Summary
Renal angiomyolipoma (AML) is a benign mesenchymal tumour. AML often leads to haemorrhagic complications such as retroperitoneal haematoma. Treatment varies from case to case, ranging from minimally invasive approaches such as selective embolization of the renal artery to invasive wedge resection, partial nephrectomy or, in more severe cases, radical nephrectomy.
Here we report a case of retroperitoneal haematoma secondary to AML, treated with conservative approach by super-selective embolization of the lower-pole segmental renal artery.
Keywords: Renal angiomyolipoma, Retroperitoneal haematoma
Introduction
Angiomyolipoma (AML) is the most frequent mesenchymal tumour of the kidney, composed of vascular, smooth muscle and fat elements. AML has an incidence of 0.1–0.22% in the general population, and is four times more frequent in women than in men (1). The lesions may present as sporadic cases or in association with tuberous sclerosis complex (TSC). TSC is an autosomal dominant neurocutaneous disorder that may affect several organs, e.g. brain, skin, eyes, heart, kidney and lungs. AMLs and renal cysts represent the renal manifestation of this syndrome (2).
TSC is diagnosed in 20% of all renal AML cases (3), 80% of which present as bilateral or multiple lesions (4–6). Sporadic variants are the most frequently encountered form of AML, and are typically diagnosed in patients with a mean age of 43 years (7).
Recent studies by Green et al. (12) and Paradis et al. (13) demonstrated the clonal nature of this neoplasm, which is associated with non-random X chromosome inactivation. In some cases of AML, abnormalities in the TSC2 region of chromosome band 16p13 have been identified (14, 15).
Perivascular epithelial cells (PEC) that give rise to the 3 cellular types identified in renal AML are the progenitor cells of AML. It has been shown that these precursors may differentiate into spindle-shaped cells with features of smooth muscle, fat and eosinophilic and epithelioid cells, giving rise to a family of neoplasms (PEComas) that includes angiomyolipoma, lipoangioleiomyoma and clear cell tumours of the lung and pancreas (14).
There is also an epithelioid variant of AML characterized by epithelioid proliferation (renal PEComa), which was recently added to the 2004 WHO classification of the renal tumours (16) and as such exhibits features of malignancy.
Since patients are usually asymptomatic, the diagnosis of AML is often incidental; this happens with lesions with a diameter of less than 4 cm. Lesions greater than 4 cm in diameter are often symptomatic and manifest with a clinical picture characterized by lumbar pain, anaemia and haematuria (7).
Retroperitoneal haemorrhage and/or bleeding into the renal collecting system are the major complications of AML; both conditions may put the patient’s life at risk.
The haemorrhagic tendency is related to the angiogenic component of AML, owing to the presence of aneurysmatic vessels with an irregular course (8), to tumour size and to the association with TSC (9).
The therapeutic strategy varies from case to case: selective embolization of the renal artery and surgical removal of the lesion are the pillars of AML management (11).
Alternatively, it is possible to follow the clinical course, with periodic surveillance of the lesion. Nephrectomy can be opted for in more severe cases. In case the aforementioned alternatives cannot be performed, a medical approach with hormonal therapy or with agents such as sirolimus, an inhibitor of the mammalian target of rapamycin (mTOR), can be chosen (10, 11).
Case report
A 41-year old female patient, weighing 73 kilograms and 158 cm tall, presented to us from another hospital with a diagnosis of retroperitoneal haematoma.
The patient had presented at the aforementioned hospital with a sudden onset of pain in the lumbar region and no evidence of fever, weight loss, anorexia, urinary retention or haematuria. The patient did not report any relevant diseases.
A non-contrast CT scan revealed a probable retroperitoneal haematoma that required angiography. Therefore, the patient was urgently transferred to our hospital.
The patient was in good general conditions. Pain was elicited upon deep palpation of the right flank, mesogastrium and right inguinal area. Giordano’s sign (renal percussion) was positive on the right side, and pain was elicited upon palpation of the right upper and middle ureteral points. Serial laboratory tests were performed, which showed a decrease in haemoglobin level from 10.8 g/dl (haematocrit 31.9%) to 9.3 g/dl (haematocrit 26.2%) over 24 hours; blood pressure was stable at about 120/70 mmHg, with a mean heart rate of 88 bpm.
