Abstract
The chemical composition and antibacterial activity of Thymus glabrescens Willd. (Lamiaceae) essential oil were examined, as well as the association between it and chloramphenicol. The antibacterial activities of geraniol and thymol, the main constituents of T. glabrescens oil, individually and in combination with chloramphenicol, were also determined. The interactions of the essential oil, geraniol, and thymol with chloramphenicol toward five selected strains were evaluated using the microdilution checkerboard assay in combination with chemometric methods. Oxygenated monoterpenes were the most abundant compound class in the oil, with geraniol (22.33%) as the major compound. The essential oil exhibited in vitro antibacterial activity against all tested bacterial strains, but the activities were lower than those of the standard antibiotic and thymol. A combination of T. glabrescens oil and chloramphenicol produced a strong synergistic interaction (FIC indices in the range 0.21–0.87) and a substantial reduction of the MIC value of chloramphenicol, thus minimizing its adverse side effects. The combinations geraniol-chloramphenicol and thymol-chloramphenicol produced synergistic interaction to a greater extent, compared with essential oil-chloramphenicol association, which may indicate that the activity of the thyme oil could be attributed to the presence of significant concentrations of geraniol and thymol.
1. Introduction
Antimicrobial resistance (AMR) represents a rapidly growing public health concern worldwide. AMR has been observed following the introduction of every antimicrobial agent into clinical practice. For example, resistance of the bacterium Staphylococcus aureus to penicillin was encountered in hospitals in the mid-1940s, only a few years after the introduction of penicillin [1]. A multifaceted approach is needed to combat AMR, including the discovery of novel antimicrobial drugs and/or new methodological concepts.
Many studies have shown significant antibacterial activity of essential oils against a wide range of resistant microbial strains [2]. The antibacterial activity of essential oils could reflect all the molecules present or only those present in high amounts. For the same reasons, no particular bacterial resistance or adaptation to essential oils has been described and secondary effects have not been confirmed. To enhance the efficacy of antimicrobial drugs and avoid their potentially toxic side effects, their combination with an essential oil may be an innovative alternative and promising strategy [3].
The genus Thymus contains about 350 species, most commonly used in traditional medicine as antibacterial and antifungal remedies [4]. The Serbian flora recognizes 30 species of the Thymus genus, with more than 60 varieties [5].
Given the importance of Thymus species as useful antibacterial remedies, the aim of the present study was to examine the chemical composition and antibacterial effect of the essential oil of Thymus glabrescens (thyme), as well as the association between it and chloramphenicol. The antibacterial activities of geraniol and thymol, the main active principles of thyme oil, in combination with chloramphenicol were also determined.
2. Materials and Methods
2.1. Plant Material and Chemicals
The aerial parts of Thymus glabrescens Willd. (Lamiaceae) were collected in June 2011 from natural populations at the Kravlje village, southeast Serbia. A voucher specimen, with the accession number 16642, is deposited at the Herbarium of the Department of Botany, Faculty of Biology, University of Belgrade Herbarium Code BEOU. All chemicals, reagents, and standards were of analytical reagent grade and were purchased from the Sigma-Aldrich Chemical Company.
2.2. Oil Isolation
The aerial parts of the plant (dried and ground) were subjected to hydrodistillation for 4 h, using a Clevenger-type apparatus to obtain the oil. The resulting essential oil was dried over anhydrous sodium sulphate and stored at 4°C.
2.3. Chemical Analysis
Quantitative and qualitative data of the essential oil were obtained by gas chromatography (GC) and gas chromatography/mass spectrometry (GC-MS) analyses.
2.4. Gas Chromatography
The GC analysis of the oil was performed on a GC HP-5890 II apparatus, equipped with the split-splitless injector, an HP-5MS capillary column (30 m × 0.25 mm, 0.25 μm film thickness) using helium as the carrier gas (1 mL/min), and an FID. Operating conditions were as follows: injector temperature 250°C, interface temperature of 280°C, temperature program from 50°C (3 min) to 250°C at a rate of 3°C/min.
2.5. Gas Chromatography/Mass Spectrometry
GC-MS analyses were performed on an Agilent Technologies apparatus, Model GS 6890N at 70 eV coupled with a mass selective detector MSD 5975C, under the same gas-chromatographic conditions.
2.6. Identification of Compounds
Identification of the compounds was based on comparison of arithmetic retention indices (applying calibrated automated mass spectral deconvolution and identification system software AMDIS ver. 2.64) in combination with the selective ion analysis (SIA) resolution method by Tan et al. [6], comparison with the spectral data from the available literature [7], and comparison of their mass spectra to those from Wiley 275 and NIST/NBS libraries using various search engines. The retention indices were obtained by coinjection with a standard aliphatic hydrocarbons C7–C40 mixture.
2.7. Antibacterial Testing
The activity of the essential oil samples was tested towards 13 different bacteria. Gram-negative bacteria were represented by Escherichia coli ATCC 25922, Salmonella enteritidis ATCC 13076, Klebsiella pneumoniae ATCC 10031, Klebsiella pneumoniae ATCC 700603, Proteus mirabilis ATCC 12453, Pseudomonas aeruginosa ATCC 9027, Pseudomonas aeruginosa ATCC 27853, and Enterobacter aerogenes ATCC 13048, while the researched Gram-positive strains were Enterococcus faecalis ATCC 19433, Bacillus cereus ATCC 11778, Staphylococcus aureus ATCC 25923, Staphylococcus aureus ATCC 29213, and Listeria monocytogenes ATCC 15313.
The inocula of the bacterial strains were prepared from overnight broth cultures and the suspensions were adjusted to 0.5 McFarland standard turbidity (corresponding to 108 CFU/mL, depending on genera-consensus standard by the Clinical and Laboratory Standards Institute) [8].
