Abstract
RB serves as a scaffold to coordinate binding of numerous proteins, including E2F and histone deacetylases, through its C-terminal domain. The amino-terminal half of RB has few known binding partners and its function is not well understood. We used the amino-terminal domain of the Drosophila retinoblastoma tumor suppressor Rbf (RbfN) to identify novel binding partners by immunoprecipitation coupled with mass spectrometry. Our experiment reveals that the RNA-binding protein Squid (Sqd) is a putative interacting partner of RbfN. Western blot confirmed that Sqd interacts with the amino-terminal domain of Rbf. We observed that Sqd colocalizes with RbfN in Drosophila salivary gland cells. We also show that double RNAi knockdown of Rbf and Sqd in the eye results in an extensive loss of eye bristles, suggesting that Rbf and Sqd function in a common pathway. We conclude from our studies that Rbf physically and genetically interacts with Sqd. We propose that the retinoblastoma tumor suppressor may play a novel role in RNA processing through interaction with RNA binding proteins.
Keywords: RB, Rbf, Rbf1, retinoblastoma, tumor suppressor, Sqd, hnRNP, RNA binding, RNA processing
Introduction
The retinoblastoma tumor suppressor (RB) is a conserved protein that plays an important role in development and cell cycle regulation in all multicellular organisms. The major function of RB is best characterized by its repression of the E2F family of transcription factors which serves to arrest cells in G1. RB also has an influence over other cellular processes, including apoptosis and tissue differentiation [1]. RB restricts cell cycle progression by serving as a scaffold to coordinate binding of numerous proteins, including E2F and histone deacetylases, through its C-terminal domain. As such, most studies have focused on elucidating the function of RB through analysis of its C-terminal half [2]. On the other hand, the amino-terminal half of RB has few known binding partners and its function is not well understood [3].
The goal of the present study is to infer new functions for the retinoblastoma protein by using the Drosophila melanogaster retinoblastoma homologue, Rbf, to identify novel binding partners of its amino-terminal domain. We immunoprecipitated the Rbf N-terminal domain (RbfN) expressed in Drosophila S2 cells and identified binding partners by mass spectrometry. We identified the RNA-binding protein Squid as a novel binding partner of Rbf and discuss the possible implications of this protein interaction in development and cancer.
Materials and Methods
S2 cell culture and protein expression
Drosophila Schneider S2 cells were grown under standard conditions in M3 medium (Sigma) supplemented with antibiotics and 12% fetal bovine serum. V5 epitope-tagged RbfN protein was expressed in S2 cells, as described previously [4].
Antibodies, immunoprecipitations, and immunoblots
Immunoprecipitations were performed as described previously [4]. Protein-A beads (Sigma) were used for all reactions. For immunoprecipitations: Anti-Sqd [5] and anti-V5 (AbD Serotec) antibodies were used at 1:50 dilutions. Anti-Rbf and its pre-immune serum were used at 1:20 [6]. Immunoblotting was done by standard techniques as described previously [4]. For immunoblots: Anti-Sqd was used at 1:200. HRP-conjugated anti-mouse was used as a secondary antibody (Jackson Immunoresearch). Chemiluminescence was used to visualize the immunoblots (Pierce). Ethidium bromide was included in the immunoprecipitations to eliminate DNA-mediated interactions. Sqd and Rbf antibodies were kind gifts from Trudi Schupbach and David Arnosti, respectively.
Microscopy
Transgenic UAS>RbfN-RFP virgins were collected and crossed to males containing salivary gland specific Sgs3>GAL4 driver (Bloomington). UAS>RbfN-RFP; Sgs3>GAL4 flies were then crossed to GFP trap lines for sqd stock #YB0291DE or betaTub56D stock #YC0063 [7, 8]. Salivary glands were dissected from wandering third instar larvae in Grace's medium and fixed with 8% formaldehyde in Buffer B [9]. Images were obtained using a Zeiss LSM 510 Meta microscope.
