Summary
Aim
To demonstrate the utility of the renal artery embolization (RAE) in the dissection of gross neoplasms and the reduction in blood loss and operative time.
Case report
We report a case of a gross left renal neoplasm (with the diameter of about 12 cm) in a 45 years old Caucasian female who underwent to renal artery embolization 24 hours before left nephroureterectomy. This procedure has determined a reduction in operative times (about 90 minutes) because of the ligature of the renal vein was facilitated. Intraoperative blood loss was of about 100 ml and the patient didn’t need of blood transfusions; the abdominal drain was removed in third postoperative day (daily drained serous fluid was about 20 ml). The patient was discharged 7 days later.
Conclusion
RAE facilitates the dissection of gross neoplasms (diameter > than 10 cm), so causing a reduction in intraoperative blood loss and in blood transfusion. The operative times are lower because the ligature of the renal vein is less difficult and the dissection is facilitated for the presence of tissue oedema. The disadvantages are the incomplete hembolyzation, coil migration, hematomes, post-infarction syndrome (nausea, vomit, abdominal pain, leucocytosis, hyperpyrexia, hematoma); other risks include the possibility of pulmonary embolism, intestinal infarction and infections. ts reduced utilization could be due to the lack of randomized prospective studies showing its potential benefits.
Keywords: Renal artery embolization, Blood loss, Post-infarction syndrome, Kidney, Malignancy
Introduction
Renal artery embolization (RAE), firstly described in 1969 by Lalli AF and Peterson N, was above all indicated in the symptomatic treatment of the hematuria and in the palliation of metastatic renal cancer (1, 2).
With technical advances and growing experience, the indications have broadened to include conditions such as vascular malformations, medical renal disease, angiomyolipomas and preoperative infarction. The introduction of smaller delivery catheters and more precise embolic agents has drastically improved the morbidity associated with this technique (3).
However, opinions on the role of preoperative RAE in the management of patients with renal clear cells carcinoma are controversial (4).
Although a significant number of studies on RAE are reported in these patients, there is no consensus on the benefits and morbidity associated with the procedure (5, 6).
Most proponents of preoperative RAE cite the facilitation of nephrectomy through decreased operative blood loss, ease of dissection secondary to oedema in tissue planes and decreased operative times (7).
It is likely that RAE is underutilized perhaps because of a lack of prospective randomized studies demonstrating these potential benefits (8).
The aim of this report is to demonstrate the utility of RAE in the dissection of gross renal neoplasms and in blood loss and operative times reduction.
Case report
A 45-years-old female was admitted to our Surgical Unit because of fever, severe anaemia (Hb:7,6 g/dl), weight loss and macroscopic hematuria.
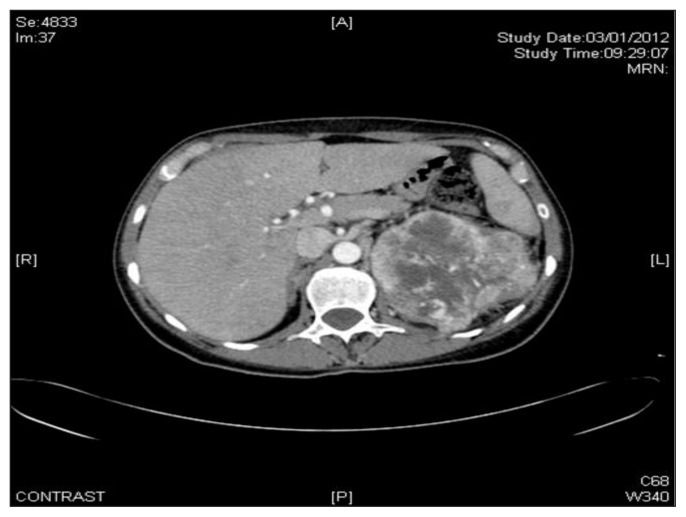
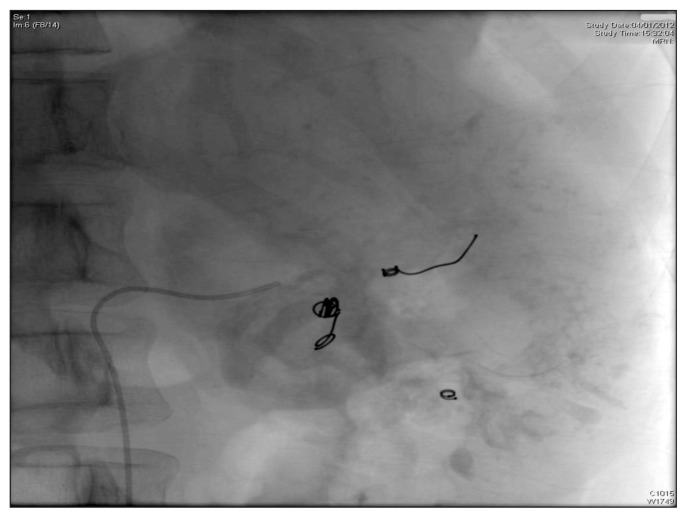
The admission CT total body showed a gross neoplasm (about mm 87 × 102) of the left kidney with intralesional vascularization associated to renal and paraortic lymphadenopathy (Fig. 1). The patient underwent to arteriography which showed an eteroplastic neoplasm and then to embolization of the distal branches of division of renal artery by metal coils (Fig. 2) such to allow surgical clamping and ligation during the subsequent nephrectomy without hindrance by metallic coils in the renal artery trunk (Fig. 3).
