Abstract
There has recently been an outbreak of injectional anthrax infection secondary to contaminated heroin use in the UK and Europe. We present a case of a 37-year-old man presenting with pain and swelling in the groin following injection of heroin into the area. He was initially treated for severe cellulitis, however, he failed to respond to appropriate antimicrobial therapy. He went onto develop a widespread rash; it was then that a diagnosis of injectional anthrax infection was considered. Appropriate investigations were initiated including serum sample and tissue biopsy, and the diagnosis was confirmed. Management included extensive surgical debridement and a prolonged course of combination antibiotic therapy. The authors summarise the important steps in diagnosis and the management options in patients presenting with this life-threatening infection.
Background
Anthrax is an infection caused by the spore-forming Gram-positive bacterium Bacillus anthracis.1 It is a worldwide disease primarily of herbivore animals that may also occur in humans.2 According to 2003 data from the WHO, the estimated number of human anthrax infections was between 2000 and 20 000 per year.3 In most of Europe and North America, animal cases are sporadic and uncommon, and human cases are rare and usually associated with exposure to infected animal products.2 Nowadays anthrax is most well-known in connection with bioterrorism, where anthrax spores are used as a bioterrorist weapon.4 5
The three primary forms of anthrax infection are cutaneous, gastrointestinal and inhalational.1 Recently, an injectional form resulting in severe soft tissue infection following injection with contaminated heroin has also been described.1 This injectional form is associated with a 30% mortality.6
An outbreak of injectional anthrax has occurred previously in the UK starting in December 2009 in Glasgow.7 Between December 2009 and December 2010 there were 47 confirmed cases of anthrax, 13 of these patients died.7 Prior to this there had only been one such case described in 2000 by Ringertz et al8 in Norway. This patient suffered a severe systemic infection including that of the cerebrospinal fluid and died.
There is currently an ongoing outbreak of anthrax among heroin users affecting more European countries. Within the UK there have now been eight cases identified (five in England, two in Scotland and one in Wales).9 The source of the outbreak is presumed to be contaminated heroin.
Case presentation
A 37-year-old man with a history of intravenous drug use presented with a 2-day history of pain and erythema in the right groin and thigh. He reported injecting heroin into his groin 1 day prior to the pain starting. He was also feeling generally unwell experiencing fever and vomiting. On examination he was tachycardic with a heart rate of 130 bpm, although non-feverish at 36.5°C. There was patchy erythema and extensive tense oedema of the right groin and thigh area. On admission white cells were raised at 27.7×109/L (4.0–11.0×109/L), C reactive protein (CRP) was mildly raised at 58 mg/L (<10 mg/L), and D-dimer was elevated at 4000 ng/mL (0–500 ng/mL).
He was started on intravenous clindamycin 600 mg thrice daily for cellulitis as per hospital policy as he was penicillin allergic. A CT scan was requested which showed extensive oedema of the subcutaneous tissue but no discrete abscess could be seen (figure 1).
Figure 1.
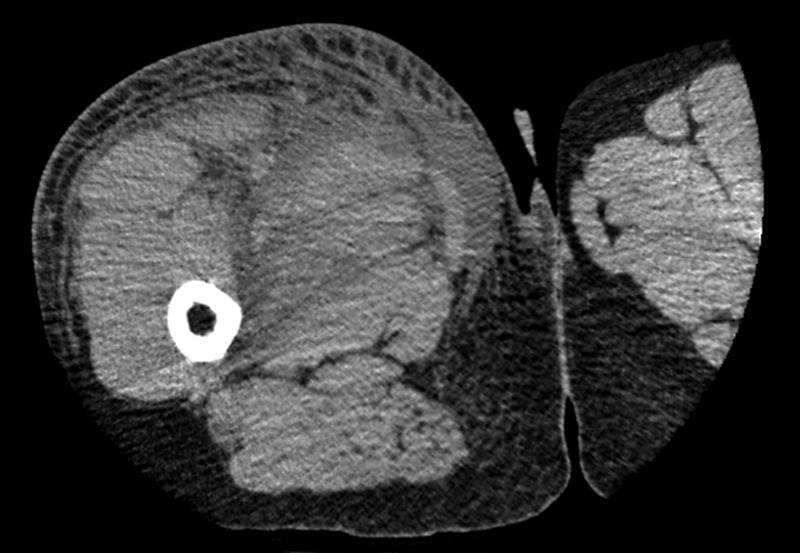
CT scan of the right thigh showing widespread tissue oedema.