Proteomics
Proteins from V5 antibody immunoprecipitations either from S2 cells expressing RbfN-V5 or from control S2 cells were eluted with SDS-PAGE sample buffer by boiling at 95 degrees for 10 minutes. Eluates were resolved by 12.5% SDS-PAGE (BioRad). The protein gels were stained with comassie and nonspecific bands found in both the experimental and control samples, including the V5 antibody, were cut out and discarded. The proteins remaining in the rest of the gel were subjected to in gel digestion using trypsin and then extracted from the gel. The extracted peptides were then submitted for mass spectrometry analysis, as previously described [10].
All MS/MS samples were analyzed using Sequest (ThermoFinnigan, San Jose, CA; version 27, rev. 12) and X! Tandem (www.thegpm.org; version 2007.01.01.1). X! Tandem was set up to search a subset of the drosoMay05 database also assuming trypsin. Sequest was set up to search the drosophila database (downloaded from NCBI on August 12, 2008 with 87625 entries) assuming the digestion enzyme trypsin. Scaffold (version Scaffold_2_01_01, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they exceeded specific database search engine thresholds. Proteins were considered as strong candidates if they contained at least 2 unique peptide identifications.
Results
Proteomic identification of Sqd as a novel binding partner of Rbf
To gain more insight into the function of the amino-terminal domain of Rbf (RbfN) we wished to indentify novel binding partners by immunoprecipitation coupled with mass spectrometry. We performed an immunoprecipitation using a V5 antibody with Drosophila S2 cell lysates expressing RbfN-V5, as described previously [4]. Lysates from untransfected cells were used for a parallel immunoprecipitation as a control for nonspecific binding to the antibody or beads. After extensive washing, the immunoprecipitated proteins were eluted and then subjected to SDS-PAGE followed by LC/LC-MS/MS (MudPIT). MudPIT spectra were manually validated for those proteins that had more than one unique peptide and that were not also identified in the control immuoprecipitation MudPIT (Table 1).
Table 1.
| Identified Proteins | Accession Number | Molecular Weight (kDa) | Unique Peptides | Coverage (%) |
|---|---|---|---|---|
| RbfN | gi|24638969| | 42a | 10 | 26.38a |
| Hsc70-4 | gi|103190| | 71 | 4 | 6.91 |
| Squid | gi|476947| | 35 | 2 | 7.17 |
RbfN amino acids 1-345.
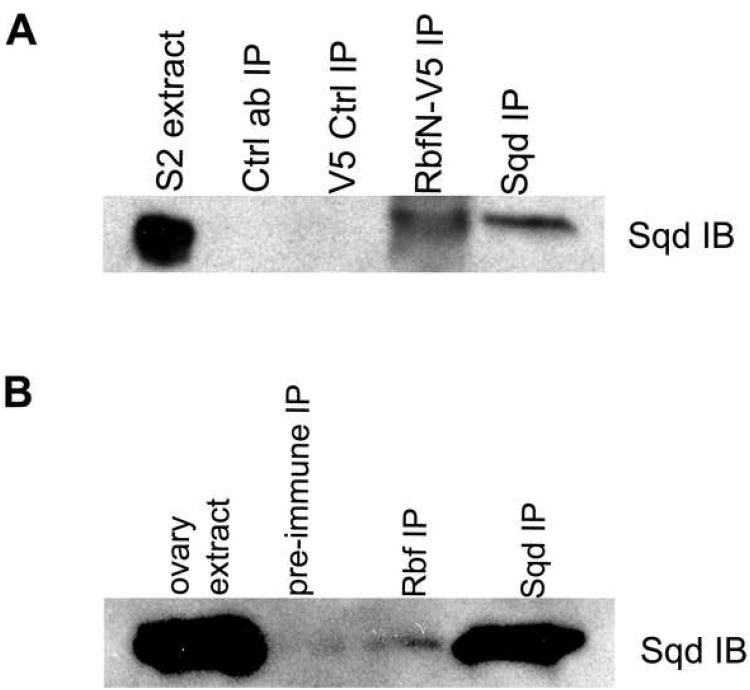
Our MudPIT experiment reveals that the heat shock cognate protein Hsc70-4 is a putative binding partner of RbfN. In addition, the RNA-binding protein Squid (Sqd) was identified as a putative interacting partner of RbfN. We also identified Sqd as a putative partner of RbfN in an independent nano-LC-MS/MS experiment (data not shown). To confirm our MudPIT data we probed a western blot of an RbfN immunoprecipitation with a Sqd monoclonal antibody [5]. The western blot showed a band of approximately 40 kDa in the cell extract that corresponds to Sqd (Figure 1A, lane 1). A control monoclonal antibody fails to pull down Sqd in extracts containing RbfN-V5 (lane 2). Immunoprecipitation with V5 antibody from cells not expressing RbfN also failed to pull down Sqd (lane 3). However, immunoprecipitation with V5 or Sqd monoclonal antibodies from RbfN lysates resulted in an enrichment of Sqd protein (lanes 4 and 5). These results confirm that Sqd interacts with the amino-terminal domain of Rbf in Drosophila S2 cells.
