Abstract
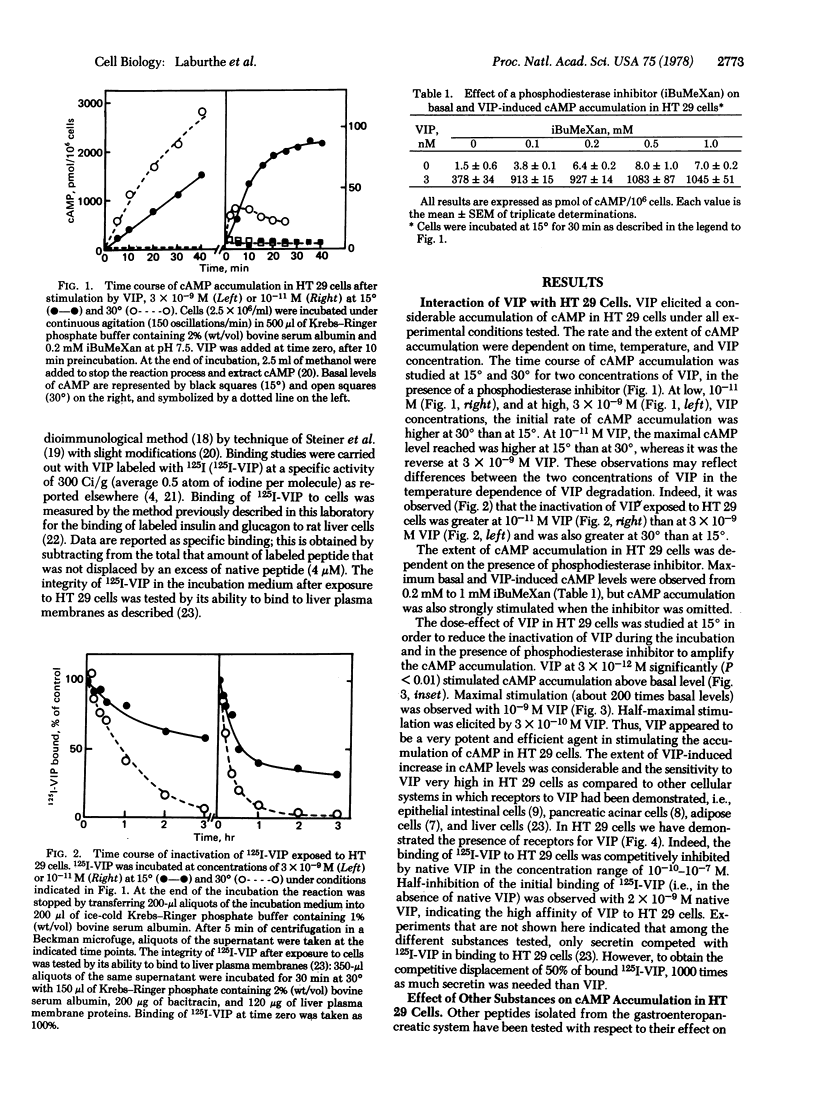
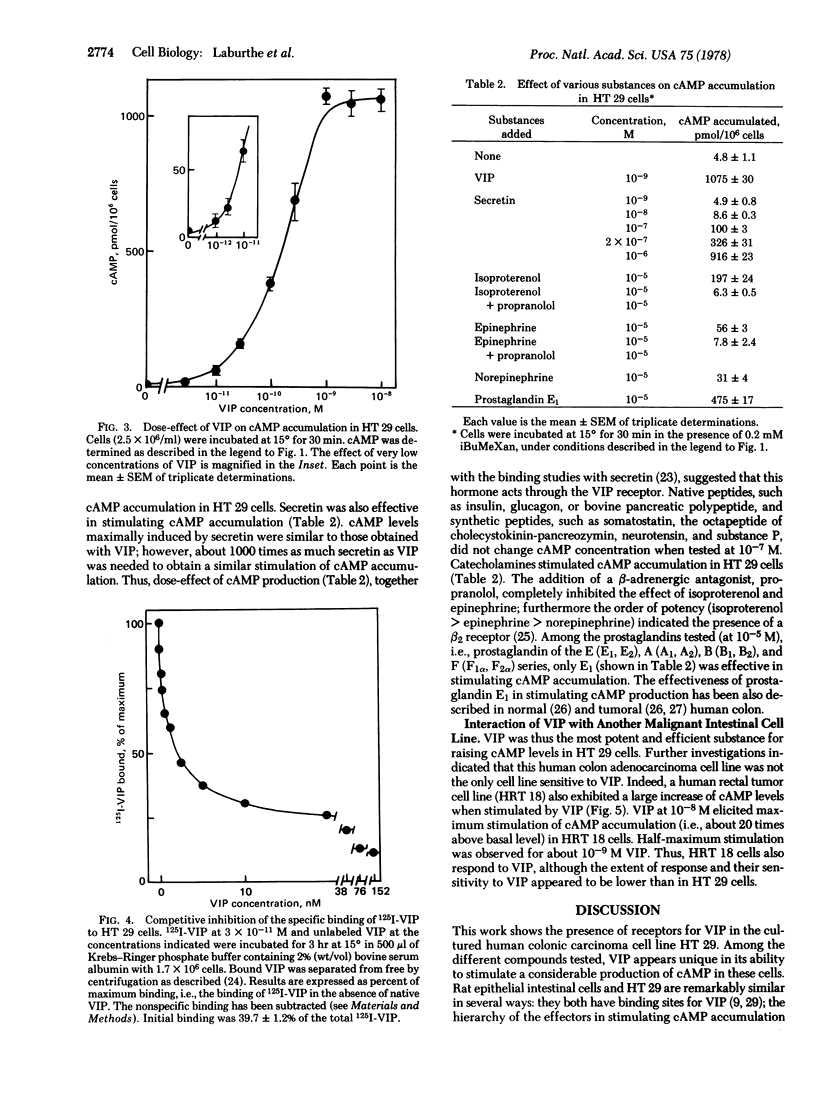
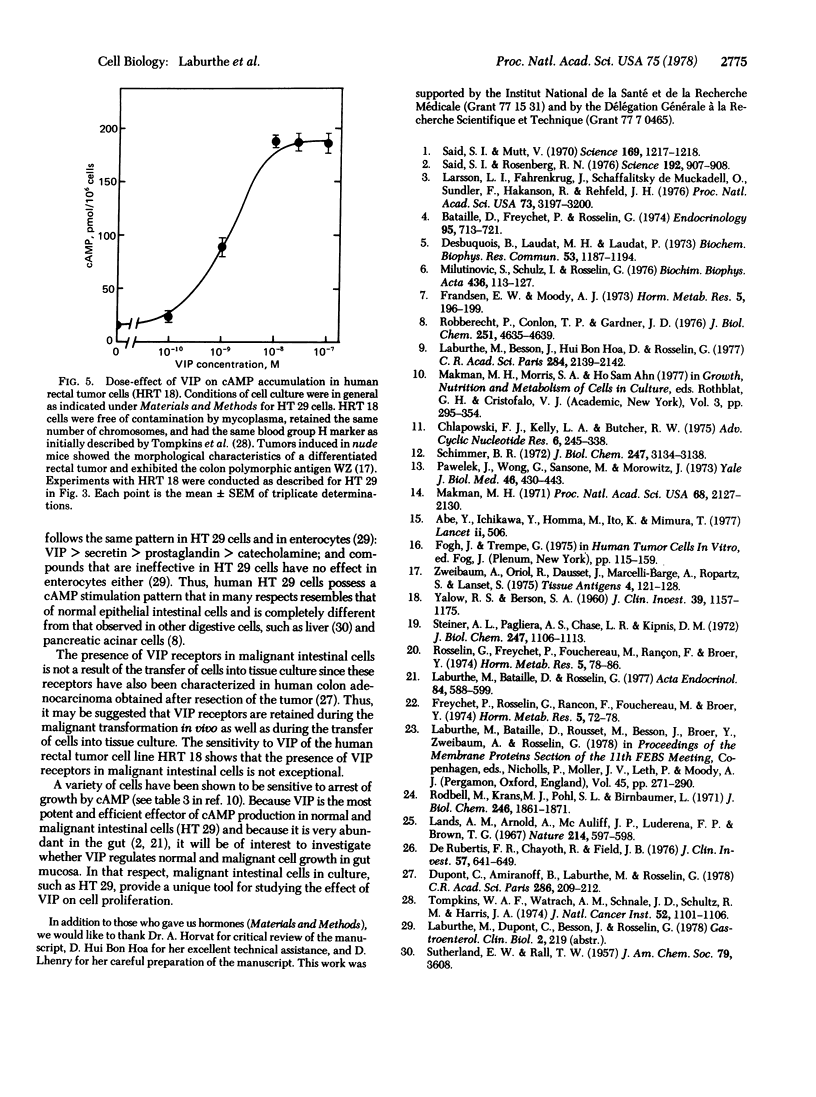
Vasoactive intestinal peptide (VIP) is a potent and efficient stimulator of adenosine 3′:5′-cyclic monophosphate (cAMP) accumulation in a human colon carcinoma cell line, HT 29. cAMP accumulation is sensitive to a concentration of VIP as low as 3×10-12 M. Maximum VIP-induced cAMP levels were observed with 10-9 M VIP and are about 200 times above the basal levels. Half-maximum cAMP production was obtained at 3×10-10 M VIP. 125I-Labeled VIP was found to bind to HT 29 cells; this binding was competitively inhibited by concentrations of unlabeled VIP between 10-10 and 10-7 M. Half-maximum inhibition of binding was observed with 2×10-9 M VIP. Secretin also stimulated cAMP accumulation in HT 29 cells, but its effectiveness was 1/1000 that of VIP. The other peptides tested at 10-7 M, such as insulin, glucagon, bovine pancreatic polypeptide, somatostatin, octapeptide of cholecystokinin, neurotensin, and substance P, did not stimulate cAMP accumulation. Prostaglandin E1 and catecholamines stimulated cAMP production but were 1/2.3 and 1/5.5 as efficient as VIP, respectively. Another malignant cell line from the gut, the human rectal tumor cell line HRT 18, is also sensitive to VIP. In HRT 18 cells, VIP stimulated cAMP accumulation with a maximal effect at 10-8 M; half-maximum stimulation was observed at about 10-9 M. These results demonstrate the presence of VIP receptors in two malignant human intestinal cell lines (HT 29 and HRT 18) in culture and provide a model for studying the action of VIP on cell proliferation.
Keywords: hormone action, receptor, prostaglandin, catecholamine, malignant digestive cell
