Abstract
A 66-year-old man, chronic smoker, presented with episodes of syncope, hypotension and constitutional symptoms. Initial evaluation revealed pre-renal azotaemia and acute secondary adrenal insufficiency.MRI performed was interpreted as a pituitary macroadenoma with enlargement of the infundibulum (stalk). Further endocrinological tests performed suggested central hypothyroidism and hypogonadism. Subsequent development of haemoptysis, headache and diplopia warranted further investigations, which revealed stage IV small-cell lung carcinoma with adrenal metastases. Subsequent brain imaging showed lesions in the brain parenchyma, pituitary and stalk, characteristic of metastases. Thus, we present a very atypical case of pituitary metastases presenting with acute secondary adrenal insufficiency.
Background
Metastases to the pituitary gland are rare, often asymptomatic and when present, they indicate an advanced stage of malignancy. It is more commonly seen in patients with a primary tumour arising from the breast or lung, more so in elderly patients. Most of these patients present with symptoms of diabetes insipidus and visual alterations. Pituitary dysfunction can also be the presenting symptom with an occult primary tumour and progression in an indolent course, thus making it difficult to differentiate from a pituitary adenoma in the absence of specific radiological and clinical features. Acute secondary adrenal insufficiency as the initial presentation due to metastatic involvement of the hypothalamic–pituitary axis is extremely rare. Also, diabetes insipidus, which is a very common finding in pituitary metastases and helpful in differentiating it from a pituitary macroadenoma, was absent in this case.
Case presentation
A 66-year-old Caucasian man, chronic smoker, with a history of hypertension and hypercholesterolaemia presented with three episodes of syncope followed by a brief loss of consciousness over the past 10 days. Systolic blood pressure measured after these episodes was 70 mm Hg. He also had symptoms of malaise, low-grade fever, non-productive cough and anorexia. His medications included lisinopril, atenolol and simvastatin. Blood pressure on presentation was 97/40 mm Hg. Systemic examination was normal. He was hydrated with normal saline, and antihypertensive medications were withheld.
Investigations
On admission, blood glucose was 96 mg/dL and haemoglobin was 13.4 g/dL. Complete blood count, coagulation panel and liver function tests were within the normal reference range. The remaining results are shown in table 1.
Table 1.
Laboratory results
| Test | Value | Reference range |
|---|---|---|
| Serum | ||
| Creatinine | 2.6 | 0.5–1.4 mg/dL |
| BUN | 47 | 7–30 mg/dL |
| Sodium | 131 | 135–146 mEq/L |
| Potassium | 5.3 | 3.5–5.3 mEq/L |
| Chloride | 97 | 98–110 mEq/L |
| TCO2 (bicarbonate) | 22 | 21–33 mM/L |
| Osmolality | 269 | 275–295 mOsm/kg |
| Urine | ||
| Sodium, random | 13 | 40–220 mEq/L |
| Creatinine, random | 78.1 | 20–370 mg/dL |
| Protein, random | 8 | 5–25 mg/dL |
| Osmolality | 438 | 200–1100 mOsm/kg |
BUN, blood urea nitrogen.
