Abstract
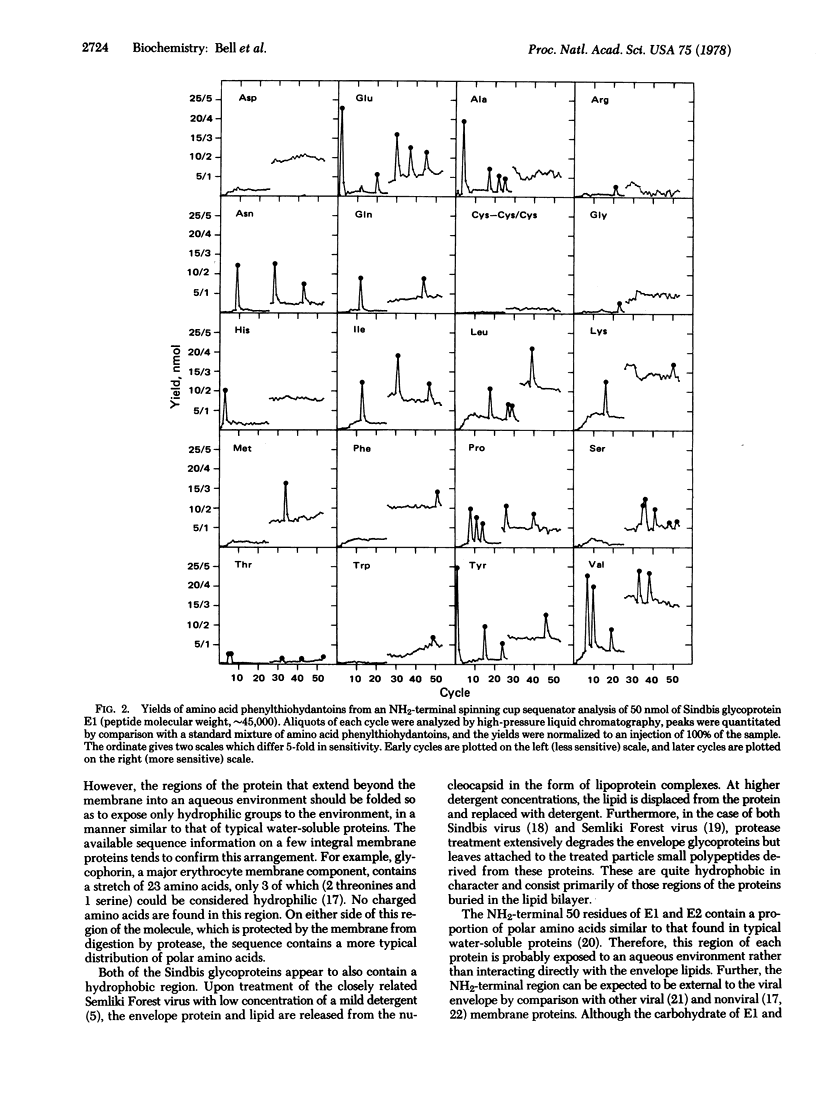
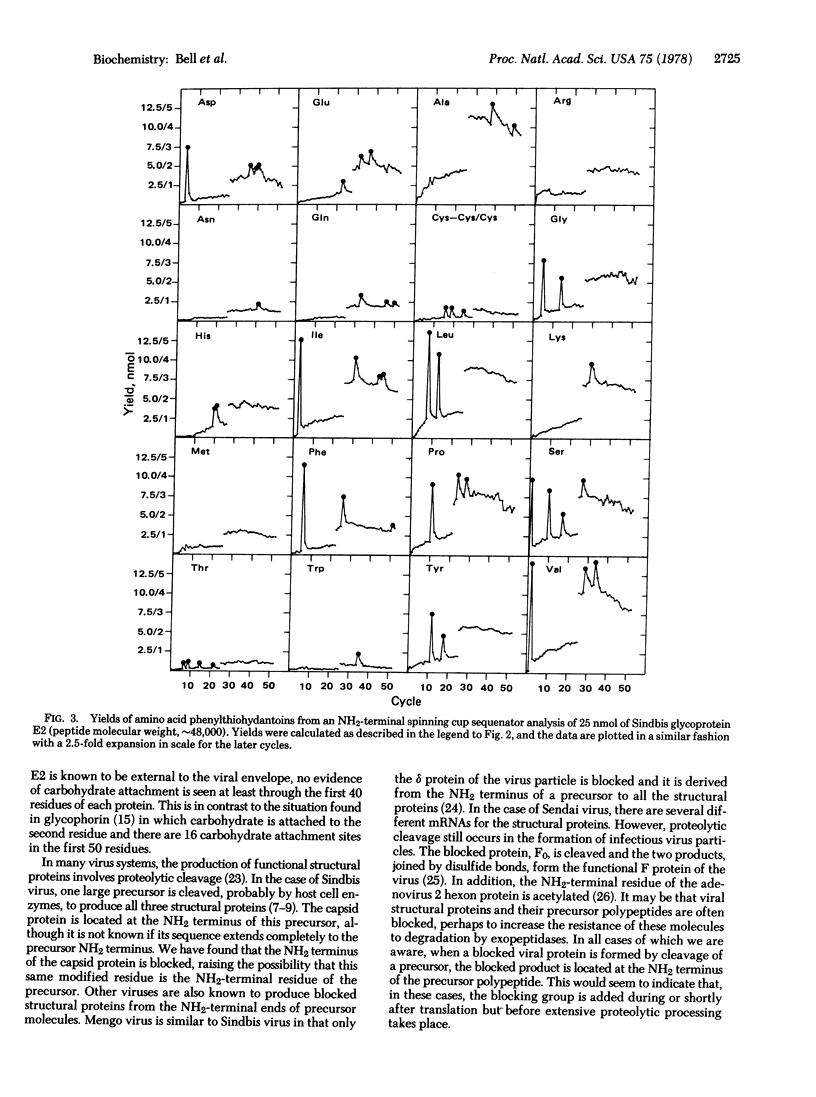
The structural proteins of Sindbis virus, an enveloped virus which belongs to the Togavirus family, have been subjected to automated Edman degradation using improved techniques. Extensive NH2-terminal sequences of about 50 residues were determined for each of the two membrane glycoproteins. In both cases the NH2 terminus of the molecule was found to be similar in composition to typical water-soluble proteins. The viral capsid protein was found to have a blocked alpha-amino group. This is consistent with other observations that viral proteins derived from the NH2 terminus of precursor molecules are often blocked.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birdwell C. R., Strauss J. H. Replication of Sindbis virus. IV. Electron microscope study of the insertion of viral glycoproteins into the surface of infected chick cells. J Virol. 1974 Aug;14(2):366–374. doi: 10.1128/jvi.14.2.366-374.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. C., Kennedy S. I. Initiation of synthesis of the structural proteins of Semliki Forest virus. J Mol Biol. 1975 Oct 5;97(4):401–411. doi: 10.1016/s0022-2836(75)80050-7. [DOI] [PubMed] [Google Scholar]
- Compans R. W. Location of the glycoprotein in the membrane of Sindbis virus. Nat New Biol. 1971 Jan 27;229(4):114–116. doi: 10.1038/newbio229114a0. [DOI] [PubMed] [Google Scholar]
- Garoff H., Simons K. Location of the spike glycoproteins in the Semliki Forest virus membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3988–3992. doi: 10.1073/pnas.71.10.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Söderlund H. Stepwise dissociation of the Semliki Forest Virus membrane with trition X-100. Biochim Biophys Acta. 1973 May 11;307(2):287–300. doi: 10.1016/0005-2736(73)90096-5. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Ohlsson H., Philipson L. An acetylated N-terminus of adenovirus type 2 hexon protein. Biochem Biophys Res Commun. 1974 Jan 23;56(2):304–310. doi: 10.1016/0006-291x(74)90842-0. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I., Burke D. C. Studies on the structural proteins of Semliki Forest virus. J Gen Virol. 1972 Jan;14(1):87–98. doi: 10.1099/0022-1317-14-1-87. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Copeland T., Summers M. R., Smythers G., Gilden R. V. Amino acid sequence homology of mammalian type C RNA virus major internal proteins. J Biol Chem. 1975 Aug 25;250(16):6232–6239. [PubMed] [Google Scholar]