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Ichikawa Y., Homma M., Ito K., Mimura T. T.S.H. receptor and adenylate cyclase in undifferentiated thyroid carcinoma. Lancet. 1977 Sep 3;2(8036):506–506. doi: 10.1016/s0140-6736(77)91629-4. [DOI] [PubMed] [Google Scholar]
- Bataille D., Freychet P., Rosselin G. Interactions of glucagon, gut glucagon, vasoactive intestinal polypeptide and secretin with liver and fat cell plasma membranes: binding to specific sites and stimulation of adenylate cyclase. Endocrinology. 1974 Sep;95(3):713–721. doi: 10.1210/endo-95-3-713. [DOI] [PubMed] [Google Scholar]
- Chlapowski F. J., Kelly L. A., Butcher R. W. Cyclic nucleotides in cultured cells. Adv Cyclic Nucleotide Res. 1975;6:245–338. [PubMed] [Google Scholar]
- DeRubertis F. R., Chayoth R., Field J. B. The content and metabolism of cyclic adenosine 3', 5'-monophosphate and cyclic guanosine 3', 5'-monophosphate in adenocarcinoma of the human colon. J Clin Invest. 1976 Mar;57(3):641–649. doi: 10.1172/JCI108320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbuguois B., Laudat M. H., Laudat P. Vasoactive intestinal polypeptide and glucagon: stimulation of adenylate cyclase activity via distinct receptors in liver and fat cell membranes. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1187–1194. doi: 10.1016/0006-291x(73)90590-1. [DOI] [PubMed] [Google Scholar]
- Dupont C., Amiranoff B., Laburthe M., Rosselin G. Récepteurs du peptide intestinal vasoactif (VIP) dans les membranes d'adénocarcinome colique humain: liaison spécifique et stimulation de l'adénylate cyclase. C R Acad Sci Hebd Seances Acad Sci D. 1978 Jan 16;286(2):209–212. [PubMed] [Google Scholar]