Figure 1. Rbf interacts with Sqd.
(A) RbfN-V5 transfected cell extracts were immunoprecipitated with anti-V5 and subjected to SDS-PAGE followed by western blotting using monoclonal Sqd antibody. Sqd appears as a band of approximately 40 kDa. V5 or Sqd antibodies from RbfN lysates pulled down Sqd protein. Note that extracts from untransfected cells treated identically with monoclonal V5 antibody failed to immunoprecipitate Sqd. A control monoclonal antibody also fails to pull down Sqd from cell extracts containing RbfN-V5, demonstrating that the interaction between Sqd and RbfN is specific. (B) Endogenous Rbf or Sqd was immunoprecipitated from wild-type ovarian extracts. Western immunoblotting with monoclonal Sqd antibody shows that Rbf antibody serum can pull down Sqd protein, whereas a pre-immune control serum did not pull down Sqd.
We next determined whether Sqd can interact with full-length endogenous Rbf. We homogenized ovaries from wild-type female flies and performed an immunoprecipitation using a polyclonal Rbf antibody and its pre-immune serum as a control antibody [6]. We performed a western blot and probed with Sqd antibody, as before. We observe a band of approximately 40 kDa in the ovarian lysate indicating the Sqd protein (Figure 1B, lane 1). The pre-immune serum antibodies were unable to pull down Sqd (lane 2), whereas Rbf and Sqd antibodies show a single protein band corresponding to Sqd (lanes 3 and 4). We conclude that Rbf physically interacts with Sqd in vivo and that this interaction is mediated by the Rbf N-terminal domain.
RbfN colocalizes with Sqd
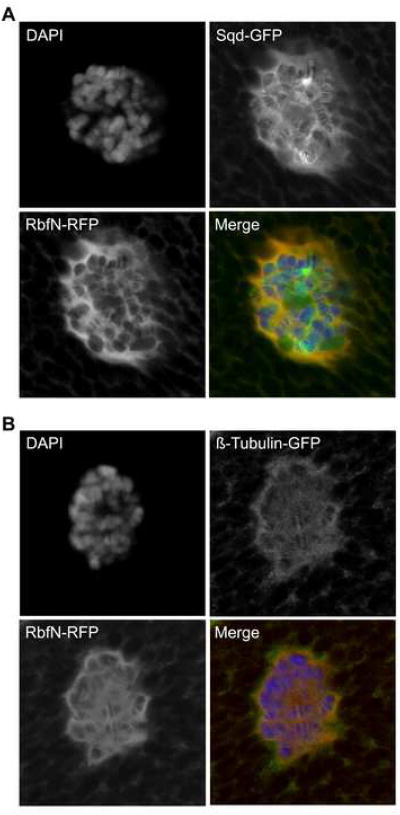
We next wished to determine if RbfN exhibited an overlapping pattern of cellular localization with Sqd. We chose to use larval salivary glands that contain highly secretory cells with large subcellular structures that are good for colocalization studies. We expressed RbfN labeled with red fluorescent protein in salivary glands that also express a GFP-tagged Sqd protein [4, 11]. We dissected salivary glands from third instar larvae, fixed them in formaldehyde, and stained them with DAPI. Confocal imaging of these glands demonstrate nuclear and cytoplasmic localization of RbfN-RFP (Figure 2A), as shown previously [4]. Sqd-GFP shows a cytoplasmic and nuclear localization that overlaps significantly with RbfN-RFP (Figure 2D). However, Sqd-GFP appears in several bright bands on the polytene chromosomes that do not show a correlated brightness in RbfN-RFP signal, which indicates that Sqd may localize to chromatin independently of Rbf. On the other hand, the RNA binding protein Rm62 did not show any cytoplasmic localization and did not colocalize with nuclear RbfN-RFP, which demonstrates the specificity of the RbfN interaction with Sqd (supplementary data). We conclude that Sqd colocalizes extensively with RbfN in the cytoplasm and throughout the nucleus.