In the following 2 days, the patient became persistently feverish upto 38.3°C and the erythema and oedema were not improving despite antimicrobial therapy. His renal function began to deteriorate; he developed diarrhoea and vomiting and was clinically deteriorating. Owing to the worsening of the swelling and extension of the cellulitis, the diagnosis of necrotising fasciitis was considered and the patient's antibiotic treatment was changed to intravenous vancomycin 1500 mg loading and 500 mg 12 hourly for maintenance and intravenous ciprofloxacin 400 mg twice daily.
On the fourth day, the patient developed a widespread rash extending proximally from the erythematous site to the patient's abdomen and arms, while the soft tissue in the proximity of the injection site began to blister (figure 2). It was thought that the rash may either be secondary to an antibiotic reaction or secondary to toxin release. Owing to the triad of the severe systemic illness, the extensive swelling and rash, and the history of intravenous heroin use, the diagnosis of injectional anthrax was suspected.
Figure 2.
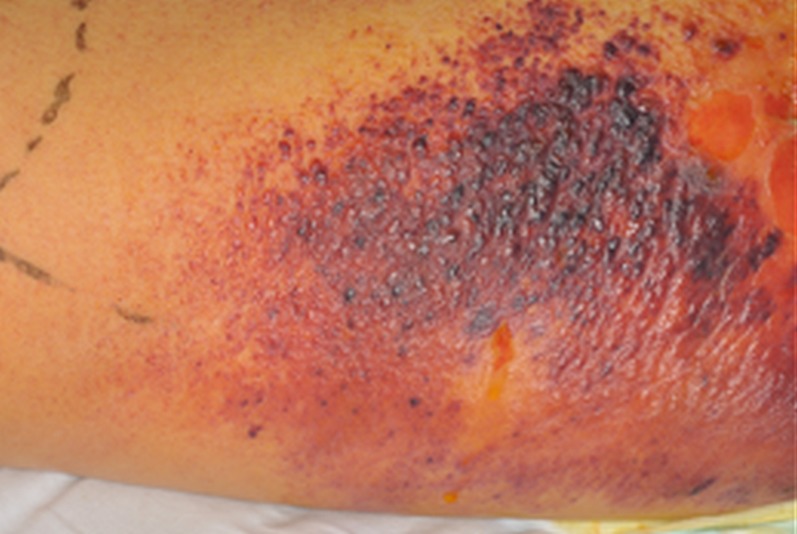
The progressive rash and blistering surrounding the primary site of infection.
Owing to the severity of his illness, the patient was escalated to the critical care unit on day 6. He underwent two extensive surgical debridements on days 8 and 10 to remove the primary source of toxin production. Following this he began to make a clinical recovery. He received a lengthy course of intravenous antibiotics requiring a prolonged hospital stay and the plastic surgery team has been closely involved in his care and follow-up.
Investigations
Blood cultures, which were taken on admission prior to starting antibiotics, were negative throughout. On suspecting anthrax infection, an EDTA blood sample was sent for anthrax toxin PCR on day 5, and a tissue biopsy was obtained and sent for microscopy, culture and PCR on day 6. Presumably secondary to the antibiotic treatment the culture remained negative, but Gram-positive bacilli could be seen during the microscopic investigation of the tissue specimen. Anthrax identification was performed by the Rare and Imported Pathogens Laboratory, Porton Down. The Anthrax PCR has previously been used extensively in prior outbreak investigations7 and was directed against three targets, one on chromosomal DNA10 and one in each virulence plasmid. Weak results were reported against the blood sample, with positive results from all three anthrax targets obtained with the thigh tissue specimen confirming the diagnosis on day 7.
