Abstract
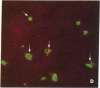
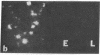
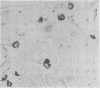
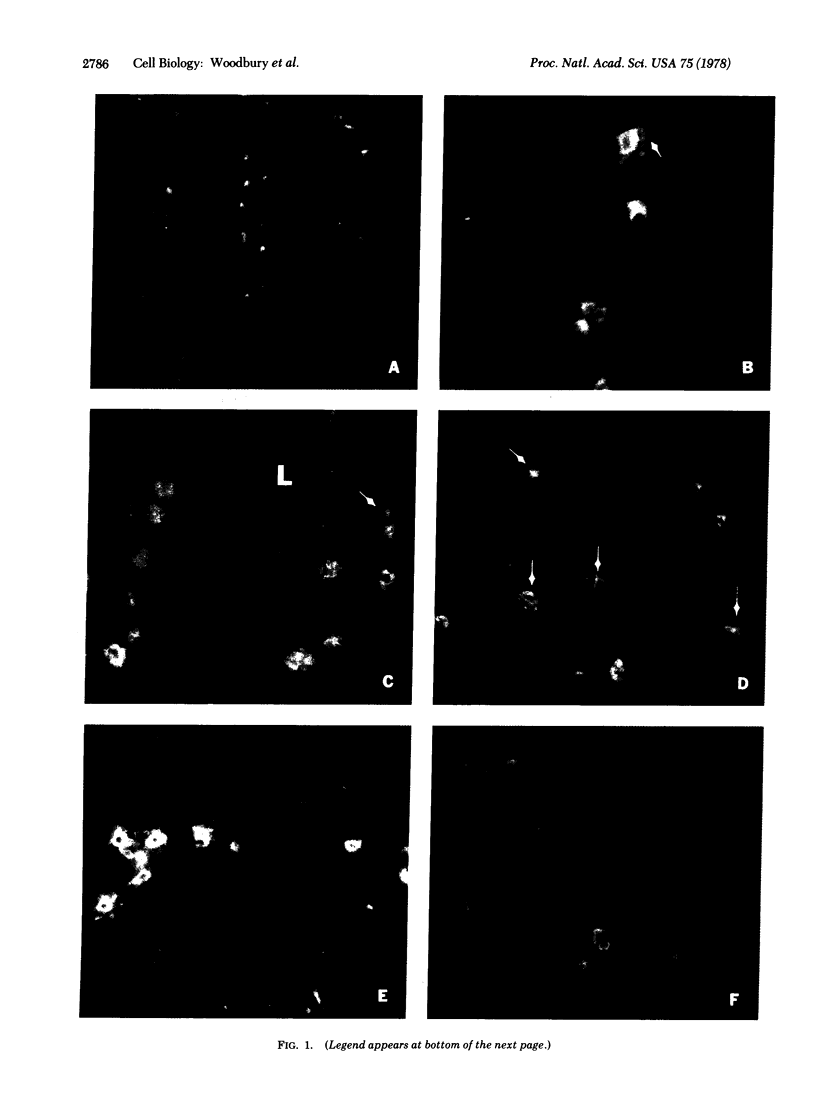
An intracellular serine protease, which is believed to initiate the degradation of several intracellular pyridoxal phosphate-dependent enzymes, was localized by immunofluorescence in atypical mast cells of the lamina propria and in intraepithelial cells of the rat small intestine. Some mucus-secreting goblet cells also contained the protease antigen. Atypical mast cells containing the enzyme were present in large numbers beneath the epithelium of bronchioles. All atypical mast cells also contained low levels of the chymotrypsin-like protease of normal mast cells. Both enzymes were consistently present in normal connective tissue mast cells. Amino acid content, molecular weight, and lack of immunologic crossreactivity indicate that the two enzymes are similar but not identical. The cell-specific localization of the intestinal serine protease makes it unlikely that the enzyme has any general role in the degradation of pyridoxal phosphate-dependent enzymes. The function of the enzyme in mast cells, atypical mast cells, and intestinal goblet cells is not known.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDITT E. P., ARASE M. An enzyme in mast cells with properties like chymotrypsin. J Exp Med. 1959 Sep 1;110:451–460. doi: 10.1084/jem.110.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerbäck L. Mast cells in rat gastrointestinal mucosa. I. Effects of fixation. Acta Pathol Microbiol Scand. 1966;66(3):289–302. doi: 10.1111/apm.1966.66.3.289. [DOI] [PubMed] [Google Scholar]
- Katunuma N., Kominami E., Kobayashi K., Banno Y., Suzuki K. Studies on new intracellular proteases in various organs of rat. 1. Purification and comparison of their properties. Eur J Biochem. 1975 Mar 3;52(1):37–50. doi: 10.1111/j.1432-1033.1975.tb03970.x. [DOI] [PubMed] [Google Scholar]
- Katunuma N., Kominami E., Kominami S. A new enzyme that specifically inactivates apo-protein of pyridoxal enzymes. Biochem Biophys Res Commun. 1971 Oct 1;45(1):70–75. doi: 10.1016/0006-291x(71)90051-9. [DOI] [PubMed] [Google Scholar]
- Lagunoff D., Pritzl P. Characterization of rat mast cell granule proteins. Arch Biochem Biophys. 1976 Apr;173(2):554–563. doi: 10.1016/0003-9861(76)90292-7. [DOI] [PubMed] [Google Scholar]
- Melville J. C., Ryan C. A. Chymotrypsin inhibitor I from potatoes. Large scale preparation and characterization of its subunit components. J Biol Chem. 1972 Jun 10;247(11):3445–3453. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]