Figure 2. RbfN colocalizes with Sqd.
RbfN-RFP localizes to the nucleus and cytyplasm in Drosophila larval salivary gland cells. (A) Sqd-GFP shows a pattern of cytoplasmic localization that overlaps extensively with RbfN-RFP. Nucleoplasmic RbfN and Sqd also significantly colocalize. However, nuclear localized Sqd-GFP shows enrichment in some chromosomal regions that appear to be independent of RbfN-RFP localization. (B) Beta-Tubulin-GFP localizes to discrete cytoplasmic structures in Drosophila larval salivary gland cells that overlaps with RbfN-RFP, indicating the RbfN potentially associates with microtubule networks.
We were intrigued by the distinct fishnet pattern shown by both RbfN and Sqd in the cytoplasm of salivary gland cells, so we investigated this further. It has been shown in the ovary that Sqd localizes gurken mRNA to the cytoplasm around the oocyte nucleus by means of microtubule-mediated transport [12]. We hypothesized that the localization pattern of Sqd and RbfN may correspond to the highly active microtubule networks of salivary glands. We expressed RbfN-RFP in salivary glands of larvae expressing a GFP-tagged Beta-tubulin and prepared the salivary glands as before. Cytoplasmic Beta-Tubulin is organized in salivary gland cells as a network that is fishnet in appearance (Figure 2B). As we predicted, RbfN-RFP colocalizes with cytoplasmic Beta-tubulin. It is interesting to note that Beta-Tubulin also localizes to the nucleus in salivary gland cells, although not as strongly as RbfN. Others have reported nuclear Beta-tubulin localization in mammalian cells, although the role of nuclear tubulin is unclear [13-15]. We also observe a cytoplasmic colocalization of RbfN-RFP with Rab11-GFP, a marker of microtubule-associated transport vesicles, which appears similar to Beta-tubulin and Sqd cytoplasmic localization (supplementary data). We believe this cytoplasmic localization is specific, since we do not see cytoplasmic localization of the RNA binding proteins Rm62-GFP or pUf68-GFP in the presence of RbfN-RFP (supplementary data). Interestingly, RB has also been shown to associate with microtubules in human cells [16, 17]. Taken together, our data suggests that Rbf can associate with cytoplasmic microtubule networks in Drosophila salivary gland cells.