- Frandsen E. K., Moody A. J. Lipolytic action of a newly isolated vasoactive intestinal polypeptide. Horm Metab Res. 1973 May;5(3):196–199. doi: 10.1055/s-0028-1093951. [DOI] [PubMed] [Google Scholar]
- Laburthe M., Bataille D., Rosselin G. Vasoactive intestinal peptide (VIP): variation of the jejuno-ileal content in the developing rat as measured by radioreceptorassay. Acta Endocrinol (Copenh) 1977 Mar;84(3):588–599. doi: 10.1530/acta.0.0840588. [DOI] [PubMed] [Google Scholar]
- Laburthe M., Besson J., Bon Hoa D. H., Rosselin G. Récepteurs du peptide intestinal vasoactif (VIP) dans les entérocytes: liaison spécifique et stimulation de l'AMP cyclique. C R Acad Sci Hebd Seances Acad Sci D. 1977 Jun 6;284(21):2139–2142. [PubMed] [Google Scholar]
- Lands A. M., Arnold A., McAuliff J. P., Luduena F. P., Brown T. G., Jr Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967 May 6;214(5088):597–598. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Fahrenkrug J., Schaffalitzky De Muckadell O., Sundler F., Håkanson R., Rehfeld J. R. Localization of vasoactive intestinal polypeptide (VIP) to central and peripheral neurons. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3197–3200. doi: 10.1073/pnas.73.9.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makman M. H. Conditions leading to enhanced response to glucagon, epinephrine, or prostaglandins by adenylate cyclase of normal and malignant cultured cells. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2127–2130. doi: 10.1073/pnas.68.9.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milutinović S., Schulz I., Rosselin G. The interaction of secretin with pancreatic membranes. Biochim Biophys Acta. 1976 Jun 4;436(1):113–127. doi: 10.1016/0005-2736(76)90224-8. [DOI] [PubMed] [Google Scholar]