Fig. 1.
CT total body showing a gross neoplasm (about mm 87 × 102) of the left kidney with intralesional vascularization, renal and paraortic lymphadenopathy.
Fig. 2.
Embolization of the distal branches of division of left renal artery, performed 24 hours before nephroureterectomy.
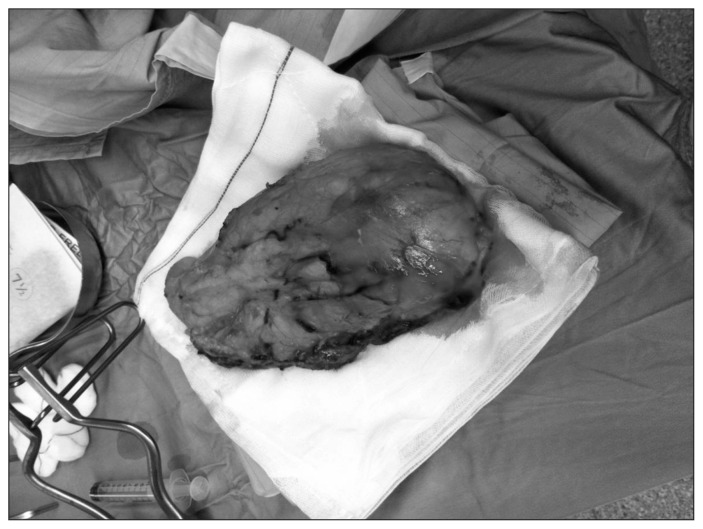
Fig. 3.
Kidney clear cells carcinoma (pT2b, pN0, G3, Stage II according to AJCC 2010); the margins of the surgical resection and the lymphnodes removed were free.
24 hours later a left nephroureterectomy was performed; however, the neoplasm was tightly sticking to the diaphragm so that it was required its removal en bloc with a small portion of this muscle. After paraortic lymphadenectomy, a drain in left renal space was inserted.
Intraoperative blood loss was of about 100 ml and the patient didn’t need of blood transfusions. The postoperative period was complicated by left apical pneumothorax, without needing a chest drain and by a left basal pneumonia with a small layer of ipsilateral pleural effusion, healed by antibiotic therapy targeted.
The abdominal drain was removed three days later (daily drained serous fluid was about 20 ml) and the patient discharged in 7th post-operative day.
The histological diagnosis was kidney clear cells carcinoma with a diameter of 12 cm, confined to renal parenchyma (pT2b, pN0, G3, Stage II according to AJCC 2010); Gerota’s fascia, margins of the surgical resection and twelve lymphnodes removed were free (Fig. 3).
The patient didn’t undergo adjuvant chemotherapy, but only a close oncological follow-up.
Discussion
Renal artery embolization (RAE), is above all utilized in malignant neoplasm, including infarction before nephrectomy, prevention or treatment of acute tumor hemorrhage and in palliation.
The rationale for renal artery embolization palliation is reduction of tumor bulk and providing symptomatic relief in patients with unresectable renal carcinoma or potentially resectable lesions in patients considered to be poor surgical candidates.
End-stage renal disease in hemodialysis, post-transplant severe nephrotic syndrome, hematuria and intractable pain, are some conditions that can benefit from complete renal artery embolization, because this procedure provides symptomatic relief by obliteration of renal function, avoiding the morbidity and mortality associated with nephrectomy (9, 10).