2.8. Microwell Dilution Assay
A broth microdilution method was used to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) according to the Clinical and Laboratory Standards Institute [8]. Serial double dilutions of the tested oil, as well as the geraniol and thymol, were prepared in 70.0% ethanol and then transferred into a 96-well microtiter plate over the concentration range of 0.025–50.0 μL/mL in inoculated nutrient broth. The final volume was 100 μL and the final bacterial concentration was 107 CFU/mL in each well. The plate was incubated for 24 h at 37°C. All experiments were performed in triplicate. Two controls were included, a medium with solvent/ethanol (negative control) and a medium with antibiotic chloramphenicol (positive control). Bacterial growth was determined by adding 20 μL of an aqueous 0.5% triphenyl tetrazolium chloride (TTC) solution. The minimal inhibitory concentration was defined as the lowest concentration of the oil inhibiting visible growth (red collared pellet on the bottom of the wells after the addition of TTC), while the minimal bactericidal concentration was defined as the lowest oil concentration killing 99.9% of the bacterial cells. To determine the MBC, the broth was taken from each well without visible growth and inoculated in Mueller Hinton agar (MHA) for 24 h at 37°C.
2.9. Microdilution Checkerboard Assay
The microdilution checkerboard method is the technique used most frequently to assess antimicrobial combinations in vitro [9, 10]. Dilutions of T. glabrescens oil, geraniol, thymol, and the examined antibiotic were made for evaluation of their combined interactions. The type of interaction was studied on the E. coli ATCC 25922, K. pneumonia ATCC 700603, P. mirabilis ATCC 12453, P. aeruginosa ATCC 27853, and S. aureus ATCC 29213. These strains were selected based on their importance in frequently occurring infections. Dilutions from the logarithmic-growth phase of the bacterial culture were prepared and distributed into microtiter trays containing combinations of varying concentrations: chloramphenicol-T. glabrescens oil, chloramphenicol-geraniol, and chloramphenicol-thymol. The CLSI [8] guidelines were used to ensure that accurate microbiological assay and transfer techniques were followed. The inoculated trays were incubated at 37°C for 24 h and then evaluated for bacterial growth. Determinations of essential oil-antibiotic interactions were based on the median-effect principle and multiple drug effect equation as described by Chou and Talalay [11]. Three effects can be highlighted: synergetic, additive, or antagonist as a result of the combined effects of the T. glabrescens oil, geraniol, thymol, and chloramphenicol. For quantitative purposes the concept of fractional inhibitory concentrations (FIC) is frequently used. In order to assess the activities of combinations of two drugs that are mutually nonexclusive (have different modes of action), the FIC indices were calculated as
| (1) |
where MICA are the minimum concentrations of the essential oil, geraniol, and thymol, while MICB are the minimum concentrations of the examined antibiotic that inhibited the bacterial growth, respectively. The FIC indices were calculated using CalcuSyn (Biosoft), and the results were interpreted as follows: synergistic (<0.90), additive (0.90 ≤ FIC ≤ 1.10), or antagonistic (>1.10) [12].
2.10. Statistical Analysis of Data
The experimental data (FIC values) were analyzed by chemometric methods: principal components analysis (PCA) and hierarchical cluster analysis (HCA), using Mathworks MATLAB.
3. Results
To eliminate any kind of subjective analysis, interpretations and discussions of the results, presented by tables and/or graphics, and the chemometric methods: principal component analysis, and hierarchical cluster analysis were employed. Furthermore, the use of chemometric methods allows the maximum number of experimental results to be obtained and moreover enables the detection of connections, similarities, and differences among variables in the researched experimental system [4].
3.1. Chemical Composition of the Essential Oil
The yield of T. glabrescens essential oil was 0.59% (w/w). Based on GC and GC-MS analysis of the thyme essential oil, 56 components were identified that represented 97.76% of the total detected constituents (Table 1). The components of T. glabrescens essential oil were separated into six classes, that is, monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, phenolic compounds, and others. The oxygenated monoterpenes were the most abundant compound class in the oil (57.14%), and they were dominated by geraniol (22.33%), geranyl acetate (19.38%), and linalool (5.49%). The group of phenolic compounds (14%) was mainly dominated by thymol (13.79%).