Rbf genetically interacts with Sqd
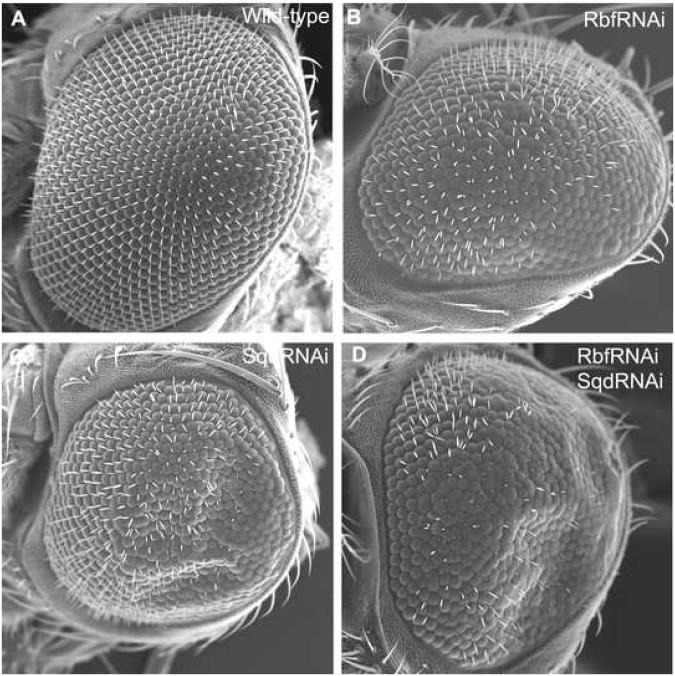
We have shown that Rbf and Sqd physically interact, and we next tested whether these proteins work together in proliferating tissues. Using the GAL4-UAS system, we expressed UAS-RNAi against rbf and/or sqd in the developing eye disc under the influence of GMR-GAL4. Scanning electron microscopy shows a wild-type adult eye in the absence of GMR-GAL4 (Figure 3A). Lowered expression by RNAi of either Rbf (Figure 3B) or Sqd (Figure 3C) results in a modest reduction of eye bristles. However, a simultaneous RNAi knockdown of Rbf and Sqd results in an extensive loss of eye bristles (Figure 3D). In addition, these eye ommatidia have lost underlying structural integrity and have a flattened appearance. These results suggests that Rbf and Sqd function in a common pathway, where reduction of both gene products results in an enhanced mutant phenotype. We conclude from our studies that Rbf physically and genetically interacts with Sqd.
Figure 3. Rbf and Sqd in genetically interact in Drosophila eyes.
We expressed RNAi against rbf and/or sqd in the developing eye disc using the GAL4-UAS system. (A) A wild-type adult eye shows a normal arrangement of ommatidia and eye bristles. (B) RNAi of rbf or (C) RNAi of sqd produces a modest reduction of eye bristles. (D) Double RNAi knockdown of rbf and sqd results in an extensive loss of eye bristles. Also note that these eye ommatidia have a flattened appearance, which may indicate a loss of underlying cells.
Discussion
The retinoblastoma tumor suppressor (RB) is an important regulator of the cell proliferation during development. Despite the volumes of research literature dedicated to RB function since its molecular identification in the 1980s [18], much remains to be learned about the scope of its function. In particular, very little is known about the function of the RB amino-terminal domain [3]. To gain further insight into the function of RB, we used the Drosophila retinoblastoma homologue Rbf to identify novel binding partners of its amino-terminal domain (RbfN) by immunoprecipitation followed by mass spectrometry.
Our mass spectrometry data indicate that Hsc70-4 interacts with RbfN (Table 1). Several lines of evidence support the validity this finding. Hsc70-4 was found in a complex with the chromatin protein Brahma that physically interacts with Rbf [19]. Hsc70-4 deficiency dominantly suppresses a cyclin E hypomorphic phenotype [19]. Since cyclin E is an upstream suppressor of Rbf, one interpretation of this data would suggest that Hsc70-4 may act as an effector of Rbf function. Finally, a protein interaction has been shown between the N-terminal domain of the human retinoblastoma protein RB and hsc73, a homologue of Hsc70-4 [20]. Thus, our data confirms that this interaction is conserved between flies and humans, although the significance of this interaction remains undefined. Additional studies will be necessary to test the functional significance of this interaction.
We also find through our proteomic analysis that the RNA binding protein Squid (Sqd) associates with RbfN (Table 1). Sqd is an RNA binding protein which has homology to human hnRNP A proteins that are involved in many processes relative to RNA metabolism and gene expression, including regulation of transcription, RNA splicing, and transport [21, 22]. We show that Sqd interacts with Rbf in Drosophila S2 cells and ovaries (Figure 1). Sqd also colocalizes with RbfN in Drosophila salivary gland cells (Figure 2). Although this colocalization is extensive, we note that several chromosomal locations are enriched with Sqd that do not correspond to an enrichment of RbfN. These genomic locations may correspond to highly transcribed regions where Sqd is recruited independently of Rbf. We also investigated whether Rbf and Sqd might genetically interact in vivo. We demonstrated that double RNAi knockdown of Sqd and Rbf in the Drosophila eye results in an enhanced phenotype (Figure 3). We therefore infer that Sqd and Rbf function together during development, which may constitute a novel mode of regulation by the retinoblastoma tumor suppressor.