RAE is also indicated in order to prevent hemorrhage from angiomyolipomas, eliminating the need for blood transfusion (11).
Before this procedure, it’s recommended prophylactic antibiotic coverage. Moderate sedation and administration of local anesthetic to the access site is typically adequate, although some authors find that the procedure can be performed more expeditiously and safely under general anesthesia (12).
Vascular access is generally obtained via either the ipsilateral or contralateral common femoral artery using an 18 or 19 Gauge puncture needle via a single-wall (modified Seldinger) puncture technique. If the femoral arteries are occluded, an alternative access site, such as the axillary or brachial artery, may be necessary (13).
Several types of materials are available for transcatheter renal artery embolization, including metallic coils, sclerosants (liquids), and particulate embolic material. Metallic coils may be composed of stainless steel, platinum, or nitinol, and some incorporate synthetic fibers for promoting thrombogenicity.
Particulate embolization agents can be composed of either biodegradable (gelfoam) or permanent materials, such as polyvinyl alcohol (PVA) and embospheres.
Liquid agents include N-butyl-2-cyanoacrylate (NBCA) glue, 98–99% ethanol, ethibloc, bucrylate, Sotradecol foam, and lipiodol (13).
It must be performed a complete aortogram to evaluate for additional arteries that may be supplying the tumor or accessory renal arteries.
Several renal artery techniques can be performed, such as partial renal artery embolization, superselective embolization or total embolizazion.
Partial renal artery embolization techniques are used when it is desirable to eliminate vascular supply to a portion of the kidney with the goal of minimizing the destruction of functioning kidney. This can be accomplished by selective catheterization of segmental/lobar renal artery branches supplying a lesion. Embolization of such arteries may cause segmental infarcts of the kidney. Alternatively, superselective embolization can provide controlled occlusion of specific minuscule renal artery branches that feed a lesion, with minimal compromise of surrounding normal vascularization.
On the other hand, the goal of total embolization is complete obliteration of renal function or elimination of blood supply to tumors that involve a large portion of the renal parenchyma (14).
The benefits of renal artery embolization before nephrectomy for renal cell carcinoma include immunologic response and decreased tumor size and vascularity, thereby enabling less extensive surgical resection and intraoperative blood loss. In addition, embolization results in oedema of the kidney and tumor, which facilitates its resection (15).
Schwartz et Al suggest that the optimum delay to surgery after RAE is 24–48 hours to maximize the benefits of tissue oedema, to allow the surgeon to proceed before collateral vessels formed and to minimize the period of post-infarction syndrome (8).
However, May et Al indicate that preoperative renal artery embolization does not improve the survival of patients after surgery in renal cell carcinoma (16).
Complications such incomplete embolization, coil migration, and groin hematomas occur in less than 2% of patients after RAE.
Inadvertent nontarget embolization can result in spine, lower extremity, and bowel infarction. Similarly, large embolization agent reflux associated with subselective techniques resulting in loss of renal function kidney and PVA embolization causing pulmonary embolism and hypertension are other known adverse outcomes of renal artery embolization.
Overall, the incidence of infection related to renal artery embolization is very low (15). Also uncommon after renal artery embolization for tumors is necrosis requiring percutaneous drainage (17).
Post-infarction syndrome is a very common occurrence after renal artery embolization, particularly with complete embolization, for which over 90% of patients are afflicted. The syndrome is generally mild and consists of flank pain, fever, nausea or vomiting, and elevated white blood cell count beginning 1–3 days after renal artery embolization.
Treatment is symptomatic and consists of analgesics, antipyretics, and antiemetics as needed, although spontaneous resolution occurs within several days (18).
Conclusions
RAE is an effective therapeutic and adjuvant tool because it facilitates the dissection of large renal tumours and tumours with extensive involvement around the renal hilum; it also decreases operative bood loss and operative time, leading to lower overall morbidity and transfusion requirement.
However, the lack of randomized prospective studies is the primary reason that RAE is not used often before surgery (8).