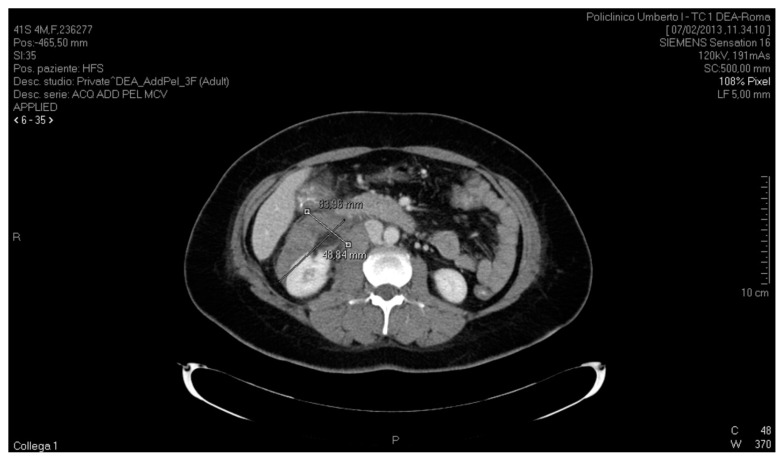
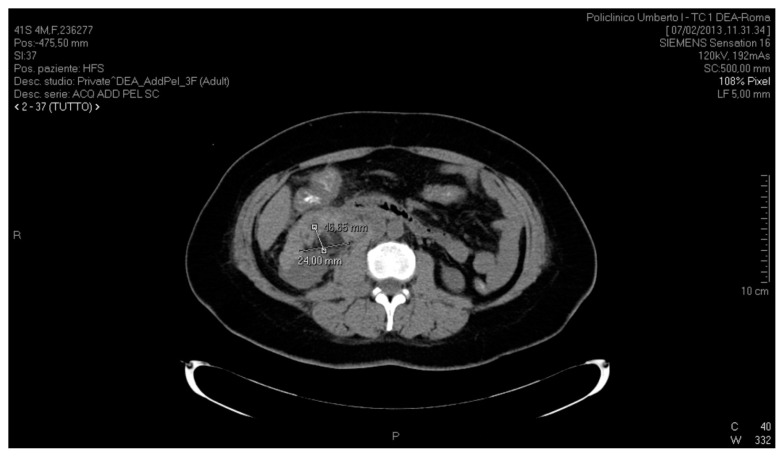
Diagnosis was confirmed both by contrast-enhanced and non-contrast CT scan of the upper and lower abdomen, which showed the presence of a copious serohaematic retroperitoneal collection mainly located in the anterior peri-renal space and along the right parietal colic sulcus, with a diameter of 83.96 mm × 48.84 mm, extending to the pelvis. The haematoma also extended to the vena cava, to the second duodenal portion, to the lower liver margin, encompassing the entire proximal-middle tract of the ureter on the same side. No active bleeding was documented during the arterial and venous contrast phases. On CT scan, right kidney contour presented irregular margins owing to the presence of a hypodense lesion at the lower third of the anterior surface of the kidney, with a maximum diameter of 46.65 mm × 24 mm, that had likely caused the adjacent bleeding (Figs. 1, 2).
Fig. 1.
CT scan of the abdomen, axial section, showing a retroperitoneal serohaematic collection in the anterior peri-renal space and along the right parietal colic sulcus, with a diameter of 83.96 mm × 48.84 mm.
Fig. 2.
CT scan of the abdomen, axial section, showing a hypodense lesion anterior to the lower third of the kidney, with a maximum diameter of 46.65 mm × 24 mm.