Although the role of Sqd during Drosophila eye development has not been studied, the function of Sqd is best defined by its role in EGFR signaling during oocyte development where it participates in dorsoventral patterning by localizing gurken mRNA [23]. Gurken is a ligand for EGFR that locally acts as a signal from the oocyte to instruct a subpopulation of the dorsal epithelium to adopt a dorsal cell fate. sqd mutants do not properly localize gurken mRNA, resulting in broad gurken signaling that produces a dorsalized egg [24]. On the other hand, mutations that reduce gurken-EGFR signaling result in reduced dorsal cell fates that give a ventralized phenotype [25].
The RB/E2F pathway may have a role in EGFR signaling during Drosophila oogenesis. Loss of the E2F binding partner DP during oogenesis results in a ventralized egg, which appears to be due to the reduced ability to transport gurken mRNA into the oocyte from the nurse cells [26]. Another study found that reduction of E2F or cyclin E enhances a dorsalization phenotype caused by overexpression of Imp, an RNA binding protein that functions in gurken mRNA localization [27]. In light of this data, our finding that the Rbf protein associates with Sqd may indicate a direct role for the retinoblastoma protein in regulation EGFR signaling. Further studies will reveal to what degree Rbf influences dorsoventral patterning during oogenesis.
The nematode C. elegans also has a system of EGFR signaling, where EGF signals locally from the adjacent gonad to induce only one of six vulval precursor cells to adopt a vulval cell fate [28]. Analogous to what is observed during Drosophila oogenesis, broad misexpression of EGF from neighboring cells induces all of the vulval precursor cells to adopt a vulval fate, resulting in a worm with multiple vulvae. RB normally suppresses EGF signaling in this context, since mutations in the C. elegans orthologues of RB/E2F/DP result in a multivulval phenotype [29]. In Drosophila, rbf mutation sensitizes cells to E2F-induced apoptosis that can be modulated by EGFR activity [30]. Studies in mammalian cell culture also demonstrate functional interplay between the EGFR/Ras and Rb/E2F signaling pathways [31-33]. Thus, it appears that the interaction of these two pathways may be conserved among multicellular organisms, and we suggest that the physical interaction of Rbf with Sqd may constitute one method by which these pathways converge. Our findings also raise the interesting possibility that the retinoblastoma tumor suppressor proteins may have a role in RNA processing, since Sqd is an RNA binding protein that functions in other pathways of RNA processing, such as alternative splicing of transcripts [34]. A role in EGFR signaling and influence on RNA processing through interaction with RNA binding proteins may be novel mechanisms for tumor suppression by RB.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grant GM069462. Mass spectrometry was performed by the Arizona Proteomics Consortium supported by NIEHS grant ES06694 to the SWEHSC and NIH-NCI grant CA02304 to the AZCC by the Bio5 Institute of the University of Arizona. We are grateful to Maureen Peterson for a critical reading of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van den Heuvel S, Dyson NJ. Conserved functions of the pRB and E2F families. Nat Rev Mol Cell Biol. 2008;9:713–724. doi: 10.1038/nrm2469. [DOI] [PubMed] [Google Scholar]
- 2.Morris EJ, Dyson NJ. Retinoblastoma protein partners. Adv Cancer Res. 2001;82:1–54. doi: 10.1016/s0065-230x(01)82001-7. [DOI] [PubMed] [Google Scholar]
- 3.Goodrich DW. How the other half lives, the amino-terminal domain of the retinoblastoma tumor suppressor protein. J Cell Physiol. 2003;197:169–180. doi: 10.1002/jcp.10358. [DOI] [PubMed] [Google Scholar]