Table 1.
Composition of the essential oil of T. glabrescens.
| Component | RTa (min) | AILb | AIEc | T. glabrescens (%) |
|---|---|---|---|---|
| Monoterpene hydrocarbons | 11.07 | |||
| α-Thujene | 8.161 | 924.0 | 925.0 | 0.33 |
| α-Pinene | 8.400 | 932.0 | 932.1 | 0.29 |
| Camphene | 8.951 | 946.0 | 948.4 | 0.15 |
| Sabinene | 9.734 | 969.0 | 971.6 | 0.09 |
| β-Pinene | 9.897 | 974.0 | 976.5 | 0.10 |
| Myrcene | 10.320 | 988.0 | 989.0 | 0.59 |
| α-Phellandrene | 10.909 | 1002.0 | 1006.1 | 0.10 |
| 3-Carene | 10.984 | 1008.0 | 1007.9 | 0.03 |
| α-Terpinene | 11.294 | 1014.0 | 1016.6 | 0.60 |
| o-Cymene | 11.620 | 1022.0 | 1025.6 | 4.73 |
| Limonene | 11.751 | 1024.0 | 1029.2 | 0.81 |
| β-cis-Ocimene | 11.980 | 1032.0 | 1035.6 | 0.15 |
| β-trans-Ocimene | 12.362 | 1044.0 | 1046.1 | 0.18 |
| γ-Terpinene | 12.822 | 1054.0 | 1058.7 | 2.75 |
| Terpinolene | 13.767 | 1086.0 | 1084.8 | 0.17 |
| Oxygenated monoterpenes | 57.14 | |||
| Eucalyptol | 11.861 | 1026.0 | 1032.3 | 0.56 |
| trans-Linalool oxide | 13.828 | 1084.0 | 1086.5 | 0.03 |
| Linalool | 14.437 | 1095.0 | 1103.2 | 5.49 |
| α-Thujone | 14.603 | 1101.0 | 1107.8 | 0.38 |
| cis-p-Mentha-2,8-dienol | 15.697 | 1133.0 | 1138.4 | 0.01 |
| Borneol | 16.956 | 1165.0 | 1173.0 | 0.47 |
| 4-Terpineol | 17.249 | 1174.0 | 1181.1 | 0.47 |
| α-Terpineol | 17.790 | 1186.0 | 1196.1 | 0.79 |
| trans-Dihydrocarvone | 18.088 | 1200.0 | 1204.5 | 0.02 |
| Nerol | 18.842 | 1227.0 | 1226.3 | 1.18 |
| Isobornyl formate | 18.990 | 1235.0 | 1230.5 | 2.87 |
| Neral | 19.311 | 1235.0 | 1239.8 | 2.25 |
| Geraniol | 19.936 | 1249.0 | 1257.8 | 22.33 |
| Geranial | 20.396 | 1264.0 | 1271.0 | 0.50 |
| Bornyl acetate | 20.924 | 1287.0 | 1286.3 | 0.20 |
| Nerol acetate | 23.364 | 1359.0 | 1359.2 | 0.21 |
| Geranyl acetate | 24.244 | 1379.0 | 1385.8 | 19.38 |
| Sesquiterpene hydrocarbons | 14.56 | |||
| α-Cubebene | 23.065 | 1345.0 | 1350.2 | 5.51 |
| α-Copaene | 23.927 | 1374.0 | 1376.2 | 0.03 |
| β-Elemene | 24.409 | 1389.0 | 1390.7 | 0.09 |
| β-Caryophyllene | 25.384 | 1417.0 | 1421.3 | 1.04 |
| α-trans-Bergamotene | 25.770 | 1432.0 | 1433.6 | 0.05 |
| Aromadendrene | 25.952 | 1439.0 | 1439.4 | 0.12 |
| (Z)-β-Farnesene | 26.376 | 1440.0 | 1452.9 | 0.22 |
| α-Humulene | 26.487 | 1452.0 | 1456.4 | 0.13 |
| γ-Muurolene | 27.083 | 1478.0 | 1475.4 | 0.16 |
| Germacrene D | 27.302 | 1484.0 | 1482.4 | 1.57 |
| β-Selinene | 27.567 | 1489.0 | 1490.8 | 0.14 |
| Bicyclogermacrene | 27.734 | 1500.0 | 1496.2 | 1.01 |
| β-Bisabolene | 28.153 | 1505.0 | 1510.0 | 4.08 |
| γ-Cadinene | 28.258 | 1513.0 | 1513.6 | 0.11 |
| δ-Cadinene | 28.410 | 1522.0 | 1518.7 | 0.21 |
| β-Sesquiphellandrene | 28.563 | 1521.0 | 1523.9 | 0.09 |
| Oxygenated sesquiterpenes | 0.37 | |||
| Spathulenol | 30.140 | 1577.0 | 1577.0 | 0.29 |
| Caryophyllene oxide | 30.300 | 1582.0 | 1582.3 | 0.08 |
| Phenolic compounds | 14.00 | |||
| Thymol | 21.350 | 1289.0 | 1298.6 | 13.79 |
| Carvacrol | 21.499 | 1298.0 | 1303.0 | 0.19 |
| Eugenol | 23.155 | 1356.0 | 1352.9 | 0.02 |
| Others | 0.62 | |||
| trans-2-Hexenal | 5.987 | 846.0 | 850.2 | 0.03 |
| 1-Octen-3-ol | 10.010 | 974.0 | 979.8 | 0.45 |
| 3-Octanol | 10.596 | 988.0 | 997.2 | 0.14 |
|
| ||||
| Total | 97.76 | |||
aRT: retention time; bAIL: arithmetic (retention) index-literature data, and cAIE: arithmetic (retention) index experimentally determined on HP-5MS column.
3.2. Antibacterial Activity
The essential oils were tested for their antibacterial activity by broth microdilution method to determine the MIC and MBC values against thirteen model bacteria (Table 2). The results from the antibacterial assay show that thyme essential oil possessed antimicrobial activities against all the tested microorganisms with MIC values ranging from 627.1 to 10033.6 μg/mL and MBC values from 627.1 to 20067.2 μg/mL. Gram-positive bacteria were generally found to be more sensitive than the Gram-negative ones. Geraniol was active with MIC values ranging from 1386.8 to 5547.2 μg/mL and MBC values from 1386.8 to 11094.4 μg/mL. Thymol exhibited antibacterial activity with MIC values ranging from 24.4 to 3123.2 μg/mL and MBC values from 24.4 to 6246.4 μg/mL. The reference antibiotic was active in the range of concentration 1 to 2048 μg/mL.
Table 2.
Antibacterial activity of T. glabrescens essential oil, chloramphenicol, geraniol, and thymol (μg/mL).