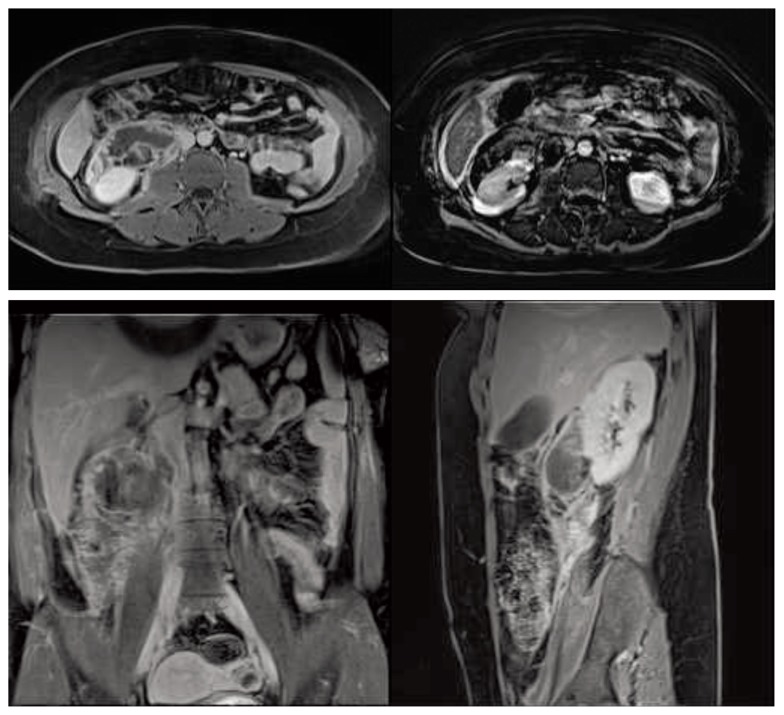
MRI was performed that confirmed the presence of the haematoma and revealed a nodular exophytic lesion arising from the lower third of the kidney on the same side, characterized by regular contours and non-homogeneous signal intensity, mainly containing fat tissue. Only slight enhancement of the lesion and no active bleeding were observed during the arterial, venous and late contrast phases (Fig. 3). Hence, the right retroperitoneal haematoma appeared to be caused by the rupture of an exophytic renal AML that could therefore be considered at high risk for re-bleeding.
Fig. 3.
MRI scan; axial, sagittal and coronal sections showing a right retroperitoneal haematoma extending to the pelvis and a nodular exophytic, fat-containing lesion of the right kidney.
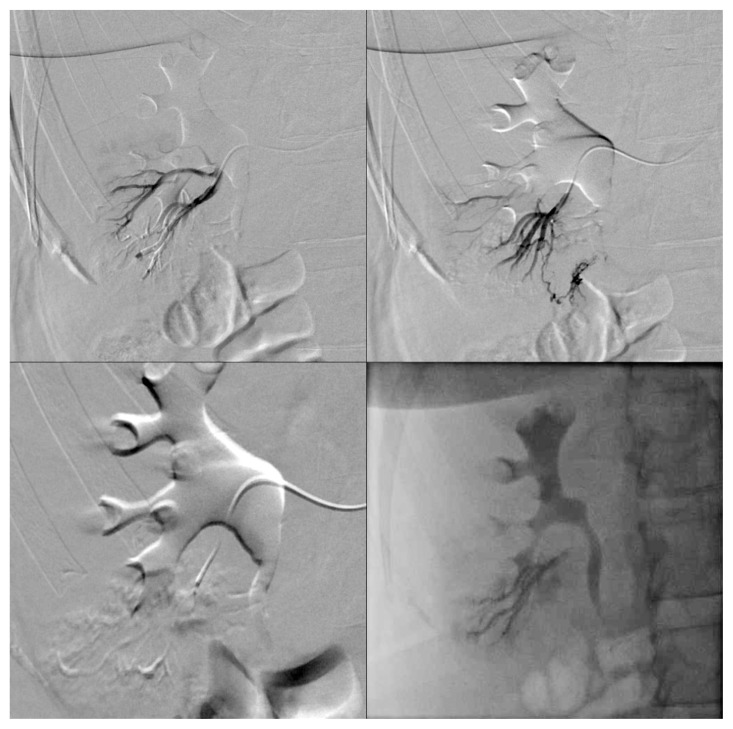
We chose to perform a superselective embolization of the afferent arteries via the right transfemoral arterial approach.