- 4.Ahlander J, Chen XB, Bosco G. The N-terminal domain of the drosophila retinoblastoma protein Rbf1 interacts with ORC and associates with chromatin in an E2F independent manner. PLoS ONE. 2008;3:e2831. doi: 10.1371/journal.pone.0002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodrich JS, Clouse KN, Schupbach T. Hrb27C, sqd and otu cooperatively regulate gurken RNA localization and mediate nurse cell chromosome dispersion in drosophila oogenesis. Development. 2004;131:1949–1958. doi: 10.1242/dev.01078. [DOI] [PubMed] [Google Scholar]
- 6.Keller SA, Ullah Z, Buckley MS, Henry RW, Arnosti DN. Distinct developmental expression of drosophila retinoblastoma factors. Gene Expr Patterns. 2005;5:411–421. doi: 10.1016/j.modgep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Kelso RJ, Buszczak M, Quinones AT, Castiblanco C, Mazzalupo S, Cooley L. Flytrap, a database documenting a GFP protein-trap insertion screen in drosophila melanogaster. Nucleic Acids Res. 2004;32:D418–20. doi: 10.1093/nar/gkh014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinones-Coello AT, Petrella LN, Ayers K, Melillo A, Mazzalupo S, Hudson AM, Wang S, Castiblanco C, Buszczak M, Hoskins RA, Cooley L. Exploring strategies for protein trapping in drosophila. Genetics. 2007;175:1089–1104. doi: 10.1534/genetics.106.065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Royzman I, Austin RJ, Bosco G, Bell SP, Orr-Weaver TL. ORC localization in drosophila follicle cells and the effects of mutations in dE2F and dDP. Genes Dev. 1999;13:827–840. doi: 10.1101/gad.13.7.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breci L, Hattrup E, Keeler M, Letarte J, Johnson R, Haynes PA. Comprehensive proteomics in yeast using chromatographic fractionation, gas phase fractionation, protein gel electrophoresis, and isoelectric focusing. Proteomics. 2005;5:2018–2028. doi: 10.1002/pmic.200401103. [DOI] [PubMed] [Google Scholar]
- 11.Buszczak M, Paterno S, Lighthouse D, Bachman J, Planck J, Owen S, Skora AD, Nystul TG, Ohlstein B, Allen A, Wilhelm JE, Murphy TD, Levis RW, Matunis E, Srivali N, Hoskins RA, Spradling AC. The carnegie protein trap library: A versatile tool for drosophila developmental studies. Genetics. 2007;175:1505–1531. doi: 10.1534/genetics.106.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delanoue R, Herpers B, Soetaert J, Davis I, Rabouille C. Drosophila Squid/hnRNP helps dynein switch from a gurken mRNA transport motor to an ultrastructural static anchor in sponge bodies. Dev Cell. 2007;13:523–538. doi: 10.1016/j.devcel.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Menko AS, Tan KB. Nuclear tubulin of tissue culture cells. Biochim Biophys Acta. 1980;629:359–370. doi: 10.1016/0304-4165(80)90108-7. [DOI] [PubMed] [Google Scholar]
- 14.Xu K, Luduena RF. Characterization of nuclear betaII-tubulin in tumor cells: A possible novel target for taxol. Cell Motil Cytoskeleton. 2002;53:39–52. doi: 10.1002/cm.10060. [DOI] [PubMed] [Google Scholar]
- 15.Yeh IT, Luduena RF. The betaII isotype of tubulin is present in the cell nuclei of a variety of cancers. Cell Motil Cytoskeleton. 2004;57:96–106. doi: 10.1002/cm.10157. [DOI] [PubMed] [Google Scholar]
- 16.Thomas RC, Edwards MJ, Marks R. Translocation of the retinoblastoma gene product during mitosis. Exp Cell Res. 1996;223:227–232. doi: 10.1006/excr.1996.0076. [DOI] [PubMed] [Google Scholar]