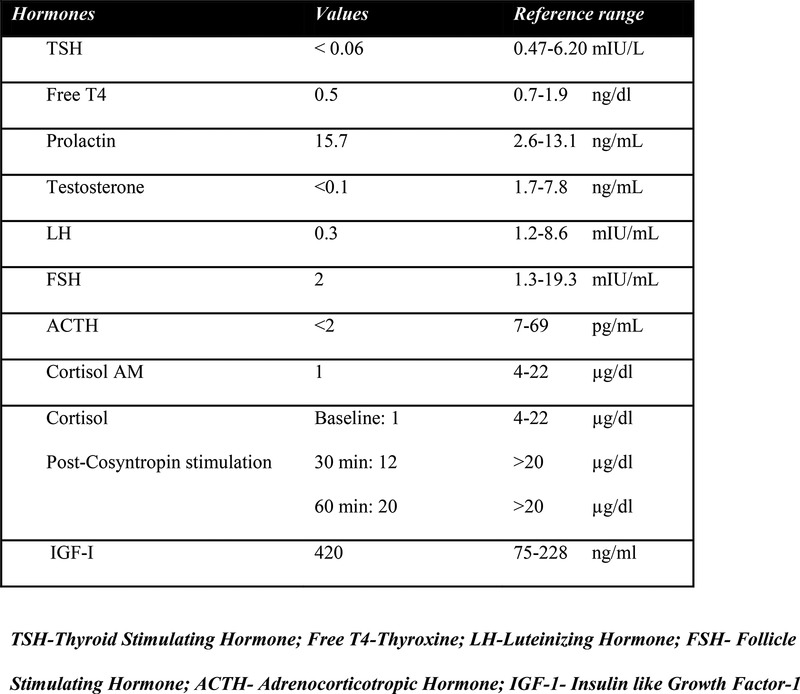
Serum creatinine and blood urea nitrogen were elevated. Pre-renal azotaemia was diagnosed. Hypotension and dehydration were considered to be the cause of pre-renal azotaemia and intravenous hydration was continued. Cardiac cause of hypotension was ruled out. Azotaemia improved but the patient was persistently hyponatraemic (serum sodium in range 123–128 mEq/L), hypotensive and symptomatic from excessive malaise. Thus, morning serum cortisol level was obtained which was significantly low at 1 µg/dL. Cosyntropin stimulation and baseline adrenocorticotropic hormone (ACTH) tests were performed before administration of hydrocortisone and a pituitary panel was obtained (table 2).
Table 2.
Results of hormonal evaluation.
 |
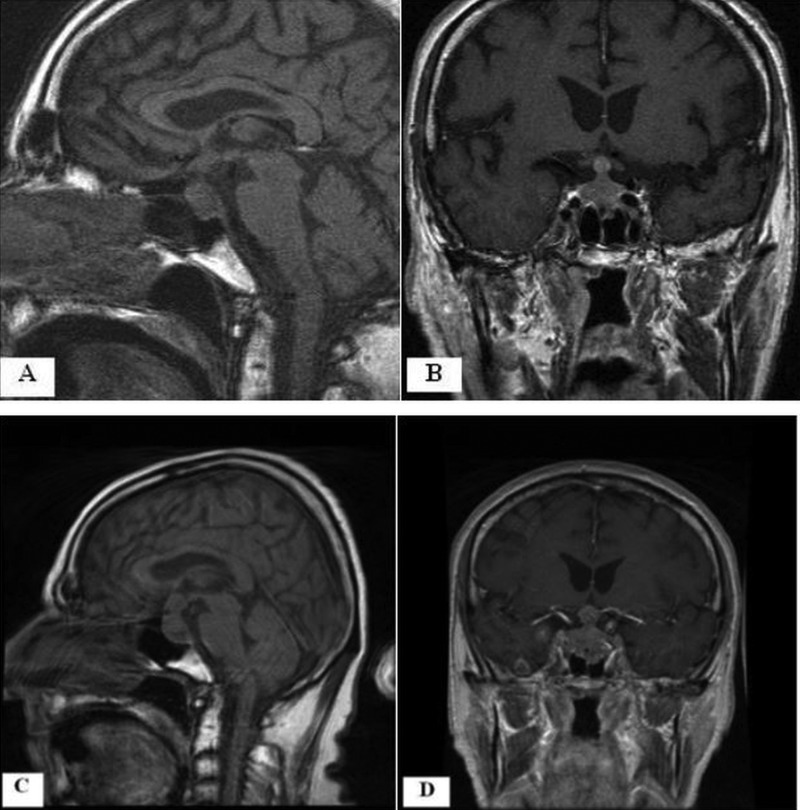
Secondary adrenal insufficiency was diagnosed. Hydrocortisone 20 mg three times a day was started and there was an excellent clinical response with resolution of hypotensive episodes. A chest X-ray showed changes consistent with chronic obstructive pulmonary disease. MRI of the brain with contrast revealed a 22×19×11 mm homogeneous mass involving the pituitary gland and the stalk, nodularity in the superior aspect of the stalk, with mild enhancement and mass effect on the optic chiasm (figure 1A,B).
