Abstract
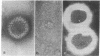
Rotational power spectrum analysis of scanning transmission electron micrographs of negatively stained herpesvirus capsids has given direct evidence for the 6-fold symmetry of the herpesvirus hexon capsomere. Individual hexons have been analyzed in situ without interference from other parts of the capsid because only one level of the capsid was in focus in the micrograph. This study found hexons to have 6-fold symmetry. The majority of the mass of each hexon subunit was seen to lie on a line connecting the centers of adjacent capsomeres. This agrees with analysis of conventional micrographs of capsid fragments, which are also presented, as well as with biochemical data and theoretical expectations.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Crewe A. V., Wall J. A scanning microscope with 5 A resolution. J Mol Biol. 1970 Mar;48(3):375–393. doi: 10.1016/0022-2836(70)90052-5. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Amos L. A. Harmonic analysis of electron microscope images with rotational symmetry. J Mol Biol. 1971 Aug 28;60(1):123–130. doi: 10.1016/0022-2836(71)90452-9. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Franklin R. M. The structure of the groups of nine hexons from adenovirus. J Mol Biol. 1972 Jul 14;68(1):181–184. doi: 10.1016/0022-2836(72)90273-2. [DOI] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973 Dec;12(6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E. L., Martin M. L., Gary G. W., Jr The ultrastructure of disrupted herpesvirus nucleocapsids. Virology. 1975 May;65(1):260–265. doi: 10.1016/0042-6822(75)90026-4. [DOI] [PubMed] [Google Scholar]
- Palmer E. L., Martin M. L. The fine structure of the capsid of reovirus type 3. Virology. 1977 Jan;76(1):109–113. doi: 10.1016/0042-6822(77)90287-2. [DOI] [PubMed] [Google Scholar]
- Vernon S. K., Lawrence W. C., Cohen G. H. Morphological components of herpesvirus. I. Intercapsomeric fibrils and the geometry of the capsid. Intervirology. 1974;4(4):237–248. doi: 10.1159/000149968. [DOI] [PubMed] [Google Scholar]
- WILDY P., RUSSELL W. C., HORNE R. W. The morphology of herpes virus. Virology. 1960 Oct;12:204–222. doi: 10.1016/0042-6822(60)90195-1. [DOI] [PubMed] [Google Scholar]
- Winkler F. K., Schutt C. E., Harrison S. C., Bricogne G. Tomato bushy stunt virus at 5.5-A resolution. Nature. 1977 Feb 10;265(5594):509–513. doi: 10.1038/265509a0. [DOI] [PubMed] [Google Scholar]