Abstract
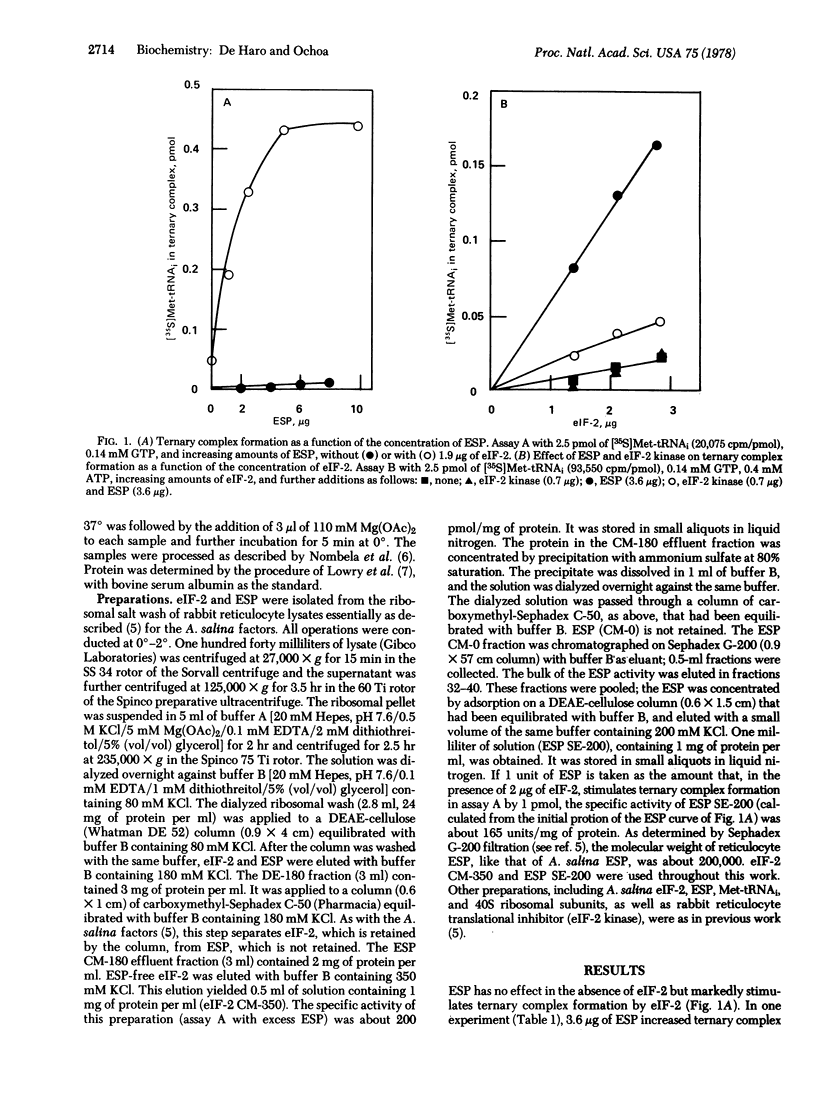
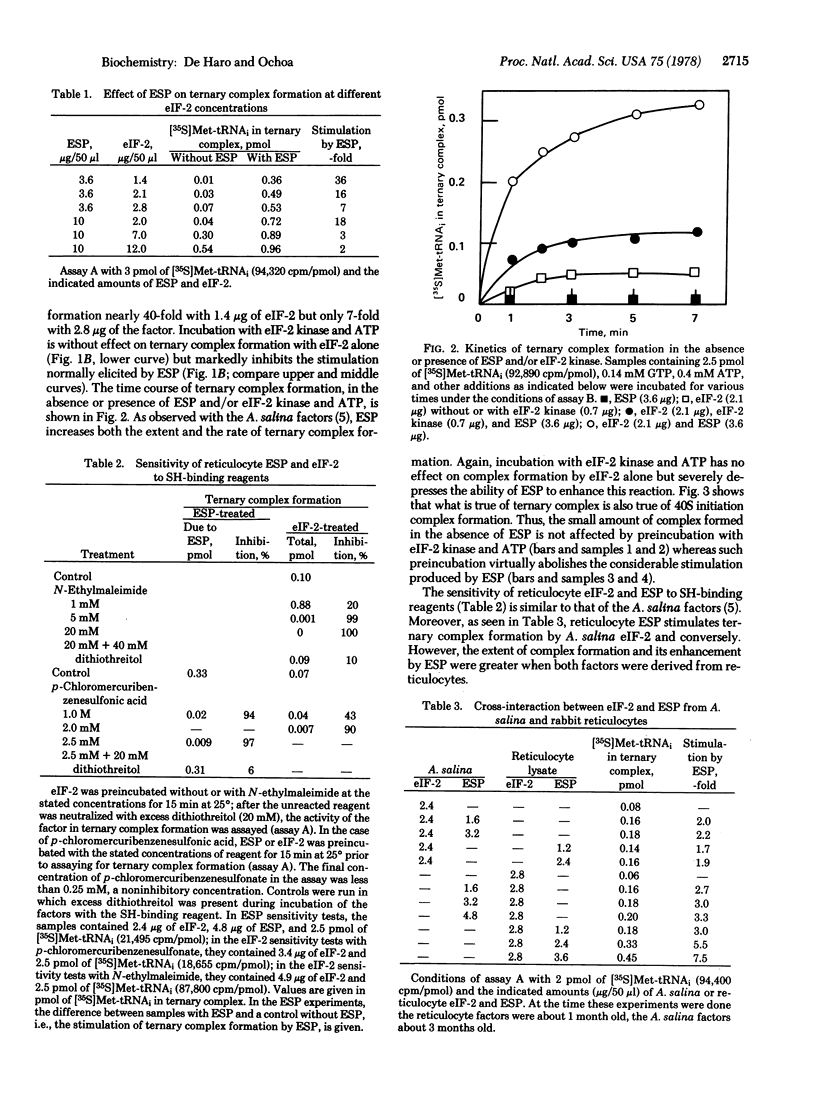
Previously [de Haro, C., Datta, A & Ochoa, S. (1978) Proc. Natl. Acad. Sci. USA 75, 243--247] it was shown with initiation factors from Artemia salina embryos that the activity of the initiator methionyl-tRNA binding factor eIF-2 is stimulated by another factor (ESP, for eIF-2 stimulating protein) present, like eIF-2, in ribosomal salt washes. Incubation of eIF-2 with translational inhibitor from rabit reticulocytes, in the presence of ATP, abolished the ESP effect. At physiological concentrations eIF-2 was virtually inactive without ESP. These observations indicated that the translational inhibitor acts by converting eIF-2 to a form that is not stimulated by ESP. The same observations have now been made with eIF-2 and ESP from rabbit reticulocytes but, in this case, the dependence of eIF-2 activity on ESP is much more pronounced than with the A. salina factors. eIF-2 from reticulocytes interacts with ESP from A. salina and conversely.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cherbas L., London I. M. On the mechanism of delayed inhibition of protein synthesis in heme-defecient rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3506–3510. doi: 10.1073/pnas.73.10.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A., Majumdar A., George A. D., Gupta N. K. Protein synthesis in rabbit reticulocytes. XV. Isolation of a ribosomal protein factor (CO-EIE-1) which stimulates Met-tRNAfMet binding to EIF-1. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1234–1241. doi: 10.1016/0006-291x(76)90786-5. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Gross M., Mendelewski J. Additional evidence that the hemin-controlled translational repressor from rabbit reticulocytes is a protein kinase. Biochem Biophys Res Commun. 1977 Jan 24;74(2):559–569. doi: 10.1016/0006-291x(77)90340-0. [DOI] [PubMed] [Google Scholar]