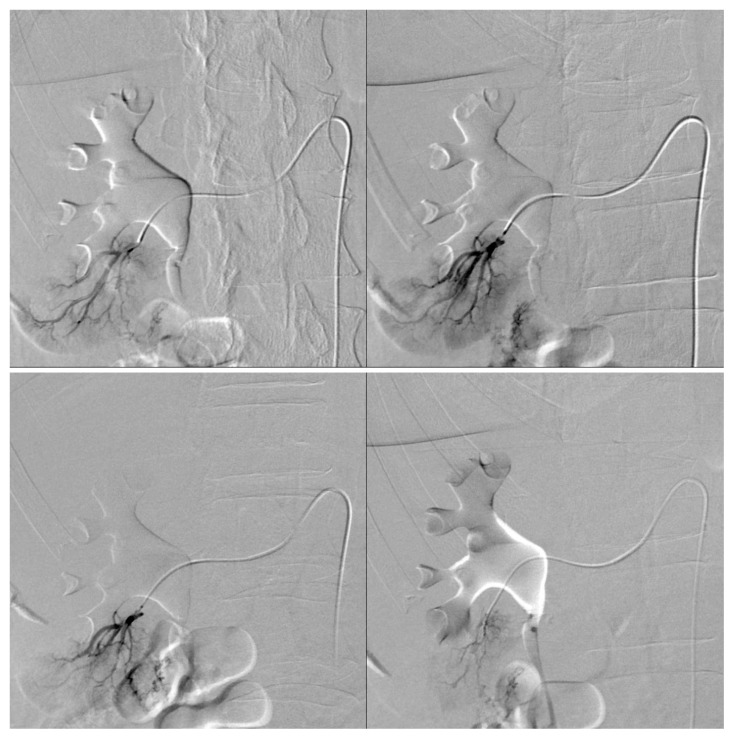
Following a selective arteriography of the right renal artery to locate the exophytic lesion, superselective catheterization of the lower-pole segmental renal artery was performed. This allowed identifying at least two small and irregular arterial branches supplying the lesion (Fig. 4). During the procedure it was possible to superselectively catheterize only one afferent arterial branch, which was embolized using 40 μm Embozene® microspheres. The final angiographic control showed cessation of blood flow in the treated vessel (Fig. 5), whereas attempts to catheterize other vessels were unsuccessful due to technical difficulties related to their tortuous and irregular course. Thus, only partial embolization was achieved due to the inability to occlude all arterial branches afferent to the AML. A follow-up CT scan will be performed three months after the procedure.
Fig. 4.
Pre-embolization angiogram showing two small arteries supplying the lesion after superselective catheterization of the lower-pole segmental renal artery.
Fig. 5.
Post-embolization angiogram showing cessation of blood flow in the branch of the right lower-pole segmental renal artery supplying the lesion.
Discussion
Renal AML is considered as a benign kidney tumour with hamartomatous features. AML is composed of heterogeneous tissues, including blood vessels, adipose tissue and smooth muscle, and may present as sporadic cases or in association with TSC.
In the case we described above, a diagnosis of TSC was ruled out due to absence of family history of TSC and lack of specific signs and symptoms, i.e. other intra-abdominal masses, facial angiofibromas, mental retardation, seizures, pulmonary lymphangiomyomatosis, subependymal calcifications, cortical, facial and periungual tubers, peau de chagrin (shagreen patches) (3, 4).
In the majority of cases, classic AML is easily diagnosed by recognition of fat tissue within the lesion, which appears hyperechoic on ultrasound, as an area of negative attenuation value on CT and as an area of high signal intensity on T1-weighted images with signal loss on MRI (17, 18). Recognizing the fat component is therefore essential to rule out a diagnosis of malignant renal tumour such as renal cell carcinoma (RCC), as well as of lipomas, liposarcomas and fat-containing RCCs (7). A percutaneous renal biopsy can be helpful in dubious cases (19).
Indications for treatment of AML include intractable pain, haematuria, suspicion of malignancy, large-size tumours, spontaneous ruptures and radiographic imaging suggestive of malignant lesions (4).
According to Oesterling et al. (20) and Steiner et al.(21) the choice of treatment should be based on both tumour size and symptoms. According to this approach, tumours >4 cm are frequently symptomatic and have a haemorrhagic tendency, and therefore require either selective embolization or surgical treatments such as partial nephrectomy, enucleation or wedge resection (4, 22), whereas tumours < 4 cm should be followed up with yearly CT scans or ultrasonography (4, 7).
SAE of renal artery is a safe and effective treatment for symptomatic and large-size AMLs.
Most of the AMLs treated with SAE show a mean reduction in size of about 43% (23). Nevertheless, in a minority of cases lesions do not shrink and, instead, they increase their size due to an increase in the nonvascular component (9). In such cases, it is advisable to confirm the initial diagnosis of AML with repeated angiography and, if needed, to re-treat the lesion by SAE (23).