References
- 1.Turini D, Nicita G, Fiorelli C, Selli C, Villari N. Selective transcatheter arterial embolization of renal carcinoma: an original technique. J Urol. 1976;116:419–21. doi: 10.1016/s0022-5347(17)58840-0. [DOI] [PubMed] [Google Scholar]
- 2.Kadir S, Marshall FF, White RI, Jr, Kaufman SL, Barth KH. Therapeutic embolization of the kidney with detachable silicone balloons. J Urol. 1983;129:11–3. doi: 10.1016/s0022-5347(17)51894-7. [DOI] [PubMed] [Google Scholar]
- 3.Keller FS. Interventional radiology: new paradigms for the new millennium. J Vasc Interv Radiol. 2000;11:677–81. doi: 10.1016/s1051-0443(07)61625-x. [DOI] [PubMed] [Google Scholar]
- 4.Teasdale C, Kirk D, Jeans WD, Penry JB, Tribe CT, Slade N. Arterial embolization in renal carcinoma: a useful procedure? Br J Urol. 1982;54:616–9. doi: 10.1111/j.1464-410x.1982.tb13608.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaisary AV, Williams G, Riddle PR. The role of preoperative embolization in renal cell carcinoma. J Urol. 1984;131:641–6. doi: 10.1016/s0022-5347(17)50556-x. [DOI] [PubMed] [Google Scholar]
- 6.Christensen K, Dyreborg U, Andersen JF, Nissen HM. The value of transvascular embolization in the treatment of renal carcinoma. J Urol. 1985;133:191–3. doi: 10.1016/s0022-5347(17)48877-x. [DOI] [PubMed] [Google Scholar]
- 7.Bakal CW, Cynamon J, Lakritz PS, Sprayregen S. Value of preoperative renal artery embolization in reducing blood transfusion requirements during nephrectomy for renal cell carcinoma. J Vasc Interv Radiol. 1993;4:727–31. doi: 10.1016/s1051-0443(93)71958-2. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz Michael J, Smith Eric B, Trost David W, Vaughan E Darracott., Jr Renal artery embolization: clinical indications and experience from over 100 cases. Upper Urinary Tract. 2006:881–885. doi: 10.1111/j.1464-410X.2006.06653.x. [DOI] [PubMed] [Google Scholar]
- 9.Zielinski H, Szmigielski S, Petrovich Z. Comparison of preoperative embolization followed by radical nephrectomy with radical nephrectomy alone for renal cell carcinoma. Am J Clin Oncol. 2000;23:6–12. doi: 10.1097/00000421-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell NJ, Saleem Amer N, Rogers E, et al. Renal artery embolization in the palliative treatment of renal carcinoma. Br J Radiol. 2007;80:96–102. doi: 10.1259/bjr/31311739. [DOI] [PubMed] [Google Scholar]
- 11.Rimon U, Duvdevani M, Garniek A, et al. Large renal angiomyolipomas: digital subtraction angiographic grading and presentation with bleeding. Clin Radiol. 2006;61:520–526. doi: 10.1016/j.crad.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Abath C, Andrade G, Cavalcanti D, et al. Complex renal artery aneurysms: liquid or coils? Tech Vasc Interv Radiol. 2007;10:299–307. doi: 10.1053/j.tvir.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Ginat Daniel T, MD, MS, Saad Wael EA, MBBCh, Turba Ulku C., MD Transcatheter Renal Artery Embolization: Clinical Applications and Techniques. Tech Vasc Interventional Rad. 2009;12:224–239. doi: 10.1053/j.tvir.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Ginat Daniel T, MD, MS, Saad Wael EA, MBBCh, Turba Ulku C., MD Transcatheter Renal Artery Embolization for Management of Renal and Adrenal Tumors. Tech Vasc Interventional Rad. 2010;13:75–88. doi: 10.1053/j.tvir.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Wallace S, Chuang VP, Swanson D, et al. Embolization of renal carcinoma. Radiology. 1981;138:563–570. doi: 10.1148/radiology.138.3.7465831. [DOI] [PubMed] [Google Scholar]
- 16.May M, Brookman-Amissah S, Pflanz S, Roigas J, Hoschke B, Kendel F. Pre-operative renal arterial embolisation does not provide survival benefit in patients with radical nephrectomy for renal cell carcinoma. The British Journal of Radiology. 2009;82:724–731. doi: 10.1259/bjr/17514226. [DOI] [PubMed] [Google Scholar]
- 17.Hamlin JA, Smith DC, Taylor FC, et al. Renal angiomyolipomas: long-term follow-up of embolization for acute hemorrhage. Can Assoc Radiol J. 1997;48:191–198. [PubMed] [Google Scholar]
- 18.Cox GG, Lee Kr, Price HI, et al. Coloni infarction following ethanol embolization of renal-cell carcinoma. Radiology. 1982;145:343–345. doi: 10.1148/radiology.145.2.7134432. [DOI] [PubMed] [Google Scholar]