- Kramer G., Cimadevilla J. M., Hardesty B. Specificity of the protein kinase activity associated with the hemin-controlled repressor of rabbit reticulocyte. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3078–3082. doi: 10.1073/pnas.73.9.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin D., Ranu R. S., Ernst V., London I. M. Regulation of protein synthesis in reticulocyte lysates: phosphorylation of methionyl-tRNAf binding factor by protein kinase activity of translational inhibitor isolated from hemedeficient lysates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3112–3116. doi: 10.1073/pnas.73.9.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nombela C., Nombela N. A., Ochoa S., Safer B., Anderson W. F., Merrick W. C. Polypeptide chain initiation in eukaryotes: mechanism of formation of initiation complex. Proc Natl Acad Sci U S A. 1976 Feb;73(2):298–301. doi: 10.1073/pnas.73.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel H., Staehelin T. Binding and release of eukaryotic initiation factor eIF-2 and GTP during protein synthesis initiation. Proc Natl Acad Sci U S A. 1978 Jan;75(1):204–208. doi: 10.1073/pnas.75.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haro C., Datta A., Ochoa S. Mode of action of the hemin-controlled inhibitor of protein synthesis. Proc Natl Acad Sci U S A. 1978 Jan;75(1):243–247. doi: 10.1073/pnas.75.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]