| Number | Bacterial species | T. glabrescens | Chloramphenicol | Geraniol | Thymol | ||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| 1 | Escherichia coli ATCC 25922 | 2508.4 | 5016.8 | 128.0 | 512.0 | 1386.8 | 2773.6 | 1561.6 | 1561.6 |
| 2 | Salmonella enteritidis ATCC 13076 | 627.1 | 627.1 | 4.0 | 8.0 | 2773.6 | 2773.6 | 24.4 | 24.4 |
| 3 | Klebsiella pneumoniae ATCC 10031 | 1254.2 | 1254.2 | 2.0 | 2.0 | 2773.6 | 5547.2 | 390.4 | 390.4 |
| 4 | Klebsiella pneumoniae ATCC 700603 | 5016.8 | 10033.6 | 512.0 | 1024.0 | 2773.6 | 5547.2 | 1561.6 | 3123.2 |
| 5 | Proteus mirabilis ATCC 12453 | 1254.2 | 2508.4 | 4.0 | 64.0 | 1386.8 | 1386.8 | 1561.6 | 1561.6 |
| 6 | Pseudomonas aeruginosa ATCC 9027 | 10033.6 | 20067.2 | 4.0 | 16.0 | 5547.2 | 11094.4 | 1561.6 | 1561.6 |
| 7 | Pseudomonas aeruginosa ATCC 27853 | 5016.8 | 5016.8 | 1024.0 | 2048.0 | 2773.6 | 2773.6 | 3123.2 | 6246.4 |
| 8 | Enterobacter aerogenes ATCC 13048 | 5016.8 | 5016.8 | 1.0 | 1.0 | 5547.2 | 5547.2 | 195.2 | 195.2 |
| 9 | Enterococcus faecalis ATCC 19433 | 2508.4 | 2508.4 | 2.0 | 4.0 | 1386.8 | 1386.8 | 195.2 | 195.2 |
| 10 | Bacillus cereus ATCC 11778 | 627.1 | 1254.2 | 1.0 | 4.0 | 1386.8 | 1386.8 | 24.4 | 24.4 |
| 11 | Staphylococcus aureus ATCC 25923 | 627.1 | 627.1 | 1.0 | 8.0 | 2773.6 | 2773.6 | 97.6 | 97.6 |
| 12 | Staphylococcus aureus ATCC 29213 | 2508.4 | 2508.4 | 8.0 | 32.0 | 2773.6 | 2773.6 | 780.8 | 1561.6 |
| 13 | Listeria monocytogenes ATCC 15313 | 627.1 | 1254.2 | 8.0 | 8.0 | 2773.6 | 2773.6 | 97.6 | 97.6 |
3.3. Interactions between the Essential Oil, Geraniol, and Thymol with the Reference Antibiotic
The results of the possible interactions between the essential oil, geraniol, and thymol with the reference antibiotic are given in Figures 1–3.
Figure 1.
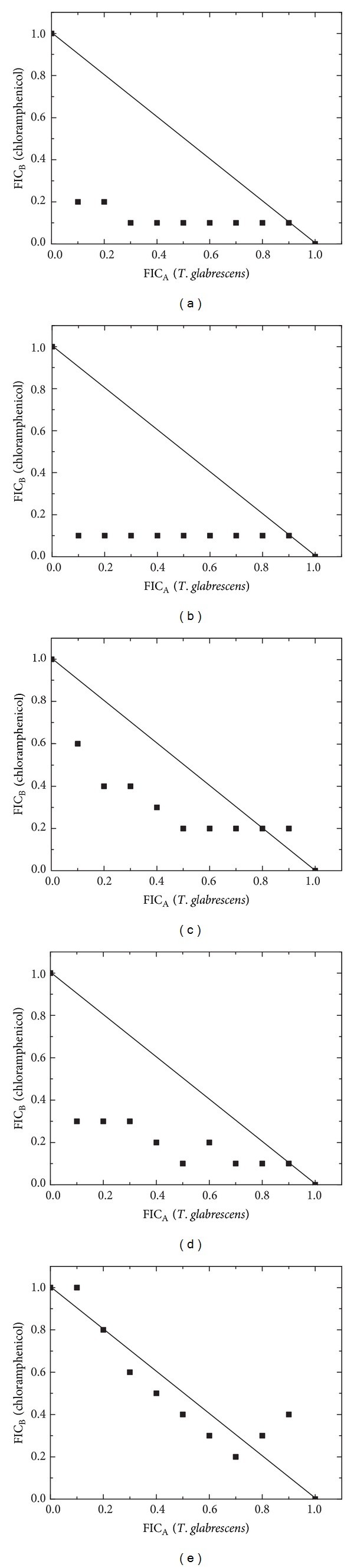
The derived isobolograms for the interaction of T. glabrescens oil-chloramphenicol and their treatment outcomes against the following: (a) E. coli ATCC 25922, (b) K. pneumoniae ATCC 700603, (c) P. mirabilis ATCC 12453, (d) P. aeruginosa ATCC 27853, and (e) S. aureus ATCC 29213.
Figure 3.
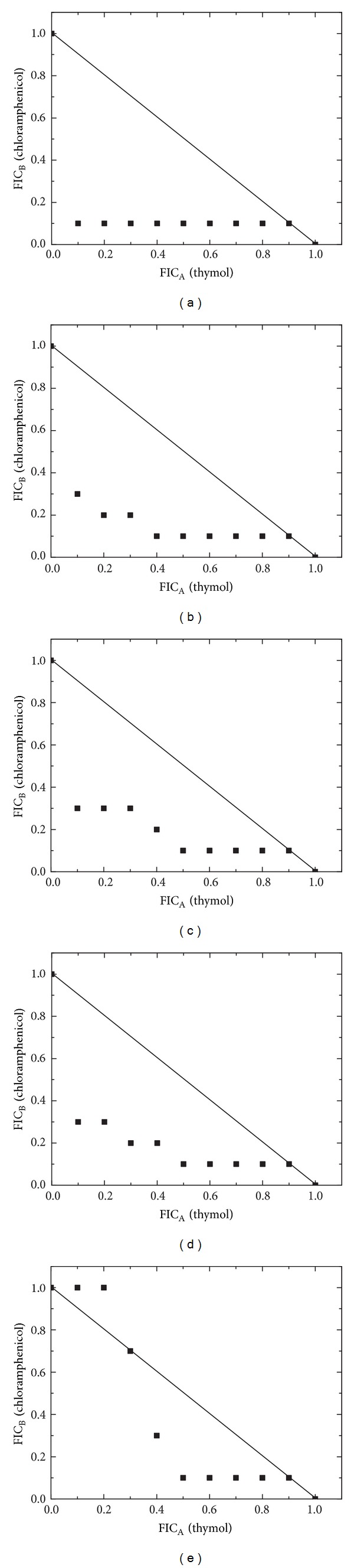
The derived isobolograms for the interaction of thymol-chloramphenicol and their treatment outcomes against the following: (a) E. coli ATCC 25922, (b) K. pneumoniae ATCC 700603, (c) P. mirabilis ATCC 12453, (d) P. aeruginosa ATCC 27853, and (e) S. aureus ATCC 29213.