Given the raised D-dimer, a duplex scan was also ordered on admission which excluded deep vein thrombosis (DVT).
Differential diagnosis
The differential diagnoses considered in this case were initially cellulitis, necrotising fasciitis and DVT. As well as B anthracis, infections with toxin producing strains of Staphylococcus aureus or Streptococcus pyogenes were also considered as these are the most frequent causative organisms of cellulitis.
Treatment
From an antibiotic point of view, the patient was initially treated as suspected cellulitis with intravenous clindamycin 600 mg thrice daily. When the possibility of necrotising fasciitis arose this was changed to intravenous vancomycin 1500 mg loading followed by 500 mg twice daily maintenance and intravenous ciprofloxacin 400 mg twice daily. Ciprofloxacin was stopped temporarily on the suspicion of an adverse drug reaction on the patient developing a rash. When anthrax was considered combined treatment with intravenous vancomycin titrated to serum levels, intravenous clindamycin 600 mg twice daily and intravenous ciprofloxacin 400 mg twice daily was restarted as per Public Health England (formerly the Health Protection Agency) guidelines.4
Extensive surgical debridement of the necrotic tissues was the main point in this patient's treatment (figure 3). Following the surgical intervention and the removal of the primary source of toxin production, the patient's clinical condition showed rapid improvement.
Figure 3.
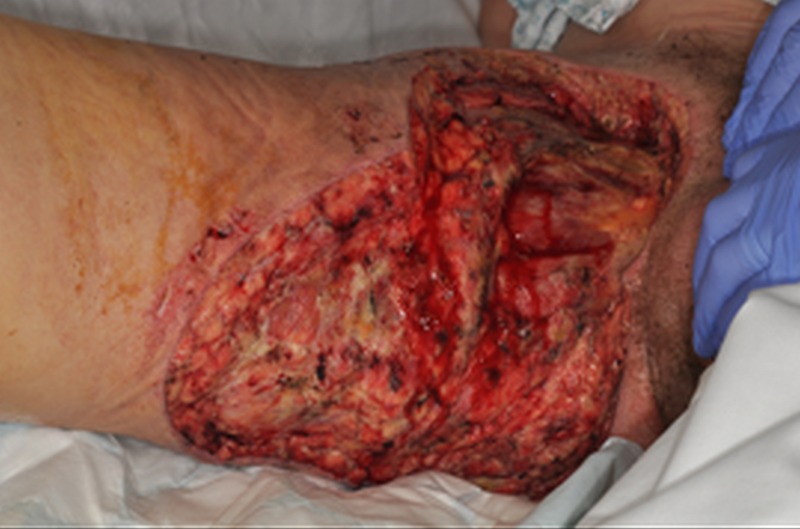
Following surgical debridement of the wound.
Intravenous vancomycin treatment was continued for 4 weeks as the patient continued to spike low-grade temperatures. When he was systematically well this was stopped. He continued with oral clindamycin 300 mg four times a day and ciprofloxacin 500 mg twice daily to complete a total of 8 weeks antibiotic therapy. The prolonged course of antimicrobial therapy was decided on due to concern regarding delayed germination of any spores.2
Outcome and follow-up
This patient survived a severe case of systemic anthrax infection secondary to injection of contaminated heroin. He underwent extensive surgical debridement of the area. The area has healed well and the patient is currently undergoing follow-up with the plastic surgery team (figure 4).
Figure 4.