SAE has been associated with less postoperative complications as compared with surgical approaches, and no haemorrhagic complications have been described during a 5-year follow up period. Furthermore, in a recent study by Ramon et al.(9) rates of post-embolization syndrome (i.e. fever and flank pain) were significantly lower as compared with other reports in the literature (9).
In the same study by Ramon et al.(9) patients initially treated with SAE were followed up for a mean of 4.8 years, during which no symptoms such as pain or bleeding occurred. Nevertheless, repeat embolization was needed in about 37% of cases due to neoangiogenesis or re-canalization of treated vessels.
No deaths have been described in relation to embolization treatment, and kidney function remains nearly unaltered after the intervention. Therefore, SAE is a minimally invasive procedure that is associated with optimal preservation of renal function (9).
Alternatively, a partial nephrectomy can be performed. This approach has higher complication rates (24), although it is associated with a lower incidence of recurrence as compared with SAE (9).
Drugs such as mTOR inhibitors (10) and anti-angiogenic agents (25) represent a non-invasive therapeutic option with a lower incidence of complications for the treatment of asymptomatic AML patients in whom SAE or nephron sparing surgery (NSS) (22) are difficult to perform.
Due to high morbidity related to the possible occurrence of renal insufficiency, radical nephrectomy is indicated only for the treatment of AMLs >8 cm (22), when suspicion of malignancy is high and when minimally invasive techniques cannot be performed (11).
Conclusions
Renal angiomyolipoma is a benign kidney tumour with hamartomatous features. Retroperitoneal haemorrhage is the most frequent complication of AML.
Among possible treatments, super-selective embolization of segmental arteries that supply the lesion is considered as the most effective minimally invasive approach in preventing haemorrhagic events and symptomatic manifestations. This procedure is advisable for AMLs with a diameter >4 cm. The procedure is well tolerated and is associated with minor complications, although it has been associated with frequent relapses as compared with surgical alternatives.
References
- 1.Fujii Y, Ajima J, Oka K, et al. Benign renal tumors detected among healthy adults by abdominal ultrasonography. Eur Urol. 1995;27:124–127. doi: 10.1159/000475142. [DOI] [PubMed] [Google Scholar]
- 2.Di Matteo G, Maturo A, Marzullo A, Peparini N, Wedard BM, Zeri KP, Di Matteo FM, Mascagni D. Giant abdominopelvic epithelioid angiomyolipoma associated with tuberous sclerosis: report of a case. Surg Today. 1999;29(11):1183–8. doi: 10.1007/BF02482270. [DOI] [PubMed] [Google Scholar]
- 3.Aruna RP, Ranjan C, Ashwani G, Brij Bhushan L. Giant aneurysm formation in sporadic renal angiomyolipoma. Radiology Case. 2010;4(6):21–27. doi: 10.3941/jrcr.v4i6.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyo CK, Won TK, Won SH, Jin SL, Hee JJ, Young DC. Trends of Presentation and Clinical Outcome of Treated Renal Angiomyolipoma Yonsei. Med J. 2010;51(5):728–734. doi: 10.3349/ymj.2010.51.5.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadley DA, Bryant LJ, Ruckle HC. Conservative treatment of renal angiomyolipomas in patients with tuberous sclerosis. Clin Nephrol. 2006;65:22–7. doi: 10.5414/cnp65022. [DOI] [PubMed] [Google Scholar]
- 6.Kim JW, Lee TW, Kim MJ, Oh MM, Bae JH, Park HS, et al. Spontaneous rupture of renal angiomyolipoma in a female tuberous sclerosis patient with pulmonary lymphangioleiomyomatosis. Korean J Urol. 2007;48:344–7. [Google Scholar]