Of the 45 combinations of T. glabrescens essential oil-chloramphenicol, 25 (55.6%) showed synergism, while 14 (31.1%) had an additive and 6 (13.3%) had an antagonistic effect (Figure 1). Studies on E. coli ATCC 25922 and K. pneumoniae ATCC 700603 showed a synergistic pattern for seven ratios (FIC indices in the range 0.21–0.87). Synergy was also noted when tested against P. aeruginosa ATCC 27853 (six ratios, FIC indices in the range 0.43–0.87) and P. mirabilis ATCC 12453 (five ratios, FIC indices in the range 0.68–0.82). Combinations with S. aureus ATCC 29213 indicated additive (five ratios) and antagonistic (four ratios) effects.
From all the tested combinations of geraniol-reference antibiotic (Figure 2), 26 (57.8%) showed synergism, 15 (33.3%) had an additive effect, and 4 (8.9%) had an antagonistic effect. Studies on E. coli ATCC 25922 and K. pneumoniae ATCC 700603 showed a synergistic pattern for seven ratios (FIC indices in the range 0.21–0.87). Synergy was also noted when tested against P. mirabilis ATCC 12453 and P. aeruginosa ATCC 27853 (six ratios, FIC indices in the range 0.43–0.87). Combinations with S. aureus ATCC 29213 indicated additive (five ratios) and antagonistic (four ratios) effects.
Figure 2.
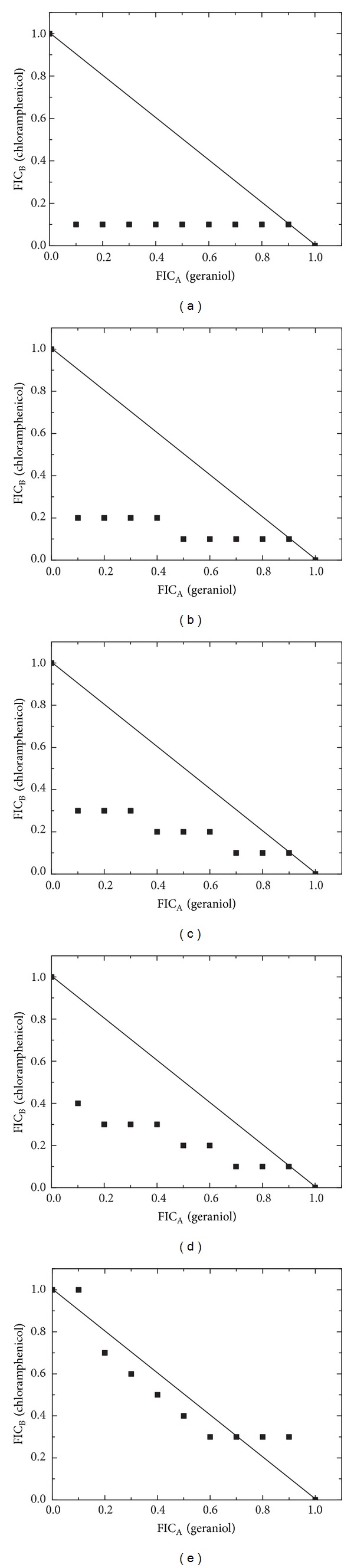
The derived isobolograms for the interaction of geraniol-chloramphenicol and their treatment outcomes against the following: (a) E. coli ATCC 25922, (b) K. pneumoniae ATCC 700603, (c) P. mirabilis ATCC 12453, (d) P. aeruginosa ATCC 27853, and (e) S. aureus ATCC 29213.
The combination profiles of thymol with chloramphenicol are presented in Figure 3. A predominantly synergistic profile was noted against all the studied pathogens. Synergy was best noted for 32 (71.1%) ratios, an additive effect was recorded for 10 (22.2%), ratios and three combinations (6.7%), against S. aureus ATCC 29213, exhibited an antagonistic effect. To evaluate the correlation among the antibacterial activities of the essential oil-chloramphenicol, geraniol-chloramphenicol, and thymol-chloramphenicol combinations, the FIC values were subjected to PCA and HCA analysis.
3.4. PCA and HCA Analysis of the Total FIC Indices of the Essential Oil, Geraniol, Thymol, and Chloramphenicol Combinations
PCA and HCA were applied on all FIC data (Figures 1–3) in order to evaluate similar antibacterial behaviour among studied combinations. According to the eigenvalues of the obtained correlation matrix, the PC1 horizontal axis explained 81.14% of the total variance among the tested interactions, while the PC2 vertical axis showed a further 12.77% (Figure 4(a)). The loading plot (Figure 4(b)) illustrates the influence of the FIC values, marked by FICA equivalents, responsible for the classification of the interaction in the score plot (Figure 4(c)). Based on the Euclidean distance and dissimilarity ≥0.42 (Figure 4(d)), the HCA method indicated two groups of interaction (A and B). Group A, constituted only by S. aureus ATCC 29213, was characterized mainly by strong antagonistic interactions with the applied combinations. In this group, only the association thymol-chloramphenicol showed some percent of synergistic interaction. In contrast, in group B, formed by the rest of the examined bacteria strains and studied combinations, mainly synergistic or additive interactions were detected.
Figure 4.