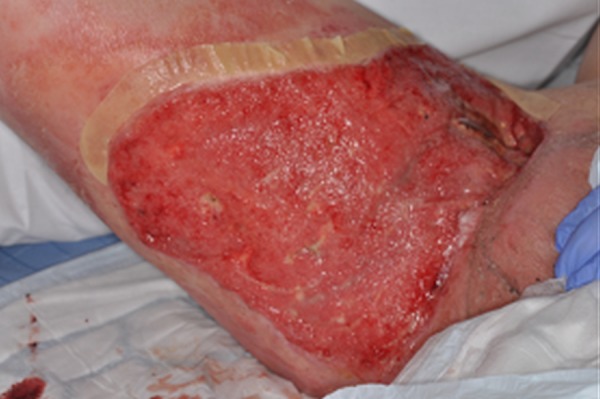
After completion of 4 weeks of vancomycin, clindamycin and ciprofloxacin and the application of a vacuum-assisted closure dressing.
Discussion
Patients with injectional anthrax infections can present with severe skin and soft tissue infections and can progress to septic shock. This is different from the typical cutaneous form of anthrax infection that is usually characterised by small, pruritic but painless papules, or vesicles containing serous fluid that are surrounded by an area of induration and marked oedema. This patient presented with the characteristic symptoms of injectional anthrax infection including severe soft tissue infection with possible necrotising fasciitis associated with marked tissue oedema and signs of severe sepsis. Public Health England, a UK body who provide advice and guidance to the national health service and department of health to aid protection of public health, advise that injectional anthrax should be suspected in any intravenous drug user who has recently injected and presents with severe soft tissue infection, severe systemic sepsis or meningitis.11
Secondary to the recent outbreak, Public Health England has set out a clear algorithm advising the initial assessment and management of drug users presenting with suspected injectional anthrax.12 In all suspected cases appropriate diagnostic samples should be taken to confirm the diagnosis of anthrax. Blood cultures should be collected in all patients with severe cellulitis and systemic signs of infection. Ideally these should be taken prior to antibiotic treatment. In this case the blood culture remained negative, despite being taken prior to starting antibiotic therapy, causing a delay in the diagnosis. Diagnosis can be confirmed by tissue cultures or PCR of the serum and of any excised tissue samples. Owing to the rarity of the condition there is limited published data available regarding the sensitivity and specificity of the PCR, however, it is believed that the PCR is more sensitive than cultures; of PCR-positive samples roughly 50% will also be positive by culture.
From a therapeutic point of view, early surgical debridement to remove the necrotic tissue and primary source of toxin production, and start of empirical intravenous antibiotics is advised.9 This should consist of ciprofloxacin, clindamycin and a further agent with adequate central nervous system cover such as penicillin, rifampicin or vancomycin.4 Antibiotic therapy should be continued for at least 3–4 weeks depending on clinical course, and intravenous therapy is advised for 10–14 days.4 As in our case, treatment was continued for up to 60 days due to the possibility of delayed germination of spores; however, it is believed that even short courses of antibiotic therapy aid an immune response which prevents death from delayed germination of spores after antibiotic therapy has stopped.2 There are currently trials taking place in the use of specific antitoxin therapies, including the anthrax immune globulin, though the full results of these have not yet been published and they should only be used as a second-line treatment.1 4 6
Microbiology involvement is necessary in all cases to advice on the diagnosis and management as well as leading the necessary communication. The local microbiology laboratory has to be notified about the possibility of anthrax infection and to organise delivery of the specimens to the reference laboratory. In all suspected cases, Public Health England and the Rare and Imported Pathogens Laboratory at Porton Down should be notified about the samples delivered and further advice should be sought. The Local Health Protection Unit should be notified as soon as possible in suspected cases.
This case demonstrates the full recovery of a severely septic patient due to systemic B anthracis infection after appropriate antibiotic therapy and surgical debridement. Although his blood culture remained negative he had signs of septic shock usually associated with high rates of mortality.