Figure 1.
(A and B) Sagittal T1-weighted non-contrast MRI and a coronal postcontrast MRI, respectively, showing a 22×19×11 mm contrast-enhancing, homogeneous mass involving the pituitary gland and the stalk. (C and D) Sagittal T1-weighted non-contrast MRI and a coronal postcontrast MRI, respectively, performed 1 month after the initial imaging (A and B) showing the mass increased to the size of 28×24×16 mm.
The prolactin elevation was attributed to stalk compression. The elevated IGF-1 level was not further addressed at that time. The patient was discharged on hydrocortisone, laevothyroxine and testosterone with a diagnosis of non-functioning pituitary macroadenoma with acute secondary adrenal insufficiency, secondary hypothyroidism and secondary hypogonadism.
MRI of the brain repeated after 3 weeks for detailed study of the pituitary region showed an increase in the pituitary and stalk mass to 27×22×14 mm, but it was still considered to be consistent with a pituitary macroadenoma. The patient presented a week later with new symptoms of diplopia, a constant, severe bitemporal headache for 5 days and cough with haemoptysis. Vital signs were normal, but on examination, he had right abducens nerve palsy, global hyper-reflexia and bilateral decreased breath sounds with scattered wheezes in the left lung field. Laboratory studies except for serum creatinine (1.7 mg/dl) were within normal range. A CT scan of the head showed new subcentimetre metastatic nodules in the brain, and chest CT showed a large paraoesophageal, mid-mediastinal mass causing extrinsic compression of the left main stem bronchus most likely from a primary lung carcinoma. MRI of the brain revealed multiple enhancing lesions bilaterally in the cerebellum and in the cerebral hemispheres. The pituitary and stalk mass had grown to 28×24×16 mm (a 134% volume increase) with extension into the suprasellar region and elevation of the chiasm (figure 1C,D). The stalk enlargement increased during the 4-week interval from 8×7 × 7 mm to 11×11×10 mm, a 209% volume increase. These findings favoured the diagnosis of metastatic disease over a primary pituitary macroadenoma. CT scan of the abdomen and pelvis for staging revealed bilateral adrenal masses (47×25 mm right, 27×23 mm left). The bronchoscopic biopsy specimen was positive for small-cell carcinoma of the lung.
Differential diagnosis
Our patient thus presented with hypotension and pre-renal azotaemia. On the basis of low baseline serum ACTH (<2 pg/mL), low baseline serum cortisol (1 µg/dL) with mild hyponatraemia (131 mEq/L) and subnormal response on cosyntropin test, without any history of steroid treatment in the recent past, acute secondary adrenal insufficiency was diagnosed. High normal serum potassium (5.3 mEq/L) on presentation was likely due to pre-renal azotaemia related to dehydration. Endocrinological evaluation did reveal secondary hypothyroidism and hypogonadotropic hypogonadism; however, he was not overtly symptomatic with these. MRI performed during the initial presentation was interpreted as a non-functioning pituitary macroadenoma with enlargement of the stalk and accompanying central hypothyroidism and hypogonadism. Development of haemoptysis, diplopia and headache prompted additional evaluation that revealed stage IV small-cell lung carcinoma with adrenal metastases. Subsequent brain imaging showed progressive lesions in the brain, pituitary and stalk characteristic of metastases. The patient subsequently developed diplopia and right abducens nerve palsy which favoured the differential of pituitary metastases over adenoma. Our patient thus had a very atypical presentation with the absence of diabetes insipidus even though the imaging studies had shown the involvement of stalk and both the pituitary lobes. This may be explained by the fact that the presence of severe secondary adrenal insufficiency could have suppressed the manifestation of diabetes insipidus, although it did not become evident even after treatment with glucocorticoids.
Treatment
Hormone replacement was continued. An external beam radiation therapy to the brain and the mediastinum was started with a planned total dose of 3000 cGy (centi-Gray).