- Pawelek J., Wong G., Sansone M., Morowitz J. Molecular biology of pigment cells. Molecular controls in mammalian pigmentation. Yale J Biol Med. 1973 Dec;46(5):430–443. [PMC free article] [PubMed] [Google Scholar]
- Robberecht P., Conlon T. P., Gardner J. D. Interaction of porcine vasoactive intestinal peptide with dispersed pancreatic acinar cells from the guinea pig. Structural requirements for effects of vasoactive intestinal peptide and secretin on cellular adenosine 3':5'-monophosphate. J Biol Chem. 1976 Aug 10;251(15):4635–4639. [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- Said S. I., Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970 Sep 18;169(3951):1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- Said S. I., Rosenberg R. N. Vasoactive intestinal polypeptide: abundant immunoreactivity in neural cell lines and normal nervous tissue. Science. 1976 May 28;192(4242):907–908. doi: 10.1126/science.1273576. [DOI] [PubMed] [Google Scholar]
- Schimmer B. P. Adenylate cyclase activity in adrenocorticotropic hormone-sensitive and mutant adrenocortical tumor cell lines. J Biol Chem. 1972 May 25;247(10):3134–3138. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Tompkins W. A., Watrach A. M., Schmale J. D., Schultz R. M., Harris J. A. Cultural and antigenic properties of newly established cell strains derived from adenocarcinomas of the human colon and rectum. J Natl Cancer Inst. 1974 Apr;52(4):1101–1110. doi: 10.1093/jnci/52.4.1101. [DOI] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweibaum A., Oriol R., Dausset J., Marcelli-Barge A., Ropartz C., Lanset S. Definition in man of a polymorphic system of the normal colonic secretions. Tissue Antigens. 1975 Sep;6(3):121–128. doi: 10.1111/j.1399-0039.1975.tb00625.x. [DOI] [PubMed] [Google Scholar]


