamic-pituitary-adrenal insufficiency, hypertension, myopathy, and osteoporosis.13 The use of immunosuppressive agents may also increase the risk of relapse due to attenuation of the graft-versus-leukemia effect. Patients with steroid-dependent or steroid-refractory cGVHD have limited treatment options, and ECP appears to be an efficient therapeutic modality to reduce the symptoms of cGVHD.4, 5 It is a simple procedure from a technical point of view; however, its mechanisms of action are not well understood. ECP induces DNA damage and apoptosis in lymphocytes and processing of these apoptotic cells is thought to have tolerogenic and anti-inflammatory effects.6, 14
The role of DCs in the response of cGVHD to ECP is not defined. DCs are key players in the immune system, with roles in the induction of immune responses and in maintenance of immune tolerance. By activating antigen-specific T cells, DCs constitute an essential link between the innate and the adaptive defense systems and are able to initiate and direct alloimmune responses.15 The main objective of the present investigation was to elucidate the in vivo effect of ECP on numbers of circulating DCs and T cells in patients with cGVHD. Our study is the first to correlate baseline numbers of circulating mDCs and CD4+ T cells with response to ECP in patients with cGVHD. Clinical studies have shown that low numbers of donor DCs in the graft or in peripheral blood on Day 30 after allogeneic HPCT predicts increased risk for cGVHD 16, 17 and death.18 In contrast to the earlier studies that showed declines in DC populations after ECP using single-marker phenotyping (that could include granulocytes or macrophages),7 and then used costimulatory molecule expression of cultured blood monocyte-derived DC progeny to quantify additional DC subsets,8 we used noncultured blood samples and multiparameter flow cytometric analysis to more precisely define DC subsets based on expression of HLA-DR, CD123, and CD11c. While we observed a decline in the numbers of CD4 T cells in responding patients, there were no other significant changes in T-cell or DC populations over a 12-month period after initiation of ECP. The current findings support a critical role for donor-derived DCs in antigen presentation and cGVHD and suggest a mechanism of ECP that involves donor-derived DCs in the generation of immunomodulatory processes that reduce effector T-cell responses in patients with cGVHD. Human pDCs can prime naïve CD4+ and CD8+ T cells to differentiate into immunosuppressive Foxp3+ regulatory T cells (Treg) that produce interleukin-1019 and strongly inhibit allospecific proliferation of naïve CD8+ T cells.20 The improved clinical response of patients with larger numbers of blood mDC and pDC precursors may be a consequence of larger numbers of immune regulatory cells that were successfully modulated by the ECP procedure. Limitations of this study include the relatively small sample size and the limited panel of monoclonal antibodies used to prospectively assess patients receiving ECP. In particular, specific markers for Treg (Foxp3) were not used, so conclusions about this important T-cell subset in the pathogenesis and treatment of GVHD cannot be made. Nevertheless, measurements of pDCs, mDCs, and T cells can be easily performed during the initial phase of ECP and may predict the probability of response. A current prospective study being conducted by the Blood and Marrow Transplant Clinical Trials Network in which patients with cGVHD are treated with ECP versus non-ECP therapies should help address the question of whether patients who are predicted to have a poor response to ECP (i.e., low baseline numbers of CD4+ T cells or mDCs) will have better response of their cGVHD to alternative immunosuppressive drug regimens. In conclusion, our results demonstrate that higher numbers of circulating DCs and T cells predict response to ECP in patients with cGVHD.
ACKNOWLEDGMENTS
CRG was supported in part by NIH Grant T32 HL069769. The authors thank Ms Kathryn Lee for management of clinical flow cytometry operations and data analysis.
ABBREVIATIONS
- cGVHD
chronic graft-versus-host disease
- DC(s)
dendritic cell(s)
- ECP
extracorporeal photopheresis
- HPCT
hematopoietic progenitor cell transplantation
- LFT(s)
liver function test(s)
- mDC(s)
myeloid dendritic cell(s)
- pDC(s)
plasmacytoid dendritic cell(s)
- ROC
receiver operating characteristic
Footnotes
NOTE ADDED IN PROOF
Recent work by Kuzmina Z, Greinix HT, et al. Blood 2009;114:744-6, shows that low frequencies of immature CD19+CD21− B cells in cGVHD patients prior to ECP therapy are correlated with response, supporting the hypothesis that monitoring circulating immune cell subsets may be useful for predicting clinical response of cGVHD to ECP therapy.
CONFLICT OF INTEREST
The authors have no conflict of interest.