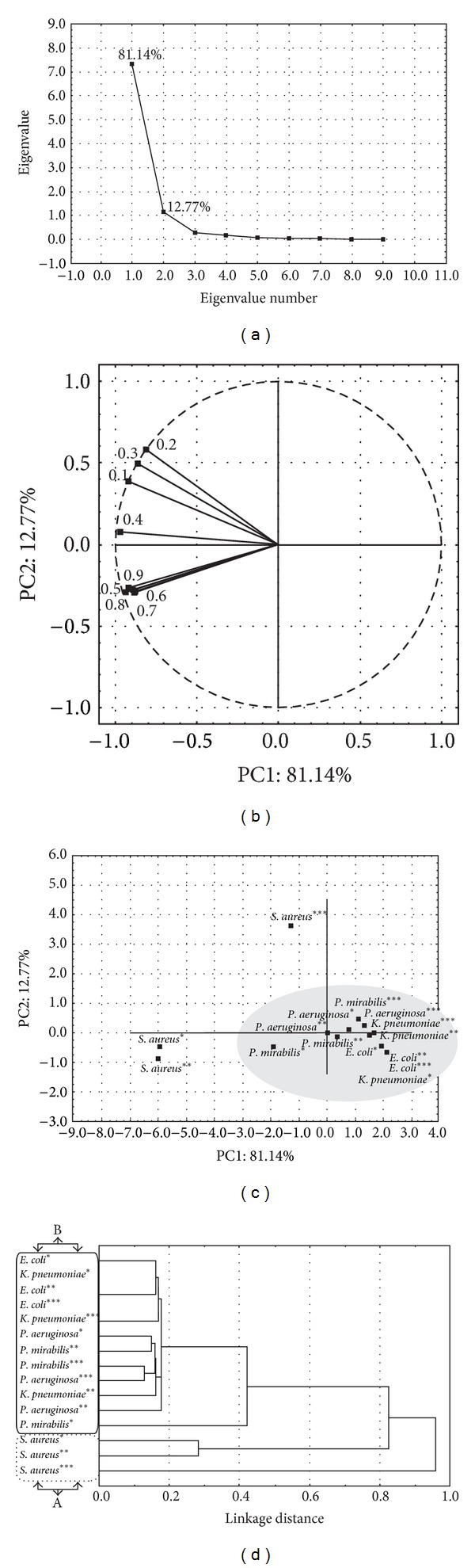
PCA and HCA of the antibacterial activity of the studied combinations {T. glabrescens oil-chloramphenicol (*); geraniol-chloramphenicol (**); thymol-chloramphenicol (***)} based on their FIC values: (a) eigenvalues of the correlation matrix, (b) the loading plot of the responsible FIC values, (c) the score plot of the examined bacteria, and (d) the corresponding dendrogram. The examined FIC values are presented in Figures 1–3.
4. Discussion
The essential oil of T. glabrescens from southeast Serbia belongs to the geraniol/geranyl acetate/thymol chemotype [13]. Chemical polymorphism of the essential oils is a characteristic of the species of the Thymus genus. Except for genetic factors, environmental conditions also have an influence on the chemical composition of an essential oil. It was established that the production of phenolic compounds is favoured in warmer and drier climatic zones, while the other, nonphenolic compounds usually accumulate in higher quantities in cooler and damper areas [14]. Geraniol is the dominant component of T. glabrescens essential oil from Romania [15]. In Hungarian T. glabrescens essential oil, the major compounds were sesquiterpenes: germacrene D, β-caryophyllene, and caryophyllene oxide [13].
The release of cellular content in the treated bacteria led to the hypothesis that the first effect of an essential oil is membrane disruption. However, the fact that some interaction with other targets of the bacterial cell might play a key role in the observed antibacterial effects of the essential oil should not be ignored [16]. The antibacterial activity of T. glabrescens essential oil displayed variation among the different bacteria species but remained lower than the activities of the standard antibiotic and thymol. A correlation of the antibacterial activity of the oil and its chemical composition suggests that the activity of the oil could be attributed to the presence of significant concentrations of geraniol and thymol. Therefore, it was decided to study also the antibacterial activity of thymol and geraniol individually and in combination with chloramphenicol.
The essential oil of T. glabrescens from Romania inhibited microbial growth in a range of concentrations from 10.8 to 27 μL/mL [15]. As noted, the main antibacterial agent of the T. glabrescens essential oil from southeast Serbia is not only geraniol but also thymol (13.79%); together they represent 36.12% of T. glabrescens essential oil. It is interesting to emphasize that the antibacterial activity of T. pulegioides essential oil with geraniol (66.59%) as the major constituent is significantly higher in comparison with antibacterial activity of T. glabrescens essential oil towards the same bacterial strains [17].
In a study of the inhibitory activity of terpenes on slime producing methicillin resistant strains, the authors found MIC values for geraniol of 5.8 mg/mL against the methicillin resistant S. aureus (MRSA) and 23.4 mg/mL against methicillin sensitive S. aureus (MSSA) [18]. In the same study, thymol exhibited inhibitory activities against MRSA and MSSA strains with an MIC value of 3.17 mg/mL. These values are generally higher compared to the values of the antibacterial activity of geraniol and thymol found in the present research.
In the current investigation, it was confirmed that Gram-positive bacteria were more sensitive with all tested antibacterial agents than Gram-negative ones. Most Gram-negative bacteria are intrinsically less susceptible to many antibiotics than are Gram-positive bacteria. This difference could be explained by the presence of an outer membrane in Gram-negative bacteria. The structure and composition of the layer of cells differ greatly between bacteria. On the outer envelope, the cells may have polysaccharide capsules or protein layers which protect bacteria under unfavourable conditions and affect their adhesion [19].
The interaction of essential oils with antibiotics is one of the novel ways to overcome bacterial resistance. Essential oils are combined with antibiotics in order to improve the antimicrobial effect and to reduce the required antibiotic concentration [20]. In the present study, the antimicrobial activity of T. glabrescens essential oil was evaluated in association with chloramphenicol on five bacterial strains. The combination of thyme oil and chloramphenicol against all the tested bacteria, except S. aureus ATCC 29213, exhibited a predominantly synergistic effect and decreased the MIC value of chloramphenicol 10-fold (5-fold for P. mirabilis ATCC 12453). Based on the present analyses, it can be assumed that in research of the antibacterial effects of essential oil-antibiotic combinations, the choice of Gram-negative or Gram-positive bacterial species is not decisively significant. In other words, the proper essential oil-antibiotic association will act equally stronger or weaker against all Gram-positive and Gram-negative bacterial strains. In this case, the outer membrane of the Gram-negative bacteria is not a predominant factor of their resistance.
The essential oil of P. graveolens and its main components (geraniol and citronellol) exhibited strong synergism with norfloxacin against B. cereus and S. aureus with FIC indices of 0.50, 0.37, and 0.38, respectively [21]. According to Prashara et al. [22], the antimicrobial action of Cymbopogon martinii essential oil (mainly attributed to its geraniol content) against S. cerevisiae occurs via a two-step process. The first step involves the passive entry of the oil into the plasma membrane in order to initiate membrane disruption. The second step is reaction with the active sites of the enzymes or action as an H+ carrier, thereby depleting the adenosine triphosphate pool.