- Ozols J., Gerard C., Nobrega F. G. Proteolytic cleavage of horse liver cytochrome b5. Primary structure of the heme-containing moiety. J Biol Chem. 1976 Nov 10;251(21):6767–6774. [PubMed] [Google Scholar]
- PFEFFERKORN E. R., CLIFFORD R. L. THE ORIGIN OF THE PROTEIN OF SINDBIS VIRUS. Virology. 1964 Jun;23:217–223. doi: 10.1016/0042-6822(64)90285-5. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Two disulfide-linked polypeptide chains constitute the active F protein of paramyxoviruses. Virology. 1977 Jul 1;80(1):54–66. doi: 10.1016/0042-6822(77)90380-4. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S., Burge B. W. Identification of a second glycoprotein in Sindbis virus. Virology. 1972 Feb;47(2):539–541. doi: 10.1016/0042-6822(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S. Large-molecular-weight precursors of sindbis virus proteins. J Virol. 1973 Jun;11(6):1013–1016. doi: 10.1128/jvi.11.6.1013-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. Z., August J. T. Proteolytic cleavage events in oncornavirus protein synthesis. Biochim Biophys Acta. 1976 Dec 23;458(4):375–396. doi: 10.1016/0304-419x(76)90008-1. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Waterfield M. D. Studies on the primary structure of the influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):93–97. doi: 10.1073/pnas.72.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Pfefferkorn E. R., Darnell J. E., Jr Identification of the membrane protein and "core" protein of Sindbis virus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):533–537. doi: 10.1073/pnas.59.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utermann G., Simons K. Studies on the amphipathic nature of the membrane proteins in Semliki Forest virus. J Mol Biol. 1974 Jan 5;85(4):569–587. doi: 10.1016/0022-2836(74)90316-7. [DOI] [PubMed] [Google Scholar]
- Wirth D. F., Katz F., Small B., Lodish H. F. How a single Sindbis virus mRNA directs the synthesis of one soluble protein and two integral membrane glycoproteins. Cell. 1977 Feb;10(2):253–263. doi: 10.1016/0092-8674(77)90219-7. [DOI] [PubMed] [Google Scholar]
- Ziola B. R., Scraba D. G. Structure of the Mengo virion. IV. Amino- and carboxyl-terminal analyses of the major capsid polypeptides. Virology. 1976 May;71(1):111–121. doi: 10.1016/0042-6822(76)90098-2. [DOI] [PubMed] [Google Scholar]