This case highlights the importance of having a high clinical suspicion of anthrax in any intravenous drug user presenting with a severe soft tissue infection and systemic sepsis. If anthrax is suspected, appropriate investigations should be initiated and antibiotics should be started immediately; surgical debridement should be performed as soon as possible. Timely treatment can be life-saving in these cases.
Learning points.
There should be a high clinical suspicion of anthrax infection in any intravenous drug user presenting with severe soft tissue infection and systemic sepsis.
Extensive communication is required in all suspected cases, including microbiological involvement, infection control input, notification of the local microbiology laboratory and the reference laboratory in Porton Down, and the local health protection unit.
Appropriate clinical samples should be collected ideally prior to antibiotic treatment to support microbiological diagnosis, including blood culture and tissue/fluid samples, EDTA blood for PCR and serum for toxin/antibody testing. Depending on clinical symptoms, cerebrospinal fluid or respiratory secretion samples may also be sent.
Empirical intravenous antibiotic therapy and urgent surgical debridement are the mainstay of treatment for such infections.
Footnotes
Contributors: JV and AK were involved equally in the production of this article.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hicks CW, Sweeney DA, Cui X, et al. An overview of anthrax infection including the recently identified form of disease in injection drug users. Intensive Care Med 2012;38:1092–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin GJ, Friedlander AM. 2010. Bacillus anthracis (anthrax). In: Mandell GL, Bennett JE, Dolin R, eds. Mandell Douglas and Bennett's principles and practice of infectious diseases. 7th edn Churchill Livingston Elsevier; 2715–31 [Google Scholar]
- 3.Louisiana State University School of Vetinary Medicine. World anthrax data site, 2003. http://www.vetmed.lsu.edu/whocc/mp_world.htm (accessed 30 Mar 2012).
- 4.Health Protection Scotland Interim clinical guidance for the management of suspected anthrax in drug user. http://www.documents.hps.scot.nhs.uk/giz/anthrax-outbreak/clinical-guidance-for-use-of-anthrax-immune-globulin-v12-1-2010-03-19.pdf (accessed 15 Oct 2013)
- 5.Martin GJ, Friedlander AM. 2010. Anthrax as an agent in bioterrorism In: Mandell GL, Bennett JE, Dolin R, eds. Mandell Douglas and Bennett's principles and practice of infectious diseases. 7th edn Churchill Livingston Elsevier, 3983–92 [Google Scholar]
- 6.Grunow R, Verbeek L, Jacob D, et al. Injection Anthax—a new outbreak in heroin users. Dtsch Ärztebl Int 2012;109:843–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Health Protection Scotland An outbreak of anthrax among drug users in Scotland, December 2009 to December 2010. A report on behalf of the National Anthrax Outbreak Control Team. December 2011. http://www.documents.hps.scot.nhs.uk/giz/anthrax-outbreak/anthrax-outbreak-report-2011-12.pdf (accessed 15 Oct 2013)
- 8.Ringertz SH, Høiby EA, Jensenius M, et al. Injectional anthrax in a heroin skin-popper. Lancet 2000;356:1574–5 [DOI] [PubMed] [Google Scholar]
- 9.Public Health England Anthrax 2012 outbreak press releases. http://www.hpa.org.uk/webw/HPAweb&Page&HPAwebAutoListName/Page/1317135273732 (accessed 15 Oct 2013)
- 10.Antwerpen MH, Zimmermann P, Bewley K, et al. Real-time PCR system targeting a chromosomal marker specific for Bacillus anthracis. Mol Cell Probes 2008;22:313–15 [DOI] [PubMed] [Google Scholar]
- 11.Health Protection Scotland and the Health Protection Agency Clinical presentations of anthrax. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1265637136474 (accessed 15 Oct 2013)
- 12.Health Protection Agency Clinical algorithm: clinical evaluation and management of drug users with possible anthrax. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1259152399460 (accessed 15 Oct 2013)