- 17.Roth DM, Moseley GW, Glover D, Pouton CW, Jans DA. A microtubule-facilitated nuclear import pathway for cancer regulatory proteins. Traffic. 2007;8:673–686. doi: 10.1111/j.1600-0854.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee WH, Bookstein R, Hong F, Young LJ, Shew JY, Lee EY. Human retinoblastoma susceptibility gene: Cloning, identification, and sequence. Science. 1987;235:1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- 19.Brumby A, Secombe J, Horsfield J, Coombe M, Amin N, Coates D, Saint R, Richardson H. A genetic screen for dominant modifiers of a cyclin E hypomorphic mutation identifies novel regulators of S-phase entry in drosophila. Genetics. 2004;168:227–251. doi: 10.1534/genetics.104.026617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue A, Torigoe T, Sogahata K, Kamiguchi K, Takahashi S, Sawada Y, Saijo M, Taya Y, Ishii S, Sato N, Kikuchi K. 70-kDa heat shock cognate protein interacts directly with the N-terminal region of the retinoblastoma gene product pRb. identification of a novel region of pRb-mediating protein interaction. J Biol Chem. 1995;270:22571–22576. doi: 10.1074/jbc.270.38.22571. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter B, MacKay C, Alnabulsi A, MacKay M, Telfer C, Melvin WT, Murray GI. The roles of heterogeneous nuclear ribonucleoproteins in tumour development and progression. Biochim Biophys Acta. 2006;1765:85–100. doi: 10.1016/j.bbcan.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Borah S, Wong AC, Steitz JA. Drosophila hnRNP A1 homologs Hrp36/Hrp38 enhance U2-type versus U12-type splicing to regulate alternative splicing of the prospero twintron. Proc Natl Acad Sci U S A. 2009;106:2577–2582. doi: 10.1073/pnas.0812826106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilson LA, Schupbach T. EGF receptor signaling in drosophila oogenesis. Curr Top Dev Biol. 1999;44:203–243. doi: 10.1016/s0070-2153(08)60471-8. [DOI] [PubMed] [Google Scholar]
- 24.Kelley RL. Initial organization of the drosophila dorsoventral axis depends on an RNA-binding protein encoded by the squid gene. Genes Dev. 1993;7:948–960. doi: 10.1101/gad.7.6.948. [DOI] [PubMed] [Google Scholar]
- 25.Neuman-Silberberg FS, Schupbach T. The drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell. 1993;75:165–174. [PubMed] [Google Scholar]
- 26.Myster DL, Bonnette PC, Duronio RJ. A role for the DP subunit of the E2F transcription factor in axis determination during drosophila oogenesis. Development. 2000;127:3249–3261. doi: 10.1242/dev.127.15.3249. [DOI] [PubMed] [Google Scholar]
- 27.Geng C, Macdonald PM. Identification of genes that influence gurken expression. Fly (Austin) 2007;1:259–267. doi: 10.4161/fly.5246. [DOI] [PubMed] [Google Scholar]
- 28.Fay DS, Yochem J. The SynMuv genes of caenorhabditis elegans in vulval development and beyond. Dev Biol. 2007;306:1–9. doi: 10.1016/j.ydbio.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ceol CJ, Horvitz HR. Dpl-1 DP and efl-1 E2F act with lin-35 rb to antagonize ras signaling in C. elegans vulval development. Mol Cell. 2001;7:461–473. doi: 10.1016/s1097-2765(01)00194-0. [DOI] [PubMed] [Google Scholar]
- 30.Moon NS, Di Stefano L, Dyson N. A gradient of epidermal growth factor receptor signaling determines the sensitivity of rbf1 mutant cells to E2F-dependent apoptosis. Mol Cell Biol. 2006;26:7601–7615. doi: 10.1128/MCB.00836-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hallstrom TC, Nevins JR. Specificity in the activation and control of transcription factor E2F-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:10848–10853. doi: 10.1073/pnas.1831408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young AP, Longmore GD. Ras protects rb family null fibroblasts from cell death: A role for AP-1. J Biol Chem. 2004;279:10931–10938. doi: 10.1074/jbc.M311814200. [DOI] [PubMed] [Google Scholar]
- 33.Hanada N, Lo HW, Day CP, Pan Y, Nakajima Y, Hung MC. Co-regulation of B-myb expression by E2F1 and EGF receptor. Mol Carcinog. 2006;45:10–17. doi: 10.1002/mc.20147. [DOI] [PubMed] [Google Scholar]
- 34.Park JW, Parisky K, Celotto AM, Reenan RA, Graveley BR. Identification of alternative splicing regulators by RNA interference in drosophila. Proc Natl Acad Sci U S A. 2004;101:15974–15979. doi: 10.1073/pnas.0407004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.