There are some generally accepted mechanisms of antibacterial interaction that produce synergism, including inhibition of protective enzymes, combination of membrane active agents, sequential inhibition of common biochemical pathways, and the use of membranotropic agents to enhance the diffusion of other antimicrobials [23]. The results obtained in the present study indicate that chloramphenicol, not currently used as a therapeutic agent against Gram-negative bacteria, in combination with an appropriate essential oil, has significant antimicrobial activity, especially against Gram-negative bacteria. Moreover, its minimum effective dose is significantly reduced, and consequently possible toxic side effects are decreased.
The results for the antibacterial activity of a combination of geraniol-chloramphenicol are very similar to the results for a combination of thyme oil-chloramphenicol. The difference is in the increased percentage of interactions that produce synergistic and additive effects, with a decrease in the percentage of antagonistic effects. The combination of geraniol and chloramphenicol against all the tested bacteria, except S. aureus ATCC 29213, exhibited predominantly synergistic effects and decreased the MIC value of chloramphenicol 10-fold. The associations of geraniol with penicillin against MRSA and E. coli were shown to be indifferent, independently of each antimicrobial activity when they were used alone [24]. In a study of changes in the antibacterial activity and the mode of action of farnesol against S. aureus when geraniol was added to a bacterial suspension, the authors assumed that geraniol increased the growth-inhibitory activity of farnesol but suppressed its ability to damage cell membranes, which is one of the predominant features of the growth-inhibitory activity of farnesol. Their results revealed that terpenes might interact with each other and with bacterial cells to increase or decrease the antibacterial activity of each other [25]. Geraniol significantly increased the efficacy of chloramphenicol by targeting efflux mechanisms and produced significant restoration of susceptibility of the multidrug resistance strain EAEP289 to chloramphenicol by as much as 16-fold [26]. Combinations of geraniol and norfloxacin toward B. cereus and S. aureus exhibit synergistic effects with FIC indices of 0.50 [21]. The present findings and published data led to the speculation that the antibacterial effect of geraniol may result through interaction with the membrane structure and the function of the bacteria. Furthermore, geraniol might cross the cell membranes penetrating into the interior of the cell and interacting with intracellular sites critical for antibacterial activity [27].
The combination of thymol and chloramphenicol against all the tested bacteria exhibited a predominantly synergistic effect and decreased the MIC value of chloramphenicol 10-fold. Antagonism (only three combinations) was evidenced only against S. aureus ATCC 29213. Studies on the antibacterial action of thymol showed that it can cause a disturbance of the cytoplasmic membrane, disrupting the proton motive force, electron flow, and coagulation of cell contents. Thymol also impaired the citrate metabolic pathway and affected the enzymes involved in the synthesis of ATP [28]. The results obtained with the combinations of thymol-penicillin toward MRSA were antagonistic, while association between thymol and penicillin against E. coli showed synergistic activity with FIC values of 0.15 [24]. It could be argued that these results correspond to the results of the present research. If the results obtained from the study of the antibacterial activity of geraniol-chloramphenicol and thymol-chloramphenicol associations are compared, a similar pattern can be found. This leads to the speculation that geraniol and thymol in combination could not show any antagonistic effect.
If all combinations of the examined essential oil, geraniol, and thymol with chloramphenicol towards the five bacterial strains are taken into consideration, a possible hypothesis is that the components of thyme essential oil, with the geraniol and thymol as the main active principles, favour the mechanism of action of chloramphenicol, the main effect of which is inhibition of the bacterial enzyme peptidyl transferase, thereby preventing the growth of the polypeptide chain during protein synthesis [29]. It could be stated that all associations against S. aureus ATCC 29213 were characterized by a number of ratios of antagonistic interactions. In the PCA and HCA analyses this strain stands out and forms a separate group. In contrast, the other combinations exhibited mostly synergistic or additive interactions toward the other bacterial strains, which may indicate, an already supposed assumption, that the activity of the thyme oil could be attributed to the presence of significant concentrations of geraniol and thymol.
5. Conclusions
In the present study, the chemical composition of T. glabrescens essential oil was examined and a correlation among the antibacterial activities of the essential oil-chloramphenicol, geraniol-chloramphenicol, and thymol-chloramphenicol combinations was realized by the utilization of chemometric methods. It was shown that oxygenated monoterpenes, with geraniol as the dominant constituent, were the most abundant compound class of the essential oil of T. glabrescens from Southeast Serbia. The researched essential oil exhibited in vitro antibacterial activity against all the tested bacterial strains, but the activities were lower than those of the standard antibiotic and thymol. The combination of thyme oil and chloramphenicol produced predominantly synergistic interactions and substantial reductions in the MIC values of chloramphenicol against Gram-negative bacteria, the pharmacological treatment of which is very difficult nowadays. The combinations geraniol-chloramphenicol and thymol-chloramphenicol produced synergistic interaction to a greater extent, compared with the essential oil-chloramphenicol association. All the examined combinations reduced the minimum effective dose of the antibiotic and, consequently, minimized its adverse side effects.
Acknowledgment
This research was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant nos. 171025 and 176006).
Conflict of Interests
The authors declare that they have no conflict of interests regarding the publication of this paper.