Outcome and follow-up
On the sixth day of admission, the patient developed progressive left-hand weakness, bilateral lateral gaze paresis consistent with bilateral sixth nerve involvement and decreased distal left arm strength. He was discharged with a plan to start carboplatin-based and etoposide-based chemotherapy. However, he died after 3 days. An autopsy was not performed.
Discussion
Many sellar and parasellar masses mimic actual pituitary and stalk metastatic lesions clinically, endocrinologically and radiologically. Thus, it becomes imperative to differentiate pituitary metastases from pituitary adenoma to avoid unnecessary surgical intervention and to plan a therapeutic approach. Clinically, rapid onset and progression of symptoms, the presence of diabetes insipidus and the presence of cranial nerve deficits or ophthalmoplegia should raise the suspicion of pituitary metastasis. Because the pituitary has a high functional reserve, pituitary metastases rarely present with acute symptomatic anterior pituitary insufficiency. With the advent of more sensitive endocrinological tests, it is now possible to diagnose overt anterior pituitary insufficiency earlier, which has led to increased prevalence of anterior pituitary insufficiency in the newer studies.1
Presentation as secondary adrenal insufficiency due to hypothalamic pituitary axis metastases is very rare and, to our knowledge, only five cases have been reported to date.2–6 Two patients had primary tumour as bronchogenic carcinoma, while renal cell carcinoma, papillary thyroid carcinoma and breast carcinoma were found in each of the remaining three patients. Four patients had pituitary metastases as the initial manifestation of the primary tumour. One patient had additional symptoms of diabetes insipidus on presentation while one patient developed it postoperatively. The patient described by Chamarthi et al6 developed hypernatraemia and diabetes insipidus during the period of fluid restriction and glucocorticoid replacement. Two patients, including our patient, had bilateral adrenal masses. Owing to the rich sinusoidal blood supply, the adrenals are the fourth most common site of metastases. However, the metastases are seldom extensive enough to cause adrenal insufficiency. Primary adrenal insufficiency is found in less than 1% of patients having adrenal metastases, as almost 90% of the adrenal cortex must be destroyed before hypofunction becomes evident.7
Learning points.
Pituitary metastases can be the initial manifestation of an occult/inapparent primary tumour.
Incidence of anterior pituitary insufficiency in pituitary metastases has increased.
Diabetes insipidus, though important in differentiating from macroadenoma, can be absent as a presenting feature.
Rare presentation as an acute secondary adrenal insufficiency can occur.
Footnotes
Funding: This study is the result of work supported with resources and the use of facilities at the VA Nebraska-Western Iowa Health Care System.
Competing interests: None.
Patient consent: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Komninos J, Vlassopoulou V, Protopapa D, et al. Tumors metastatic to the pituitary gland: case report and literature review. JCEM 2004;89:574–80 [DOI] [PubMed] [Google Scholar]
- 2.Modhi G, Bauman W, Nicolis G. Adrenal failure associated with hypothalamic and adrenal metastases: a case report and review of the literature. Cancer 1981;47:2098–101 [Google Scholar]
- 3.Sziklas J, Mathews J, Spencer R, et al. Thyroid carcinoma metastatic to pituitary [letter]. J Nucl Med 1985;26:1097. [PubMed] [Google Scholar]
- 4.Beckett D, Gama R, Wright J, et al. Renal carcinoma presenting with adrenocotical insufficiency due to a pituitary metastasis. Ann Clin Biochem 1998;35:542–4 [DOI] [PubMed] [Google Scholar]
- 5.Bouaziz H, Kaffel N, Charfi N, et al. Panhypopituitarism revealing metastasis of small-cell lung carcinoma associated with sarcoidosis. Ann Endocinol (Paris) 2006;67:259–64 [DOI] [PubMed] [Google Scholar]
- 6.Chamarthi B, Morris C, Kaiser U, et al. Stalking the diagnosis. Clinical problem-solving. N Engl J Med 2010;362:834–9 [DOI] [PubMed] [Google Scholar]
- 7.Redman B, Pazdur R, Zingas A. Prospective evaluation of adrenal insufficiency in patients with adrenal metastasis. Cancer 1987;60:103–7 [DOI] [PubMed] [Google Scholar]