REFERENCES
- 1.Hossain MS, Roback JD, Pollack BP, Jaye DL, Langston A, Waller EK. Chronic GvHD decreases antiviral immune responses in allogeneic BMT. Blood. 2007;109:4548–4556. doi: 10.1182/blood-2006-04-017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 3.Edelson R, Berger C, Gasparro F, Jegasothy B, Heald P, Wintroub B, Vonderheid E, Knobler R, Wolff K, Plewig G, McKiernan G, Christiansen I, Oster M, Honigsmann H, Wilford H, Kokschka E, Rehle T, Perez M, Stingl G, Laroche L. Treatment of cutaneous T cell lymphoma by extracorporeal photochemotherapy: preliminary results. N Engl J Med. 1987;316:297–303. doi: 10.1056/NEJM198702053160603. [DOI] [PubMed] [Google Scholar]
- 4.Apisarnthanarax N, Donato M, Korbling M, Couriel D, Gajewski J, Giralt S, Khouri I, Hosing C, Champlin R, Duvic M, Anderlini P. Extracorporeal photopheresis therapy in the management of steroid-refractory or steroid-dependent cutaneous chronic graft-versus-host disease after allogeneic stem cell transplantation (feasibility and results) Bone Marrow Transplant. 2003;31:459–465. doi: 10.1038/sj.bmt.1703871. [DOI] [PubMed] [Google Scholar]
- 5.Flowers ME, Apperley JF, Van Besien K, Elmaagacli A, Grigg A, Reddy V, Bacigalupo A, Kolb HJ, Bouzas L, Michallet M, Prince HM, Knobler R, Parenti D, Gallo J, Greinix HT. A multicenter prospective phase II randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008;112:2667–2674. doi: 10.1182/blood-2008-03-141481. [DOI] [PubMed] [Google Scholar]
- 6.Xia CG, Campbell KA, Clare-Salzler MJ. Extracorporeal photopheresis-induced immune tolerance: a focus on modulation of antigen-presenting cells and induction of regulatory T-cells by apoptotic cells. Curr Opin Organ Transplant. 2009;14:338–343. doi: 10.1097/MOT.0b013e32832ce943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alcindor T, Gorgun G, Miller KB, Roberts TF, Sprague K, Schenkein DP, Foss FM. Immunomodulatory effects of extracorporeal photochemotherapy in patients with extensive chronic graft-versus-host disease. Blood. 2001;98:1622–1625. doi: 10.1182/blood.v98.5.1622. [DOI] [PubMed] [Google Scholar]
- 8.Gorgun G, Miller KB, Foss FM. Immunologic mechanisms of extracorporeal photochemotherapy (ECP) in chronic graft-versus-host disease. Blood. 2002;100:941–947. doi: 10.1182/blood-2002-01-0068. [DOI] [PubMed] [Google Scholar]
- 9.Greinix HT, Socié G, Bacigalupo A, Holler E, Edinger MG, Apperley JF, Schwarz T, Ullrich SE, Albert ML, Knobler RM, Peritt D, Ferrara JL. Assessing the potential role of photopheresis in hematopoietic stem cell transplant. Bone Marrow Transplant. 2006;38:265–273. doi: 10.1038/sj.bmt.1705440. [DOI] [PubMed] [Google Scholar]
- 10.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner M, Vogelsang G, Flowers ME. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–955. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Athanassopoulos P, Vaessen LM, Maat AP, Balk AH, Weimar W, Bogers AJ. Peripheral blood dendritic cells in human end-stage heart failure and the early post-transplant period: evidence for systemic Th1 immune responses. Eur J Cardiothorac Surg. 2004;25:619–626. doi: 10.1016/j.ejcts.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 12.Shodell M, Shah K, Siegal FP. Circulating human plasmacytoid dendritic cells are highly sensitive to corticosteroid administration. Lupus. 2003;12:222–230. doi: 10.1191/0961203303lu362xx. [DOI] [PubMed] [Google Scholar]
- 13.Axelrod L. Glucocorticoid therapy. Medicine. 1976;55:39–65. doi: 10.1097/00005792-197601000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Blandon J, Taylor PC. Extracorporeal photopheresis: a focus on apoptosis and cytokines. J Dermatol Sci. 2006;43:85–94. doi: 10.1016/j.jdermsci.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 16.Rajasekar R, Mathews V, Lakshmi KM, Sellathamby S, George B, Viswabandya A. Plasmacytoid dendritic cell count on day 28 in HLA-matched related allogeneic peripheral blood stem cell transplant predicts the incidence of acute and chronic GVHD. Biol Blood Marrow Transplant. 2008;14:344–350. doi: 10.1016/j.bbmt.2007.12.494. [DOI] [PubMed] [Google Scholar]
- 17.Waller EK, Rosenthal H, Jones TW, Peel J, Lonial S, Langston A, Redei I, Jurickova I, Boyer MW. Large number of CD4brightdendritic cells in donor bone marrow are associated with increased relapse after allogeneic bone marrow transplantation. Blood. 2001;97:2948–2956. doi: 10.1182/blood.v97.10.2948. [DOI] [PubMed] [Google Scholar]
- 18.Mohty M, Blaise D, Faucher C, Bardou VJ, Gastaut JA, Viens P, Olive D, Gaugler B. Impact of plasmacytoid dendritic cells on outcome after reduced-intensity conditioning allogeneic stem cell transplantation. Leukemia. 2005;19:1–6. doi: 10.1038/sj.leu.2403558. [DOI] [PubMed] [Google Scholar]
- 19.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 20.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]