References
- 1.Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nature Reviews Microbiology. 2009;7(9):629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miladinović DL, Ilić BS, Mihajilov-Krstev TM, Nikolić ND, Milosavljević VN. Antibacterial potential of the essential oil from Sideritis montana L., (Lamiaceae) Hemijska Industrija. 2012;66(4):541–545. [Google Scholar]
- 3.Wagner H. Synergy research: approaching a new generation of phytopharmaceuticals. Fitoterapia. 2011;82(1):34–37. doi: 10.1016/j.fitote.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Miladinović DL, Ilić BS, Mihajilov-Krstev TM, Nikolić ND, Miladinović LC, Cvetković OG. Investigation of the chemical composition-antibacterial activity relationship of essential oils by chemometric methods. Analytical and Bioanalytical Chemistry. 2012;403(4):1007–1018. doi: 10.1007/s00216-012-5866-1. [DOI] [PubMed] [Google Scholar]
- 5.Diklić N. Thymus L. In: Josifović M, editor. Flora of the Republic of Serbia. Belgrade, Serbia: Serbian Academy of Sciences and Arts; 1974. pp. 475–509. [Google Scholar]
- 6.Tan B, Liang Y, Yi L, et al. Identification of free fatty acids profiling of type 2 diabetes mellitus and exploring possible biomarkers by GC-MS coupled with chemometrics. Metabolomics. 2010;6(2):219–228. [Google Scholar]
- 7.Adams RP. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Carol Stream, Ill, USA: Allured Publishing Corporation; 2007. [Google Scholar]
- 8.CLSI. M07-A08. Wayne, Mich, USA: Clinical and Laboratory Standards Institute; 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. [Google Scholar]
- 9.Dougherty PF, Yotter DW, Matthews TR. Microdilution transfer plate technique for determining in vitro synergy of antimicrobial agents. Antimicrobial Agents and Chemotherapy. 1977;11(2):225–228. doi: 10.1128/aac.11.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Vuuren SF, Suliman S, Viljoen AM. The antimicrobial activity of four commercial essential oils in combination with conventional antimicrobials. Letters in Applied Microbiology. 2009;48(4):440–446. doi: 10.1111/j.1472-765X.2008.02548.x. [DOI] [PubMed] [Google Scholar]
- 11.Chou T-C, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in Enzyme Regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 12.Wyles DL, Kaihara KA, Vaida F, Schooley RT. Synergy of small molecular inhibitors of hepatitis C virus replication directed at multiple viral targets. Journal of Virology. 2007;81(6):3005–3008. doi: 10.1128/JVI.02083-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simkó H, Sárosi S, Ladányi M, et al. Studies on occurence, essential oil data and habitat conditions of Hungarian Thymus pannonicus and Thymus glabrescens populations. Medicinal and Aromatic Plants. 2013;2(1):1–7. [Google Scholar]
- 14.Ložiené K, Šakalyté J, Paškevičius A, Venskutonis PR. Anti-Candida activity of Thymus pulegioides (Lamiaceae) essential oils depends on the plant chemotype. Herba Polonica. 2008;54(4):79–92. [Google Scholar]
- 15.Pavel M, Ristić M, Stević T. Essential oils of Thymus pulegioides and Thymus glabrescens from Romania: chemical composition and antimicrobial activity. Journal of the Serbian Chemical Society. 2010;75(1):27–34. [Google Scholar]
- 16.Fadli M, Saad A, Sayadi S, et al. Antibacterial activity of Thymus maroccanus and Thymus broussonetii essential oils against nosocomial infection–bacteria and their synergistic potential with antibiotics. Phytomedicine. 2012;19(5):464–471. doi: 10.1016/j.phymed.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Miladinović DL, Ilić BS, Miladinović LC, et al. Antibacterial activity of Thymus pulegioides essential oil and its synergistic potential with antibiotics: a chemometric approach. In: Govil JN, editor. Recent Progress in Medicinal Plants. Vol. 38. Houston, Tex, USA: Studium Press; 2013. pp. 101–136. [Google Scholar]
- 18.Gallucci N, Oliva M, Carezzano E, Zygadlo J, Demo M. Terpenes antimicrobial activity against slime producing and non-producing staphylococci. Molecular Medicinal Chemistry. 2010;21:132–136. [Google Scholar]
- 19.Alakomi HL. Weakening of the Gram-Negative Bacterial Outer Membrane: a Tool for Increasing Microbiological Safety. Helsinki, Finland: University of Helsinki; 2007. [Google Scholar]
- 20.Wagner H, Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16(2-3):97–110. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Rosato A, Vitali C, de Laurentis N, Armenise D, Antonietta Milillo M. Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine. 2007;14(11):727–732. doi: 10.1016/j.phymed.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Prashara A, Hili P, Veness RG, Evans CS. Antimicrobial action of palmarosa oil (Cymbopogon martinii) on Saccharomyces cerevisiae . Phytochemistry. 2003;63(5):569–575. doi: 10.1016/s0031-9422(03)00226-7. [DOI] [PubMed] [Google Scholar]
- 23.Sokolova SM, Buzuk GN, Lovkova MY, Tyutekin YV. Membranotropic compounds and alkaloid accumulation in plants. Doklady Biochemistry and Biophysics. 2005;402(1–6):220–222. doi: 10.1007/s10628-005-0075-x. [DOI] [PubMed] [Google Scholar]
- 24.Gallucci N, Casero C, Oliva M, Zygadlo J, Demo M. Interaction between terpenes and penicillin on bacterial strains resistant to beta-lactam antibiotics. Molecular Medicinal Chemistry. 2006;10:30–32. [Google Scholar]
- 25.Togashi N, Inoue Y, Hamashima H, Takano A. Effects of two terpene alcohols on the antibacterial activity and the mode of action of farnesol against Staphylococcus aureus. Molecules. 2008;13(12):3069–3076. doi: 10.3390/molecules13123069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorenzi V, Muselli A, Bernardini AF, et al. Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrobial Agents and Chemotherapy. 2009;53(5):2209–2211. doi: 10.1128/AAC.00919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdel Rasoul MA, Marei GIK, Abdelgaleil SAM. Evaluation of antibacterial properties and biochemical effects of monoterpenes on plant pathogenic bacteria. African Journal of Microbiology Research. 2012;6(15):3667–3672. [Google Scholar]
- 28.Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Frontiers in Microbiology. 2012;3(12):1–24. doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hopps HE, Wisseman JCL, Hahn FE, Smadel JE, Ho R. Mode of action of chloramphenicol IV.: failure of selected natural metabolites to reverse antibiotic action. Journal of Bacteriology. 1956;72(4):561–567. doi: 10.1128/jb.72.4.561-567.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]