- 7.Bakshi SS, Vishal K, Dnb, Kalia V, Gill JS. Aggressive Renal Angiomyolipoma Extending Into The Renal Vein And Inferior Vena Cava- An Uncommon Entity. The British Journal Of Radiology. 2011;84:166–168. doi: 10.1259/bjr/98449202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang JQ, Fielding JR, Zou KH. Etiology of spontaneous peri-renal hemorrhage: a meta-analysis. J Urol. 2002;167:1593–1596. doi: 10.1097/00005392-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Ramon J, Rimon U, Garniek A, Golan G, Bensaid P, Kitrey ND, Nadu A, Do ZA. Renal Angiomyolipoma: Long-term Results Following Selective Arterial Embolization. European Urology. 2009;55(5):1155–1162. doi: 10.1016/j.eururo.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Bissler JJ, McCormack FX, Young LR, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–51. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faddegon S, So A. Treatment of angiomyolipoma at a tertiary care centre: the decision between surgery and angioembolization. Can Urol Assoc J. 2011;5(6):138–141. doi: 10.5489/cuaj.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green AJ, Sepp T, Yates JR. Clonality of tuberous sclerosis hamartomas shown by non-random X-chromosome inactivation. Hum Genet. 1996;97:240–243. doi: 10.1007/BF02265273. [DOI] [PubMed] [Google Scholar]
- 13.Paradis V, Laurendeau I, Vieillefond A, et al. Clonal analysis of renal sporadic Angiomyolipomas. Hum Pathol. 1998;29:1063–1067. doi: 10.1016/s0046-8177(98)90414-2. [DOI] [PubMed] [Google Scholar]
- 14.Varma S, Gupta S, Talwar J, Forte F, Dhar M. Renal Epithelioid Angiomyolipoma: A Malignant Disease. Review Jnephrol. 2011;24(01):18–22. doi: 10.5301/jn.2010.5451. [DOI] [PubMed] [Google Scholar]
- 15.Kattar MM, Grignon DJ, Eble JN, et al. Chromosomal analysis of renal angiomyolipoma by comparative genomic hybridization: evidence for clonal origin. Hum Pathol. 1999;30:295–299. doi: 10.1016/s0046-8177(99)90008-4. [DOI] [PubMed] [Google Scholar]
- 16.Antonio LB, Scarpelli M, Montironi R, et al. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49:798–805. doi: 10.1016/j.eururo.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Merran S, Vieillefond A, Peyromaure M, Dupuy C. Renal angiomyolipoma with calcification: CT-pathology correlation. Br J Radiol. 2004;77:782–783. doi: 10.1259/bjr/33776173. [DOI] [PubMed] [Google Scholar]
- 18.Maizlin Z, Gottlieb P, Corat-Simon Y, Strauss S. Various Appearances of Multiple Angiomyolipomas in the Same Kidney in a Patient Without Tuberous Sclerosis. J Ultrasound Med. 2002;21:211–213. doi: 10.7863/jum.2002.21.2.211. [DOI] [PubMed] [Google Scholar]
- 19.Metro MJ, Ramchandani P, Banner MP, Siegelman ES, Stolpen AH, Wein AJ, et al. Angiomyolipoma of the renal sinus: diagnosis by percutaneous biopsy. Urology. 2000;55(2):286. doi: 10.1016/s0090-4295(99)00427-6. [DOI] [PubMed] [Google Scholar]
- 20.Oesterling JE, Fishman EK, Goldman SM, Marshall FF. The management of renal angiomyolipoma. J Urol. 1986;135:1121–4. doi: 10.1016/s0022-5347(17)46013-7. [DOI] [PubMed] [Google Scholar]
- 21.Steiner MS, Goldman SM, Fishman EK, Marshall FF. The natural history of renal angiomyolipoma. J Urol. 1993;150:1782–6. doi: 10.1016/s0022-5347(17)35895-0. [DOI] [PubMed] [Google Scholar]
- 22.Shirotake S, Yoshimura I, Kosaka T, Matsuzaki S. A case of angiomyolipoma of the renal sinus. Clin Exp Nephrol. 2011;15:953–956. doi: 10.1007/s10157-011-0519-9. [DOI] [PubMed] [Google Scholar]
- 23.Harabayashi T, Shinohara N, Katano H, Nonomura K, Shimizu T, Koyanagi T. Management of renal angiomyolipomas associated with tuberous sclerosis complex. J Urol. 2004;171:102–105. doi: 10.1097/01.ju.0000100100.36354.61. [DOI] [PubMed] [Google Scholar]
- 24.Stephenson AJ, Hakimi AA, Snyder ME, Russo P. Complications of radical and partial nephrectomy in a large contemporary cohort. J Urol. 2004;171:130–134. doi: 10.1097/01.ju.0000101281.04634.13. [DOI] [PubMed] [Google Scholar]
- 25.Simmons JL, Hussain SA, Riley P, Wallace DM. Management of renal angiomyolipoma in patients with tuberous sclerosis complex. Oncol Rep. 2003;10:237–41. [PubMed] [Google Scholar]